Abstract
An earlier review of the toxicity of glyphosate and the original Roundup™-branded formulation concluded that neither glyphosate nor the formulation poses a risk for the production of heritable/somatic mutations in humans. The present review of subsequent genotoxicity publications and regulatory studies of glyphosate and glyphosate-based formulations (GBFs) incorporates all of the findings into a weight of evidence for genotoxicity. An overwhelming preponderance of negative results in well-conducted bacterial reversion and in vivo mammalian micronucleus and chromosomal aberration assays indicates that glyphosate and typical GBFs are not genotoxic in these core assays. Negative results for in vitro gene mutation and a majority of negative results for chromosomal effect assays in mammalian cells add to the weight of evidence that glyphosate is not typically genotoxic for these endpoints in mammalian systems. Mixed results were observed for micronucleus assays of GBFs in non-mammalian systems. Reports of positive results for DNA damage endpoints indicate that glyphosate and GBFs tend to elicit DNA damage effects at high or toxic dose levels, but the data suggest that this is due to cytotoxicity rather than DNA interaction with GBF activity perhaps associated with the surfactants present in many GBFs. Glyphosate and typical GBFs do not appear to present significant genotoxic risk under normal conditions of human or environmental exposures.
Introduction
Glyphosate is an active ingredient (a.i.) in very widely used herbicide formulations. Accordingly, the toxicity of glyphosate and glyphosate-based formulations (GBFs) has been extensively studied. An earlier extensive review of glyphosate and glyphosate formulation safety and risk assessment included descriptions and analyses of genetic toxicology studies of glyphosate and Roundup™-branded and other glyphosate formulations (Williams et al., Citation2000). These studies included a wide variety of test systems and endpoints. Subsequent to this review a number of genotoxicity studies of glyphosate and GBFs have been published in the literature. Additionally, there are large number of genetic toxicology studies of glyphosate and GBFs sponsored by companies that were not included in the previous review. The number and diversity of these studies warrant careful examination and integration of their findings with previous results to produce an updated assessment of the overall genotoxicity profile for glyphosate and a genotoxicity profile that is typical of the GBFs.
Identification and analysis of published studies
The published studies for review consideration were identified by literature searches for published reports containing references to glyphosate that also contained searchable terms which indicated that genotoxicity studies were performed. Details of search procedures are provided in the “online supplementary material”. Each identified publication was evaluated to verify that it contained original results of one or more experimental genotoxicity studies on glyphosate or GBFs. Monitoring studies are not included in this review. Emphasis was placed on publications in peer-reviewed journals. Abstracts or other sources with incomplete information were not considered. Reviews without original data were not considered for the evaluation; however, these reviews were examined to determine if there were any cited publications that had not been detected in the literature searches.
Each relevant publication was examined using several criteria to characterize the scientific quality of the reported genetic toxicology studies. Useful, objective criteria for this purpose were international guidelines for genetic toxicology studies formulated by expert groups. These include principles for conducting studies, reporting results, and analyzing and interpreting data. Some of the principles of the guidelines are generally applicable to all studies, while others are specific for a particular type of test system and endpoint. Some of the specific types of studies encountered in the review do not yet have international guidelines; however, some of the guideline elements should be generically applicable to these studies. The guidelines for genetic toxicology tests developed for the Organization for Economic Co-operation and Development (OECD) are a pre-eminent source of internationally agreed guidelines. Other international and national guidelines for regulatory genetic toxicology testing are usually concordant with the OECD guidelines. The “online supplementary material” contains a summary table of some key OECD guideline criteria that were found to be relevant to the analysis of the studies considered in this review.
Comparison of the published studies to the criteria in guidelines used for regulatory purposes does not represent an absolute judgment standard but can provide a way for evaluating the quality of the protocols used in various published studies. Some of the criteria are rarely met in scientific publications and should be given little or no weight in evaluating the studies. For example, data for individual cultures and individual animals are not commonly included in publications in scientific journals. These data are presumably collected but are usually summarized as group means with a measure of variance for the treatment and control groups. This is not considered to be a significant omission in a scientific publication. However, other guideline features are more essential as scientific quality standards and should be considered as having greater weight in evaluating a study. For example, there are consistent recommendations that assays involving visual scoring (e.g. chromosomal aberration, micronucleus and sister chromatid exchange (SCE) endpoints) should use slides that are independently coded so that scoring is performed without any knowledge of the treatment or control group being scored. This guidance is good scientific practice and studies that do not explicitly include a description of coding or “blind” scoring in the methodology would appear to have a deficiency either in the methodology, or perhaps a limitation in the description of the methodology used if coding was actually used and either not indicated or was assumed to be indicated by a reference citation. Other examples of guideline features that have clear experimental scientific value are the use of concurrent negative and positive controls and concurrent measurement and reporting of toxicity endpoints in main experiments, especially in in vitro mammalian cell assays.
Review and analysis of sponsored regulatory studies
Reports of sponsored genetic toxicology studies were provided by the companies. The studies were sponsored by companies for regulatory purposes and were conducted at in-house or contract toxicology laboratories. For brevity, the industry-sponsored regulatory studies will be subsequently referred to as regulatory studies.
Each study examined was stated to have been conducted in accordance with Good Laboratory Practice (GLP) standards with almost all studies citing the OECD Principles of Good Laboratory Practice (OECD GLP, Citation1982, Citation1997). Reports also cited compliance with various national and regional GLP Guidelines (e.g. European Commission GLP Directives 87/18/EEC or 88/320/EEC; U.S. Environmental Protection Agency Good Laboratory Practice Standards, 40 CFR Part 160; Japanese Ministry of Agriculture, Forestry, and Fisheries (MAFF) Good Laboratory Practice Standards, 11 Nousan No. 6283). Variations from GLPs were considered not to have significantly impacted the study results.
Almost all the studies were reported to have been conducted in accordance with the relevant OECD test guidelines applicable at the time of the study. Study reports were examined to determine that the protocols and experimental methods for the report were consistent with the OECD guidelines and any deviations were noted and considered. Report data were examined to confirm the conclusion of the report regarding whether treatment-related activity had been observed.
Glyphosate structure activity analysis
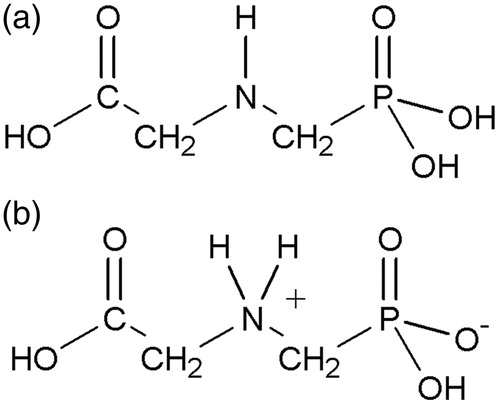
Glyphosate consists of the amino acid glycine joined with a phosphonomethyl group (). Glyphosate was evaluated for mutagenic structural alerts using Derek for Windows software (Llhasa Ltd., Leeds, UK, Version 11.0.0, 24 October 2009). No structural alerts were identified for chromosomal damage, genotoxicity, mutagenicity or carcinogenicity. The structural components of the glyphosate molecule are not known to be genotoxic; therefore, the lack of structure activity alerts for glyphosate was expected.
GBF compositions
Glyphosate-based formulations are herbicide formulations which, by definition, contain the a.i. glyphosate typically in a salt form (e.g. isopropylamine or potassium glyphosate), but the % glyphosate may be expressed in acid equivalents (a.e.) as percent weight of glyphosate acid without the counter ion. In addition to the a.i., other compounds are included in the formulation to help achieve or improve the herbicidal activity for the desired application. A very common functional component, especially for terrestrial applications, is a compound (or compounds) with surfactant activity that enables better penetration of the a.i. through leaf surfaces. Because formulation compositions are considered proprietary, their specific compositions are not generally indicated in literature reports and are not publicly available for regulatory studies. GBF test materials are usually identified with names or designations and should include either % a.i. or a.e. detail.
It should be noted that a common problem encountered in the published literature is the use of the terms “glyphosate”, “glyphosate salt” or “Roundup” to indicate any kind of GBF that contains additional components such as surfactants. Published results from studies with different formulations have sometimes been incorrectly or inappropriately attributed to the a.i. The original Roundup™-branded formulation (MON 2139), containing 41% isopropylamine glyphosate salt and 15.4% MON 0818 (a polyethoxylated tallowamine based surfactant blend), is no longer sold in many markets. However, other GBFs are sold under the Roundup™ brand name with varying glyphosate forms, concentrations and surfactant systems. Clear identification of the test material is very important in toxicology studies because the toxicity of formulations can be dramatically different from the a.i. The fact that test materials identified as Roundup™-branded formulations may actually have different compositions should be considered when comparing results of different studies, as should the possibility that any observed effects may be due to specific GBF components other than the glyphosate active ingredient.
Gene mutation endpoint
Bacterial reversion assays
Glyphosate and glyphosate salts
As reviewed by Williams et al. (Citation2000), six reports of bacterial reversion assays for glyphosate were all negative. No reports of bacterial reversion assays for glyphosate were encountered in the subsequent literature.
A large number of regulatory bacterial reversion assays have been conducted on technical glyphosate and glyphosate salt solutions. These 18 assays are presented in . Summary data tables and associated information for the regulatory studies are available in “online supplementary material”. Methodology and experimental design for these studies was generally in compliance with OECD Guideline 471 (OECD 471, Citation1997) for studies conducted in or after 1997. The previous guidelines (OECD 471, Citation1983, for Salmonella strains; OECD 472, Citation1983, for Escherichia coli strains) were used for studies conducted before 1997. All of the assays employed a core battery of Salmonella typhimurium test strains (TA98, TA100, TA1535 and TA1537 or TA 97a) and most of the assays employed additional S. typhimurium TA102 or E. coli WP2-derived strains to detect oxidative and cross-linking effects as recommended in OECD 471 (Citation1997). Limitations for some of the studies included three studies using larger than half-log dose level spacing and some studies did not employ a confirmatory assay. One study used positive controls not requiring exogenous metabolic activation for two strains in the presence of S9 (9000×g liver homogenate supernatant). Although this may be considered as a deficiency, in that the activity of the S9 was not thoroughly checked, it is only in one of the 18 studies. The top concentration employed in the assays ranged from 1000 to 5000 µg/plate with most of the studies using the OECD guideline limit dose of 5000 µg/plate. With only a couple of exceptions, the top dose tested produced the toxicity as evidenced by thinning of the background lawn, reduction in revertants/plate or both.
Table 1. Bacterial reversion assays.
None of the studies exhibited revertants/plate exceeding threshold criteria for a positive response: greater than three times the control value for strains with low spontaneous revertants/plate (TA1535 and TA1537) or exceeding two times the control value for the other strains (Kier et al., Citation1986). Some studies reported statistical effects. However, none of these cases involved as much as two-fold elevations in revertants per plate and the observations were not consistent with biologically plausible dose-responses. In cases with repeated experiments, any increases in revertants/plate were generally not reproducible between experiments. Therefore, none of the statistically significant effects were judged to indicate mutagenic activity of the test material. Thus, all of the 18 bacterial reversion studies were concluded to be negative as judged by the absence of significant, reproducible, dose-related increases in revertants/plate. These studies provide abundant weight of evidence that glyphosate and glyphosate salt solutions are negative in bacterial reversion assays under experimental conditions that generally satisfy the OECD guidelines.
Glyphosate-based formulations
As reviewed by Williams et al. (Citation2000) most bacterial reversion studies (Ames/Salmonella test strains) for GBFs were negative. Four studies reported negative results for Roundup™-, Rodeo™- and Direct™-branded GBFs. A reported positive Ames/Salmonella result for a Roundup™-branded formulation was not replicated in these studies.
Subsequent to the Williams et al. (Citation2000) review only one published GBF bacterial reversion assay was reviewed (). This publication reported a negative Ames/Salmonella assay result for a GBF of undefined glyphosate composition, Percozyd 10 SL (Chruscielska et al., Citation2000). Although this result is consistent with the majority of negative Ames/Salmonella results for GBFs, the reported study results have significant limitations. One of the recommended test strains, TA1535, was not used and results were only presented as “−” without a presentation of revertants/plate data.
A large number of regulatory bacterial reversion assays have been conducted on GBFs. These are presented in with summary data tables in “online supplementary material”. Methodology and experimental design for these studies was generally in compliance with the OECD Guideline 471 (OECD 471, Citation1997) and with other guidelines. However, two of the studies used some dose level spacings that were larger than the recommended maximum half-log spacing and four studies did not employ a confirmatory assay. All of the assays employed a core battery of S. typhimurium test strains (TA98, TA100, TA1535 and TA1537) and employed an additional S. typhimurium TA102 or E. coli WP2-derived strain to detect oxidative and cross-linking DNA effects as recommended in OECD 471 (Citation1997). The top concentration employed in the assays ranged from 100 to 5000 µg/plate for plate incorporation methodology. With only two exceptions the top dose tested produced the toxicity as evidenced by thinning of the background lawn, reduction in revertants/plate or both. For the two exceptions, the toxicity was noted at higher concentrations per plate in rangefinder assays but the toxicity was not noted for the maximum dose selected for the mutagenicity assays.
Only one of the studies exhibited revertants/plate for some strains exceeding up to three-fold of the control value (Mecchi et al., Citation2003a). However, these increases were not reproducible between experiments and did not exhibit a dose–response pattern. These results were therefore judged to be due to low vehicle control revertants/plate and not to indicate treatment-related mutagenic activity. All of the 15 regulatory bacterial reversion studies of GBFs were concluded to be negative as judged by the absence of significant, reproducible, dose-related increases in revertants/plate. These studies provide abundant weight of evidence that a variety of GBFs are negative in properly conducted bacterial reversion assays.
In vitro mammalian cell assays
Glyphosate and glyphosate salts
As reviewed by Williams et al. (Citation2000), a CHO/HGPRT in vitro mammalian cell gene mutation assay was reported negative for glyphosate when tested up to toxic dose levels of 22.5 mg/mL (≈133 mM), i.e. well above the current top limit of 10 mM (appropriate for glyphosate and glyphosate salts), in the presence and absence of mammalian metabolic activation.
Two regulatory mouse lymphoma tk locus gene mutation studies were reviewed ( and “online supplementary material”). One study was conducted according to the 1984 OECD guideline for in vitro mammalian gene mutation assays (Jensen, Citation1991b; OECD 476, Citation1984). Somewhat fewer cells were exposed (3 × 105 −S9, 1.8 × 105 + S9) than the 106 cells recommended in the updated OECD guideline (OECD 476, Citation1997) but this was not considered as a significant deficiency. Cells were exposed at four concentrations up to 4200 µg/mL with S9 (≈24.8 mM) or 5000 µg/mL without S9 (≈ 29.6 mM). Although no toxic effects (reduction in cloning efficiency) were seen on day 0 or day 2, these dose levels exceed the currently recommended upper dose level of 10 mM (1.69 mg/mL for glyphosate) for relatively non-toxic test materials (OECD 476, Citation1997). It should be noted that most OECD guidelines for in vitro mammalian cell genotoxicity assays specify an upper limit dose for soluble, relatively non-toxic substances of 10 mM or 5 mg/mL, whichever is lower. The lower and appropriate upper limit dose for glyphosate and glyphosate salts is 10 mM. A second study conducted later followed several updated recommendations for in vitro mammalian cell gene mutation assays adopted in 1997 (Clay, Citation1996; OECD 476, Citation1997). These included the use of at least 106 cells in exposed cultures and consideration of test material effects on pH and osmolality. The latter consideration proved to be important because concentrations of 1500 and 2000 µg/mL (≈8.9–11.8 mM) produced large (>1 pH unit) decreases in pH and the maximum dose level employed for mutation measurement (1000 µg/mL, ≈5.9 mM) was appropriate to avoid excessive effects on pH. This dose level did not produce effects on the day 0 cloning efficiency. Although three dose levels were used in the initial experiment, four dose levels (as recommended in OECD 476, Citation1997) were used in the confirmatory experiment.
Table 2. In vitro mammalian cell assays of glyphosate, glyphosate salt solutions and GBFs.
Both of the regulatory mouse lymphoma studies were negative for glyphosate when tested up to dose levels that either exceeded the current limit dose or avoided excessive pH effects. These negative results provide important corroboration of a lack of gene mutation activity in the earlier negative CHO/HGPRT study. They also indicate a lack of induction of effects such as large deletions in DNA that may be detected in the autosomal tk locus assay (Aaron et al., Citation1994).
Glyphosate-based formulations
No in vitro mammalian cell gene mutation assays of GBFs were observed in the published literature or the regulatory study reports.
Other non-mammalian assays
Glyphosate and glyphosate salts
No gene mutation assays on glyphosate other than bacterial reversion or in vitro mammalian test systems were reported in Williams et al. (Citation2000) or as regulatory studies. A positive result for glyphosate was reported in the Drosophila wing spot assay which can indicate both gene mutation and mitotic recombination endpoints (Kaya et al., Citation2000). Small increases in small wing spot frequencies were observed in one of four crosses of larvae treated with up to 10 mM (≈1.69 mg/mL) of glyphosate. Negative or inconclusive results were observed for the other crosses. The lack of a positive response in the balancer–heterozygous cross offspring, which are insensitive to mitotic recombination events, suggests that there is no evidence for effects on gene mutation endpoint events such as intragenic mutations or deletions in this publication.
Glyphosate-based formulations
Williams et al. (Citation2000) described one report of a positive result for a GBF in the Drosophila sex-linked recessive lethal assay but this was contradicted by a negative result for the same GBF in this assay reported by another laboratory. Further, the positive study had some features that hampered interpretation, including the lack of concurrent negative controls (Williams et al., Citation2000). No non-mammalian cell gene mutation assays of GBFs other than bacterial reversion assays were observed in the published literature or the regulatory study reports.
Chromosomal effects endpoints
In vitro mammalian cell assays
Glyphosate and glyphosate salts
Two human and one bovine in vitro peripheral lymphocyte chromosomal aberration studies of glyphosate were considered in the earlier review (Williams et al., Citation2000). One human lymphocyte in vitro study had negative results for glyphosate tested up to 0.33 mg/mL and 0.56 mg/mL (≈2–3 mM) in the absence and presence of an exogenous mammalian activation system, respectively. The other two studies with human and bovine lymphocytes and no metabolic activation system reported positive results at concentrations more than two orders of magnitude lower. The reasons for the conflicting results are unclear, but the Williams et al. (Citation2000) review noted several unusual features about the positive studies including an unusual exposure protocol and discordant positive results for another chemical found negative in other laboratories.
Subsequent to the Williams et al. (Citation2000) review, four publications have reported results for glyphosate salt solutions using cytokinesis block micronucleus (CB MN) or chromosomal aberration endpoints with cultured bovine lymphocytes (). These publications used a test material reported as 62% by weight isopropylamine salt of glyphosate from a Monsanto source. This test material appears to be a manufacturing batch of the isopropylamine salt of glyphosate in water without surfactants, which is not sold as a formulation. In two publications from one laboratory, no statistically significant increases in the frequencies of micronucleated binucleate cells were observed following the treatment with up to 560 µM (≈94.7 µg/mL acid equivalent, a.e.) for 24 h in the absence of S9 (Piesova, Citation2004) or 2 h in the absence and presence of a mammalian metabolic activation system (Piesova, Citation2005). These two studies report a statistically significant increase in micronucleus frequency with 48 h of treatment without S9 in one donor at 280 µM (≈47.3 µg/mL a.e.) but not at 560 µM and in a second donor at 560 µM but not 280 µM. The lack of a consistent response pattern between donors suggests that the results after 48 h of treatment are questionable. Two other publications found negative results for the chromosomal aberration endpoint in cultured bovine lymphocytes with what appears to be the same isopropylamine glyphosate salt solution (Holeckova, Citation2006; Sivikova & Dianovsky, Citation2006). Both of these studies used a maximum concentration of 1.12 mM (≈0.189 mg/mL a.e.), which was reported to induce a decrease in mitotic index of >50%, and treatments of 24 h without S9. These two studies have several limitations including no use of an exogenous mammalian metabolic activation system. In addition, Holeckova (Citation2006) only examined effects detectable by staining of chromosome 1 and apparently did not use a positive control. These four studies consistently indicated the lack of chromosomal damaging effects in bovine lymphocytes in the absence of metabolic activation following up to 24 h of exposure to 0.56–1.12 mM (≈0.094–0.189 mg/mL a.e.) concentrations of glyphosate isopropylamine salt.
Three publications reported testing of technical glyphosate for micronucleus or chromosomal aberration endpoints in cultured human lymphocytes (; Manas et al., Citation2009; Mladinic et al., Citation2009a,b). The treatment schedule of the Mladinic et al. publications is not clear. Although standard procedures for human lymphocyte assays recommend the treatment of exponentially growing cells at 44–48 h after mitogenic stimulation (OECD 487, Citation2010), the methodology described in the Mladinic et al. publications suggests that the 4 h treatment took place before mitogen stimulation. The cultures were then centrifuged and washed before mitogen was added. Thus, only non-dividing cells would have been exposed and this is clearly not in accordance with the OECD guideline. It is also unclear how long the cultures were maintained after the treatment. It appears that they may have been cultured for 72 h after the treatment, which suggests that the cells would have passed through the required 1.5–2 cell cycles after reaching the exponential growth (OECD 487, Citation2010) even though it appears they were not exposed during the exponential growth. Negative or equivocal results for the micronucleus and chromosomal aberration endpoints were observed in the absence of exogenous metabolic activation (S9) in all three publications. The maximum exposure concentration in the absence of S9 was in the range of 3–6 mM (≈0.51–1.01 mg/mL) in these studies.
In contrast to the cultured bovine and human lymphocyte results, Koller et al. (Citation2012) reported positive results for glyphosate in a CB MN assay using cultured human buccal epithelial cells in the absence of S9. Limitations of this study include no explicit indication of coding of slides or control of pH. However, pH effects would probably not have been observed at the concentrations used. Statistically significant effects were observed at treatment levels of 15–20 mg/L (≈0.09–0.12 mM) for 20 minutes. Statistically significant effects on nuclear morphology (nuclear buds and nucleoplasmic bridges) were observed at 10–20 mg/L and statistically significant increases in apoptosis and necrosis were observed at 20 mg/L. The concentrations and exposure times reported as producing effects in this study are substantially lower than the upper dose levels and exposure times used in the previously discussed studies. The results for this discrepancy are not clear, although Koller et al. (Citation2012) suggest that epithelial cells may be more sensitive to the effects of glyphosate than cells of the hematopoietic system such as lymphocytes. It should be noted that negative genotoxicity results have been observed in a number of regulatory in vitro mammalian cell genotoxicity studies using cultured cells other than lymphocytes (mouse lymphoma and CHL cells).
Mladinic et al. (Citation2009a,b) reported increases in micronucleated cells using the cytokinesis-block method in cultured human lymphocytes exposed to glyphosate for 4 h in the presence of an exogenous human liver metabolic activation system (S9). As discussed above, the methodology used in these studies is unclear, but it appears that cells were treated before mitogenic stimulation and cultured for 72 h. In both publications, a statistically significant increase in micronuclei was observed with S9 at the highest dose level of glyphosate tested (580 µg/mL, ≈3.4 mM), but how this could be possible when undividing cells were exposed is unclear. Increased proportions of centromere- and DAPI-positive micronuclei were observed for the high-dose with S9 suggesting that the induced micronuclei were derived from chromosome loss rather than chromosomal fragments. This observation is somewhat unusual, because there do not appear to be any known aneuploidy-inducing agents that require metabolic activation (Kirsch-Volders et al., Citation2003). Statistically significant increases in the frequency of nuclear abnormalities (buds and bridges) and DNA strand breakage were also observed at the highest dose tested in both publications. In parallel experiments cytotoxic effects such as early apoptosis, late apoptosis and necrosis were observed and these effects tended to be enhanced in the presence of S9 (Mladinic et al., Citation2009a). Also, the negative control levels of such endpoints as necrosis and comet tail moment were significantly increased in the presence of S9 (Mladinic et al., Citation2009a). It should be noted that glyphosate is mostly excreted unmetabolized in vivo in mammals with only very small levels of aminomethylphosphonic acid (AMPA) or an AMPA-related structure observed (Anadon et al., Citation2009, Brewster et al., Citation1991). There is also one report that glyphosate is essentially unmetabolized in vitro in the presence of a rat liver S9 homogenate (Gohre et al., Citation1987). It also does not seem likely that human S9, used by Mladinic et al., would be expected to be more active than much more commonly used induced rat liver S9. These observations suggest that the S9 mediated effects reported by Mladinic et al. are not likely to be due to in vivo relevant metabolites. Given the unusual methodology in these studies, the chromosomal-damaging effects of glyphosate in the presence of S9 are not convincing, and it is possible that artifacts due to low pH in the presence of S9 (Cifone et al., Citation1987; Morita et al, 1989; Scott et al., Citation1991) may be responsible. Such effects would not be relevant to in vivo exposures.
Three regulatory in vitro mammalian cell chromosomal aberration studies were conducted on technical glyphosate ( and “online supplementary material”). These studies were conducted in accordance with the 1983 OECD Guideline 473 for the in vitro mammalian chromosomal aberration test (OECD 473, Citation1983). The study protocols employed exposures in both the presence and absence of an exogenous mammalian metabolic activation system. Treatment and harvest times were appropriate to assess cells exposed in different stages of the cell cycle. Treatment times included a shorter treatment with and without S9 and extended treatments without S9. Appropriate media and culture conditions for these assays were confirmed by experimental results for negative and positive control exposures. In these studies slides were coded before the analysis and 200 metaphases per treatment were scored for chromosomal aberrations, as recommended in the updated OECD Guideline 473 (OECD 473, Citation1997). The maximum dose levels used in two of the studies (1250 µg/mL, ≈7.4 mM; Fox, Citation1998; Wright, Citation1996) were set so as to avoid excessive pH shifts as recommended in the updated OECD Guideline 473. The third study (Matsumoto, Citation1995) used maximum dose levels (500–1000 µg/mL, ≈3–5.9 mM) set by rangefinder results but noted pH-related medium color changes at dose levels of 500 µg/mL and higher.
No induction of chromosomal aberrations was observed in these regulatory studies employing cultured Chinese hamster lung (CHL) cells (two studies) or in two experiments with cultured human lymphocytes from different donors (third study). The two CHL studies also reported negative results for polyploidy induction. Taken together, these three studies provide clear evidence for the lack of in vitro mammalian cell clastogenic activity of glyphosate in robust assays for two different mammalian cell types conducted under a variety of exposure conditions in the absence and presence of S9.
The reviewed results for mammalian in vitro chromosomal effect assays demonstrate a weight of evidence that technical glyphosate and glyphosate salt concentrates are generally negative for this endpoint in cultured mammalian cells in the absence of an exogenous mammalian metabolic activation system. Three publications from three laboratories and three regulatory studies report negative in vitro mammalian cell chromosomal aberration or micronucleus results in the absence of exogenous activation. Two of the CHL regulatory studies also reported negative results for polyploidy induction. Two publications from one laboratory have questionably equivocal results for the micronucleus endpoint in human lymphocytes in the absence of exogenous activation, while two publications from another laboratory reported positive results for bovine lymphocytes only with extended treatment but these results did not exhibit a consistent dose–response between donors. One publication reported positive results for human epithelial cells in the absence of S9 with a short exposure time. The negative studies were conducted at upper dose levels and with treatment times that were the same or higher than the studies with positive or equivocal results and include different cell types. These results reinforce the Williams et al. (Citation2000) conclusion that positive chromosomal aberration results reported for glyphosate in cultured human lymphocytes in the absence of an exogenous metabolic activation system are not convincing.
Recent reports of positive chromosomal effect results for glyphosate in the presence of an exogenous mammalian activation system in cultured human lymphocytes in one laboratory (Mladinic et al., Citation2009a,b) were not reproduced in three in vitro mammalian cell chromosomal aberration regulatory studies, including a study that employed cultured human lymphocytes. These positive results are also discordant with one previously reviewed result demonstrating a negative result for glyphosate in cultured human lymphocytes with mammalian metabolic activation using the chromosomal aberration endpoint (Williams et al., Citation2000) and a negative result in the presence of S9 for the micronucleus endpoint in bovine lymphocytes (Piesova, Citation2005). They are also discordant with negative results for three in vitro mammalian cell gene mutation studies that included an exposure to S9. The unusual methodology used for cultured human lymphocytes in the Mladinic et al. studies further complicates the interpretation of results from these studies. Thus, the weight of evidence for the in vitro chromosomal effect assays generally indicates a lack of chromosomal effects in either the presence or absence of S9.
Glyphosate-based formulations
No in vitro mammalian cell chromosomal aberration assays of GBFs are described in Williams et al. (Citation2000).
Only two publications with data from in vitro mammalian cell chromosomal aberration assays of GBFs have been found since the review of Williams et al. (Citation2000). Results are in . Amer et al. (Citation2006) reported positive in vitro chromosomal aberration effects in mouse spleen cells for a test material described as “herbazed” herbicide, which was reported to contain 84% glyphosate and 16% solvent, an unusually high glyphosate concentration for a formulation. The test material is not further characterized in the publication but is considered a GBF in this review. The glyphosate or GBF concentrations to which the cells in the study were exposed are not entirely clear because the most consistent concentration unit used in the report is M glyphosate/ml which is an unusual concentration unit. Assuming this means, moles of glyphosate per mL the maximum exposure would be 5 × 10-5 M glyphosate/mL medium or 50 mM. An upper exposure concentration of 50 mM (≈8.45 mg/mL glyphosate) would be well in excess of the limit level of 10 mM or 5 mg/mL currently recommended in the OECD guidelines (OECD 473, Citation1997). In addition to the uncertainty regarding the concentrations used, there are several other limitations to the reported study including no indication that pH of treatment solutions was controlled, no use of a mammalian metabolic activation system and no reported use of coded slides for scoring. Given these limitations, the uncertainty about the concentrations used and the nature of the test material, these results should not be considered to have significant relevance with respect to typical GBFs.
Another publication reported positive results for Roundup™ UltraMax GBF for the CB MN assay in cultured human buccal epithelial cells (Koller et al., Citation2012). Limitations in conduct or reporting of this study included no indication that pH of treatment solutions was controlled and no explicitly reported use of coded slides for scoring. As noted earlier, pH effects would not be likely at the low concentrations used. Increased MN frequencies were reported for 20 minute treatments with 10–20 mg/L of glyphosate a.i. (≈0.06–0.12 mM glyphosate). Statistically significant effects on nuclear morphology (nuclear buds and nucleoplasmic bridges) were also observed at 10–20 mg/L and increases in apoptosis and necrosis were observed at 20 mg/L but only the necrosis effect was statistically significant.
There were no regulatory studies of GBFs in in vitro mammalian cell chromosomal aberration or micronucleus assays. Thus, there are only the two studies of different GBFs (discussed above) with uncertainties and limitations in this endpoint category. While the published literature reports suggest the possibility of activity of GBFs in in vitro chromosomal damage assays, the paucity of studies and their limitations do not permit a generic conclusion regarding this endpoint for in vitro mammalian cells for GBFs in general.
In vivo mammalian assays
Micronucleus and chromosomal aberration
Glyphosate and glyphosate salts
The Williams et al. (Citation2000) glyphosate toxicity review presented results from in vivo mammalian chromosomal effect assays. Results from several mouse bone marrow erythrocyte studies of glyphosate were negative for micronucleus induction. These included the studies from different laboratories mostly following modern guidelines. The intraperitoneal (i.p.) route was used for most of the negative studies. In addition to i.p. studies, a 13-week mouse feeding study was also negative for the micronucleus endpoint with an estimated maximum daily glyphosate dose of over 11 000 mg/kg body weight/day. There was one published report of a weak positive mouse bone marrow micronucleus response observed for glyphosate. This study, which employed a smaller number of animals per group than other negative studies, clearly conflicted with the numerous other negative studies, not only in terms of increased micronucleus frequencies but also the finding of altered polychromatic erythrocyte to normochromatic erythrocyte (PCE/NCE) ratios. The overall weight of evidence from the earlier reviewed studies was that glyphosate and glyphosate formulations were negative in the mouse bone marrow erythrocyte micronucleus assay. The earlier review also noted a negative mouse dominant lethal result for glyphosate administered by gavage at a maximum dose level of 2000 mg/kg body weight.
As indicated in , two publications reported results for glyphosate in the mouse bone marrow erythrocyte micronucleus assay. It should be noted that there are some fairly consistent limitations in the reported conduct of these studies compared to the OECD guidelines. In these studies, concurrent indications of the toxicity other than PCE/NCE ratio effects on the bone marrow and mortality are not reported, coding of slides for scoring is not explicitly reported and fewer than the currently recommended number of 2000 PCEs or erythrocytes per animal were scored. As noted earlier, failure to explicitly report coding of slides in the methodology may reflect either failure to code slides or failure to explicitly indicate this in the methodology description in the publication.
Table 3. In vivo mammalian chromosomal effect studies.
Negative results were reported in one study which used a dose of 300 mg/kg body weight of glyphosate administered once i.p. with sacrifices at 24, 48 and 74 h after dosing (Chruscielska et al., Citation2000). This study had some limitations including the use of only one dose level (several dose levels should be used except when there is no toxicity up to the limit dose), and no explicit reported coding of slides for scoring and scoring of only 1000 PCEs per animal. A second publication reported positive results for glyphosate administered at 50, 100 and 200 mg/kg body weight via two i.p. injections 24 h apart, with sacrifice at 24 h after the second dose (Manas et al., Citation2009). A statistically significant increase in micronucleated erythrocytes was observed in the high-dose group in this study. A particular concern with this second publication is that “erythrocytes” rather than polychromatic erythrocytes were indicated as scored for micronuclei. This does not appear to be a case of using “erythrocytes” to mean polychromatic erythrocytes because the term “polychromatic erythrocytes” is used elsewhere in the publication describing measurements of PCE/NCE ratios. Scoring of all erythrocytes instead of immature polychromatic erythrocytes for micronuclei would be inappropriate in an assay with the stated treatment and harvest times because of the transient nature of micronucleated PCEs in bone marrow (OECD 474, Citation1997). PCEs containing micronuclei would not have reached maturity in such a short time, so micronuclei in matured erythrocytes could not have been induced by the chemical treatment.
There is no definitive explanation for the discrepancy between the two publications. Although one study used a single dose with multiple harvest times and the second used two doses and a single harvest time, both are acceptable protocols and would not be expected to lead to such discordant results (OECD 474, Citation1997). The negative result reported for the 13-week feeding study in the earlier review (Williams et al., Citation2000) confirms that positive results are not simply due to the repeated dosing. The reported negative result (Chruscielska et al., Citation2000) seems to be in accordance with a majority of earlier reviewed mouse bone marrow micronucleus studies of glyphosate using similar doses and the i.p. or feeding routes (Williams et al., Citation2000). Also, the apparent scoring of micronuclei in erythrocytes at such an early time point raises questions regarding the reported positive study.
A large number of regulatory rodent bone marrow assays were conducted on technical glyphosate or glyphosate salt solutions ( and “online supplementary material”). Most of these were mouse bone marrow erythrocyte micronucleus studies, but there is also one rat bone marrow erythrocyte micronucleus assay and one mouse bone marrow chromosomal aberration study. Most of the rodent bone marrow erythrocyte micronucleus studies were reported to be conducted in accordance with the OECD Guideline 474 (Citation1983) for studies conducted prior to 1997 and the OECD Guideline 474 (Citation1997) for studies conducted after 1997. The mouse bone marrow chromosomal aberration study was reported as conducted according to the OECD Guideline 475 (OECD 475, Citation1984). Protocol features for the micronucleus studies included single dosing with harvest at 24 and 48 h after the treatment (also 72 h in one study) or two treatments 24 h apart with a single harvest at 24 h after the last treatment. These treatment and harvest time alternatives are both considered acceptable in the most recent guideline (OECD 474, Citation1997) for bone marrow erythrocyte studies. For the bone marrow chromosomal aberration study, the use of a single 24 h sampling time after two treatments separated by 24 h deviates from an earlier recommendation to have 6 h and 24 h sampling times with multiple dosing (OECD 475, Citation1984), but differs slightly from more recent recommendations to sample approximately 1.5 cell cycles (usually around 12–18 h) after two daily doses (OECD 475, Citation1997). Some studies used only males when there was no evident difference in toxicity to both sexes, which is acceptable under the most recent guideline (OECD 474, Citation1997). Three treatment groups were generally used but some studies only used a single high-dose group when a limit dose had little or no toxicity as accepted in OECD 474 (Citation1997). In most studies, 2000 PCEs per animal were scored as recommended in the most recent guideline (OECD 474, Citation1997). The earlier guideline had recommended scoring 1000 PCEs per animal (OECD 474, Citation1983). In the mouse bone marrow chromosomal aberration study, 50 metaphases per animal were scored, which is lower than the currently recommended 100 metaphases per animal (OECD 475, Citation1997).
Eleven mouse and one rat bone marrow erythrocyte micronucleus regulatory studies for technical glyphosate or glyphosate salt solutions were conducted. The upper dose levels for orally administered glyphosate were, with one exception, the earlier suggested limit dose of 5000 mg/kg body weight or the more recently recommended limit dose of 2000 mg/kg body weight. In these studies little or no toxicity was observed at the limit dose. One study (Zoriki Hosomi, Citation2007) observed considerable toxicity and lethality at an oral dose of 50 mg/kg body weight and employed a lower maximum dose level for the main study (30 mg/kg body weight). The reason for the higher reported toxicity in this study compared to other glyphosate studies is not apparent. Studies of glyphosate employing the intraperitoneal route generally employed lower maximum dose levels (62.5 to 3024 mg/kg body weight) and the maximum dose levels were set by observations of toxicity and lethality in rangefinder studies.
Micronucleated PCE frequency results for the maximum dose levels of the regulatory rodent bone marrow micronucleus studies of glyphosate and glyphosate salts are presented in . For eight of the 12 regulatory bone marrow erythrocyte micronucleus studies there were no statistically significant increases in micronucleated PCEs observed for any of the glyphosate treated groups. Three studies had small statistically significant increases in micronucleated PCE frequency that were judged not to be treatment related because the frequencies were well within historical control values (Durward, Citation2006; Jones, Citation1999; Zoriki-Hosomi, Citation2007).
Table 4. High-dose and control MN PCE frequencies for regulatory glyphosate and glyphosate salt studies.
A statistically significant increase in the micronucleated polychromatic erythrocyte (MN PCE) frequency was observed for females, but not for males, treated with 5000 mg/kg in the study of Suresh (1993b). This increase was only about two-fold over the concurrent control and no increase was observed for frequencies of micronucleated normochromatic erythrocytes for this group, although at such an early sampling time this would not be expected. Historical control data were not presented. Suresh (1993b) employed a high level of glyphosate treatment, 5000 mg/kg body weight, which is well above the currently recommended limit dose of 2000 mg/kg body weight (OECD 474, Citation1997) as well as an unusual use of groundnut oil as a vehicle for a water soluble test material. The negative control MN PCE frequencies in this study (4.9 and 6.7 MN per 1000 PCEs for females and males, respectively) exceeded control MN PCE frequencies commonly observed in mice (Salamone & Mavournin, Citation1994). The recommendation by Salamone & Mavournin (Citation1994) is that MN PCE frequencies above 5/1000 MN PCE should be questioned and in most cases confirmed. Two other bone marrow erythrocyte studies which employed 5000 mg/kg body weight treatment did not observe any statistically significant increases in MN PCE frequency (Fox & MacKay, Citation1996; Jensen, 1991c). A mouse bone marrow chromosomal aberration study conducted in the same laboratory using the same vehicle and a 5000 mg/kg body weight dose level (Suresh, Citation1994) was negative. These observations provide a strong weight of evidence that the statistically significant increase observed in Suresh (1993b) is not evidence of a treatment-related effect.
The results presented in clearly indicate a very strong overall weight of evidence that glyphosate or glyphosate salt solutions do not induce micronucleated PCEs in rodent bone marrow erythrocyte micronucleus assays conducted with maximum dose levels which are appropriate either because of toxic effects or are recommended limit doses for relatively non-toxic compounds. Statistically significant increases in MN PCE frequency in isolated studies were not reproducible in a number of other studies. Furthermore, these studies include several examples of negative results for i.p. administration at maximum doses that exceed those employed by Manas et al. (Citation2009). It should also be noted that the i.p. route of administration is not relevant to human exposure. In combination with the results presented in Williams et al. (Citation2000), there is overall a strong weight of evidence that technical glyphosate and glyphosate salt solutions are not genotoxic in in vivo mammalian micronucleus assays at high dose levels.
Glyphosate-based formulations
The Williams et al. (Citation2000) glyphosate toxicity review presented results from several mouse bone marrow erythrocyte micronucleus studies of GBFs (e.g. Roundup™, Rodeo™ and Direct™-branded formulations) that were mostly negative for micronucleus induction. The i.p. route was used for most of the negative studies and maximum doses for many of the studies were toxic or appropriately close to LD50 values. There was one published report of a weak positive mouse bone marrow micronucleus response observed for a Roundup™-branded GBF. This study, which employed a smaller number of animals per group than other negative studies, was clearly aberrant from the numerous other negative studies not only in micronucleated cell frequency finding but also the finding of altered polychromatic erythrocyte to normochromatic erythrocyte (PCE/NCE) ratios. The overall weight of evidence from the earlier reviewed studies was that GBFs were negative in the mouse bone marrow erythrocyte micronucleus assay.
As indicated in , seven publications reported results for GBFs in in vivo mammalian micronucleus or chromosomal aberration assays. It should be noted that there are some fairly consistent limitations in the reported conduct of these studies compared to the OECD guidelines. In most studies, concurrent indications of toxicity other than effects on bone marrow are not reported, coding of slides for scoring is not explicitly indicated and, in many studies, fewer than the currently recommended number of 2000 polychromatic erythrocytes or 100 metaphases per animal were scored.
Three publications report negative results for Roundup™-branded GBFs in mouse chromosomal aberration or micronucleus assays. In two of these publications, negative results in mouse bone marrow erythrocyte micronucleus assays were reported for different Roundup™-branded GBFs administered at 200 mg/kg body weight twice 24 h apart by the i.p. route (Coutinho do Nascimento & Grisolia, Citation2000; Grisolia, Citation2002). The third publication reported negative results in mouse bone marrow studies for both the chromosomal aberration and erythrocyte micronucleus endpoints using a single oral dose of 1080 mg/kg body weight of a Roundup™-branded GBF (Dimitrov et al., Citation2006).
In contrast, one publication reported positive results for a Roundup™-branded GBF in mouse bone marrow for the chromosomal aberration and erythrocyte micronucleus endpoints using a single maximum dose of 50 mg glyphosate/kg body weight i.p. (Prasad et al., Citation2009). Both the positive results and the magnitude of the increases in frequencies of chromosomal aberrations and micronuclei reported in this study are remarkably discordant with other reported results for Roundup™-branded and other GBFs in mouse bone marrow chromosomal aberration and micronucleus studies in a number of laboratories and publications ( and Williams et al., Citation2000). The reasons for this discordance are not clear. One unusual feature of the Prasad et al. (Citation2009) study is that the Roundup™-branded GBF was administered in dimethylsulfoxide (DMSO) vehicle. This is an unusual vehicle to use in in vivo genotoxicity studies, particularly using the i.p. route and for a test material which is water soluble. A published toxicity study has reported that use of a DMSO/olive oil vehicle by the i.p. route dramatically enhanced the toxicity of glyphosate formulation or the formulation components without glyphosate compared to saline vehicle (Heydens et al., Citation2008). The enhanced toxicity observed with this vehicle was not observed when the oral route was used. DMSO has also been shown to enhance the toxicity of other hydrocarbons when administered via the i.p. route (Kocsis et al., Citation1968). These observations suggest that use of DMSO as a vehicle for administration of chemicals or formulations by the i.p. route might produce unusual toxic effects that are not relevant to normally encountered exposures. Furthermore, the i.p. route is considered by many regulatory agencies to be an unphysiological route and is not recommended for the safety evaluation of chemicals. Regardless of the reasons for the discordant positive results, it is clear that a large preponderance of evidence indicates that Roundup™-branded GBFs are typically negative in mouse bone marrow chromosomal aberration and erythrocyte endpoints.
One publication reported positive results for bone marrow chromosomal aberration in rabbits administered Roundup™-branded GBF in drinking water at 750 ppm for 60 days (Helal & Moussa, Citation2005). This study is unique in terms of species and route of administration. The publication does not report water intake in the test and control groups. Given the potential for water palatability issues with a formulated product, this is a significant shortcoming, as any effects noted might be attributable to dehydration (Saunders, Citation2005). This study had further limitations including the use of only a single dose level and not explicitly indicating the coding of slides for scoring. This study did not include a positive control for chromosomal aberration effects. Examination of the chromosomal aberration scoring results showed that, for the treated group, large increases were observed for gaps and “centromeric attenuation” that were included in the summation and evaluation of structural chromosomal aberration effects. Ordinarily gaps are scored but are not included in the total aberration frequency, and centromeric attenuation is not included in conventional identification of structural aberrations (OECD 475, Citation1997; Savage, Citation1976). These unusual scoring and interpretive features raise significant questions about using this study to make conclusions about clastogenicity of the GBF tested.
Two other publications report in vivo mammalian chromosomal aberration or micronucleus results for non-Roundup™-branded GBFs. In one of these, an uncharacterized GBF, Percozyd 10 L, was reported to be negative in a mouse bone marrow erythrocyte micronucleus assay (Chruscielska et al., Citation2000). The maximum dose level tested, 90 mg/kg i.p., was reported to be 70% of the i.p. LD50 as determined experimentally by the authors, and so may have exceeded the maximum tolerated dose. This study had several limitations including use of less than three dose levels and no explicit reported coding of slides for scoring.
In an other study, positive results were reported for another uncharacterized GBF, herbazed, in mouse bone marrow and spermatocyte chromosomal aberration studies (Amer et al., Citation2006) using oral and i.p. routes and treatments from 1 to up to 5 d (i.p.) or 21 d (oral). Although i.p. exposures of 1, 3 and 5 d produced statistically significant increases in bone marrow abnormal metaphase frequency when gaps were included, the increases were not significant excluding the gaps and the OECD 475 (Citation1997) recommends not including gaps in total aberration frequency. Statistically significant positive results were observed after multiple i.p. exposures (3–5 d bone marrow only including gaps; 5 d for spermatocytes) and after extended oral treatments (14–21 d, bone marrow; 7–21 d spermatocytes). Although not a genotoxic endpoint per se, it should be noted that statistically significant increases in frequency of sperm with abnormal morphology were observed in mice treated with 100 and 200 mg/kg body weight glyphosate p.o. for 5 d. The fact that positive results were not observed in an erythrocyte micronucleus test of mice treated with glyphosate up to 50 000 ppm in feed for 13 weeks (Williams et al., Citation2000) indicates that, by contrast, extended glyphosate treatment by the oral route does not induce detectable chromosomal effects. This treatment was longer and up to much higher glyphosate exposures than those used for the Amer et al. (Citation2006) studies. Thus, it appears likely that these effects were due to some component(s) of the specific herbazed GBF tested rather than glyphosate. It is noteworthy that the Amer et al. (Citation2006) publication is unique in reporting positive responses for such a large number of endpoints for a single test material.
A total of 12 mouse bone marrow erythrocyte micronucleus regulatory studies of GBFs were available ( and “online supplementary material”). These studies were designed to be in compliance with the OECD 474 (Citation1997) guidance for rodent erythrocyte micronucleus assays. The treatment regimen was either a single oral dose with harvests at 24 and 48 h after dosing or two oral doses 24 h apart with a single sacrifice at 24 h after the last dose. Either of these treatment regimens is acceptable under the most recent OECD guideline for this assay (OECD 474, Citation1997). Many of the studies used only males but reported no significant differences in gender response in preliminary toxicity studies. All of these studies employed a maximum dose of 2000 mg/kg body weight and most of the studies also used lower doses. This is consistent with a limit dose recommendation of 2000 mg/kg body weight in the OECD guideline. The upper dose level was not reported to induce mortality in any of the studies but in a few studies clinical signs were observed in high-dose animals. No toxic effects on bone marrow were generally observed in these studies as judged by PCE/NCE ratios. A decrease in PCE/NCE for 48 h high-dose animals was observed in one study (Xu, Citation2009a) but this may not have been treatment-related because the control PCE/NCE ratio was unusually high.
Ten of the studies did not exhibit a statistically significant increase in MN PCE for any treatment group. Two studies had statistically significant increases in MN PCE frequency at the 48 h time point but the MN PCE frequencies were within historical control levels and judged in each case to be due to a statistical anomaly from a low vehicle control MN PCE frequency and is not treatment-related (Erexson, Citation2003a; Xu, Citation2008a). Thus, none of these 12 studies indicated treatment-related increases in MN PCE frequencies and all studies were considered negative for this endpoint.
In summary, in addition to the in vivo rodent bone marrow chromosomal effect studies presented in Williams et al. (Citation2000), a majority (three of four) of the rodent bone marrow studies in the subsequent published literature are negative for Roundup™-branded formulations at maximum dose levels that significantly exceed the maximum dose level of the study reporting positive results. One noteworthy feature of the positive study is the use of a DMSO vehicle which is unusual, if not inappropriate, for a water soluble test material. A rabbit drinking water study found positive effects for a Roundup™-branded GBF; however, this study had a large number of limitations including not presenting information on palatability and no positive control. Publication reports for other GBFs included a negative study for Perzocyd 10 SL and positive chromosomal aberration results for both bone marrow and spermatocytes for a herbazed GBF using extended oral and i.p. treatments. A very large number of well-conducted regulatory mouse bone marrow micronucleus studies indicated that a variety of GBFs are negative in this assay system up to the limit dose of 2000 mg/kg body weight. While the possibility that GBFs with different compositions might have different properties cannot be excluded, the overall data certainly indicate that a typical GBF is negative for the induction of chromosomal damage in vivo.
Rodent dominant lethal
The Williams et al. (Citation2000) review notes a negative result in a mouse dominant lethal assay of glyphosate using a maximum treatment level of 2000 mg/kg body weight administered by gavage.
No rodent dominant lethal assays of glyphosate or GBFs were encountered in the subsequent literature.
One regulatory rat dominant lethal study was available (Suresh, 1992; “online supplementary material”). This study was reported to be conducted in accordance with the OECD 478 (Citation1984). In this study, groups of 30 male Wistar rats were given a single oral administration of glyphosate (suspension in groundnut oil vehicle) at dose levels of 200, 1000 and 5000 mg/kg body weight. Control groups received vehicle only or ethyl methane sulfonate as a positive control. Each week for 10 consecutive weeks males were mated 1:1 to separate groups of untreated virgin females. Each week’s paired females were removed after co-housing for 6 d and were sacrificed on the 16th day after pairing and reproductive parameters were measured (pregnancy status, corpora lutea, early and late resorptions, and live implants). One unusual aspect of this study is that mean body weights of all treatment groups were initially statistically higher than the control group mean body weight and this pattern persisted throughout the study. The following effects were observed in the first group of week 1 females mated to high-dose males: reductions in pregnancy rate, decreases in live implants and increases in pre- and post-implantation loss. There were also increases in embryonic resorptions (“small moles”) in week 1 females mated to mid-dose males. These effects were attributed to significant acute toxic effects of glyphosate (not dominant lethal effects) exhibited after the treatment in week 1 as evidenced by body weight loss in the mid and high-dose males and clinical signs. Although some statistically significant findings in post-implantation loss were sporadically observed in subsequent weeks these were not considered to be treatment-related because they were not consistent with a biologically plausible dose-response or a biologically plausible time course (see post-implantation loss data table in “online supplementary material”). This conclusion was also indicated in an EU monograph report (BBA, Citation1998–2000). This study appears to be in accordance with the study noted in Williams et al. (Citation2000) indicating that glyphosate is not active as a rodent germ cell mutagen.
Non-mammalian assays
Glyphosate and glyphosate salts
The Williams et al. (Citation2000) review reported negative results for isopropylamine salt of glyphosate in an onion root tip chromosomal aberration assay.
One subsequent published study reported a weak positive result for technical glyphosate in a Drosophila wing spot assay (Kaya et al., Citation2000). Statistically significant positive increases were found only in one of four crosses for small twin spots and not for the two other wing spot categories (large wing spots and twin wing spots). As discussed above, only negative or inconclusive results were observed for crosses that were not subjected to mitotic recombination effects. If the result was actually treatment-related it would only indicate an increase in recombination events and not in somatic mutations.
Glyphosate-based formulations
The Williams et al. (Citation2000) review reported a positive result for a Roundup™-branded GBF for chromosomal aberrations in an onion root tip assay and it was noted that this may have been caused by toxic effects of the GBF surfactant.
Negative results were observed in subsequently published in vitro assays for the chromosomal aberration and micronucleus endpoints in Crepis capillaris root meristems exposed to a Roundup™-branded GBF at concentrations up to 0.5% a.i. (Dimitrov et al., Citation2006).
Subsequent to the earlier review a number of publications have reported discordant results for blood erythrocyte micronucleus assays conducted on GBFs in several non-mammalian fish, reptile and amphibian species (). One publication reported what might arguably be considered as equivocal results for the erythrocyte micronucleus test in Oreochromis niloticus (Nile tilapia), administered a test material described as Roundup™ 69 GBF at an upper dose of 170 mg/kg i.p. (Coutinho do Nascimento & Grisolia, Citation2000). Although there was a statistically significant increase in micronucleated erythrocyte frequency at the mid-dose level, a significant increase was not observed at the high-dose level and considerable variability in frequencies in different groups was noted. Negative results were reported in another fish species (Prochilodus lineatus) exposed to 10 mg/L Roundup™-branded GBF for 6, 24 and 96 h (Cavalcante et al., Citation2008). This concentration was reported to be 75% of a 96-h LC50. Negative results were also reported for the micronucleus endpoint in the fish Corydoras paleatus exposed to 6.7 µg/L Roundup™-branded GBF (calculated 3.2 µg/L glyphosate) for 3, 6 and 9 days (de Castilhos Ghisi & Cestari, Citation2012). Positive results were reported for the erythrocyte micronucleus assay conducted in the fish T. rendalli exposed to up to 170 mg/kg body weight i.p. of another Roundup™-branded GBF (Grisolia, Citation2002). Examination of the micronucleus frequencies in this publication indicated that the negative control micronucleus frequency was considerably lower than the frequencies for all but one of 21 treatment groups for seven different test materials. This suggests an unusually low control frequency and at least one treatment group had statistically significant increases in MN frequencies for each of the seven test materials. In the absence of historical negative control data and few publications from which to estimate negative control ranges, the possibility that the apparently significant increases were due to a low negative control value that should be considered for this publication. Another publication reported positive erythrocyte micronucleus results in goldfish (Carassius auratus) exposed to 5 to 15 ppm glyphosate concentration of a Roundup™-branded GBF for 2 to 6 d (Cavas & Konen, Citation2007).
Table 5. Blood erythrocyte micronucleus assays in non-mammalian systems.
The reasons for the discordant results are not clear for the fish erythrocyte micronucleus assays of Roundup™-branded GBFs. Although different species and GBFs were used in different studies there were pairs of studies with positive and negative or equivocal results that used similar treatment conditions (e.g. 170 mg/kg i.p. or 10–15 mg/L in water).
An amphibian erythrocyte micronucleus study reported questionable effects of a Roundup™-branded GBF (Bosch et al., Citation2011). For one species (O. cordobae), toxicity and lethality were observed at exposures to concentrations of 200–800 mg/L a.i. (glyphosate active ingredient) of Roundup™-branded GBF. The surviving 100 mg/L a.i. treatment group had an increase in micronucleated erythrocyte frequency after 5 d but the increase was not statistically significant. A second species (R. arenarum) tolerated exposure up to 800 mg/L a.i. Roundup™-branded GBF. No statistically significant differences were found in the experimental groups by the analysis of variance. Although a statistically significant correlation between dose and micronucleated erythrocyte frequency was observed at day 2 of the treatment this analysis apparently omitted the high-dose group which had a mean micronucleus frequency comparable to negative control values. The downturn in dose-response and apparent omission of the high-dose from the statistical analysis is peculiar, because significant toxicity was not reported in this species at the 2-day sampling time. The results reported in this publication do not clearly support a conclusion of a micronucleus effect of a GBF in these species.
Results for an unusual test system of exposed caiman eggs are reported in two publications. In one study, eggs were topically exposed in a laboratory setting to Roundup™ Full II GBF, and erythrocyte micronucleus formation was measured in hatchlings (Poletta et al., Citation2009). The tested GBF was reported to contain the potassium salt of glyphosate. Statistically significant increases in micronucleated erythrocytes were observed in hatchlings from eggs treated with 500–1750 µg/egg. This system is quite unusual in the species tested and even more so in using an egg application with measurement of effects in hatchlings. Although there is some experience with a hen’s egg erythrocyte micronucleus assay using in ovo exposure, the erythrocytes were evaluated in embryos only a few days after the treatment (Wolf et al., Citation2008). In the caiman egg assay reported by Poletta et al. (Citation2009), there was presumably a single topical exposure followed by an egg incubation period of about 10 weeks before hatching. It is difficult to envisage that genotoxic events in ovo could produce elevated micronucleated erythrocyte frequencies detectable after 10 weeks, given the number of cell divisions occurring in development of a hatchling, and dilution of any micronucleated cells in a larger population as a result of this.
A second publication by Poletta et al. (Citation2011) described two field experiments evaluating caiman hatched from eggs in artificial nests that were sprayed with Roundup™ Full II GBF. Increases in micronucleated erythrocyte frequency in hatchlings were reported for both experiments. Additional measurements of growth in one experiment showed small but statistically significant differences in total length and snout-vent length in 3-month-old, but not 12-month-old, animals. Alanine aminotransferase and creatine kinase enzyme levels in serum of 3-month-old animals were significantly elevated (>two-fold control values). Alterations in these parameters suggest that the treated groups have some persistent biological differences or toxic effects either as a result of the treatment or some other factor. It is certainly possible that the micronucleus effects in both publications are associated with these persistent biological differences or toxic effects rather than from genotoxic effects induced in the embryos.
There were no regulatory reports of non-mammalian chromosomal effect assays.
In summary, the above in vivo micronucleus assays in non-mammalian systems have given discordant results for reasons that cannot be precisely defined. Typically these results would be given lower weight than mammalian systems in terms of prediction of mammalian effects, especially since there is very little experience with these systems in comparison with in vivo mammalian chromosomal effect assays, such as the rat or mouse bone marrow chromosomal aberration or erythrocyte micronucleus assays.
DNA damage
In vitro mammalian cell assays
Glyphosate and glyphosate salts
Some positive results for glyphosate for induction of SCE were reported in cultured human and bovine lymphocytes in the earlier review (Williams et al., Citation2000). These results tended to be weak, inconsistent and with limited evidence for dose–response. A number of limitations were observed for these studies such as the failure to control pH and abnormally low control values. Negative results were reported for technical glyphosate in a B. subtilis DNA damage assay and a rat primary hepatocyte unscheduled DNA synthesis (UDS) assay.
Subsequent to the review there is one publication of a positive in vitro SCE result in cultured bovine lymphocytes (; Sivikova & Dianovsky, Citation2006). It is noteworthy that negative effects for the chromosomal aberration endpoint were reported in this publication.
Table 6. DNA damage assays of glyphosate, glyphosate salts and GBFs in in vitro and in vivo mammalian systems.
Positive results for technical glyphosate have been reported for the comet (alkaline single cell gel electrophoresis, alkaline SCGE) endpoint in in vitro mammalian cell assays in four publications subsequent to the Williams et al. (Citation2000) review (). Some general protocol concerns for these studies are failure to explicitly indicate the assessment or control of pH or to explicitly indicate the coding of slides for scoring. It is possible that these may be deficiencies or limitations in reporting rather than conduct. Positive Comet results were observed for two mammalian cell lines exposed to glyphosate for 4 h at concentrations of 4.0–6.5 mM (≈0.68–1.10 mg/mL, GM38 cells) and 4.75–6.5 mM (≈0.80–1.10 mg/mL, HT1080 cells) (Monroy et al., Citation2005). These concentrations are close to the upper limit dose of 10 mM (appropriate for glyphosate) generally recommended for in vitro mammalian cell assays in the current OECD guidelines. Positive Comet results were also reported in Hep-2 cells exposed for 4 h to 3.0–7.5 mM (≈0.51–1.27 mg/mL) glyphosate (Manas et al., Citation2009). This publication reported negative results for the chromosomal aberration endpoint in cultured human lymphocytes exposed to up to 6 mM (≈1.01 mg/mL) glyphosate for 48 h and it should be noted that pH control of the culture medium was reported for the chromosomal aberration endpoint. Positive Comet results have also been reported for cultured human lymphocytes exposed to glyphosate at concentrations of up to 580 µg/mL (≈3.4 mM) for 4 h (Mladinic et al., Citation2009a). Effects were observed both in the presence and absence of S9. A modification of the Comet assay by employing a human 8-hydroxyguanine DNA-glycosylase (hOGG1) to detect an oxidative damage indicated only statistically significant effects on comet tail length for 580 µg/mL with S9. Measurements of total antioxidant capacity and thiobarbituric acid reactive substances showed statistically significant increases at 580 µg/mL in the presence or absence of S9. Interpretation of the significance of metabolic activation effects is complicated by the observation that several of the endpoints (e.g., comet tail intensity and nuclear abnormalities) tended to show increases in the presence of S9 in negative controls or at the very lowest concentrations of glyphosate (0.5–3.5 µg/mL, ≈2.9–20.7 µM). A reasonable summation of the results in this publication is that comet effects and other effects such as nuclear abnormalities, early apoptosis, necrosis and oxidative damage were consistently observed at 580 µg/mL. Positive Comet effects were also reported in a human epithelial cell line at dose levels up to 2000 mg/L (≈11.8 mM) (Koller et al., Citation2012). An unusual feature of these results is that statistically significant increases in comet tail intensity were reported as low at 20 mg/L (0.118 mM) with not much dose-response between 40 and 2000 mg/L. These dose levels of glyphosate were observed to produce little or no effects on a cellular integrity marker but statistically significant effects on necrosis and apoptosis markers were observed at 20 mg/L in parallel experiments.
One regulatory study of technical glyphosate was reported for a primary rat hepatocyte UDS assay (Rossberger, Citation1994; and “online supplementary material”). In this study, cultures of hepatocytes were exposed to glyphosate concentrations of 0.02–48.98 mM (≈0.34–8.28 mg/mL) and 0.14–111.69 mM (≈0.19–18.88 mg/mL) for 18 h in two experiments. Radio-labeled and halogen-substituted nucleosides were used to enable replicative and unscheduled DNA synthesis to be identified by density-gradient centrifugation and radioactivity counting. No effects on an unscheduled DNA synthesis were observed in this study in two separate experiments. Measurements of replicative DNA synthesis indicated that cytotoxic concentrations were tested and the maximum concentrations were in any case much higher than recommended for other in vitro mammalian cell assays (10 mM for glyphosate). This study is limited by the use of only single cultures per experimental point, although there were two separate experiments. The relatively narrow distribution of repair synthesis values with no dose-response in glyphosate-treated cultures, and the clear increases in repair induced by the positive control, suggest that this study provides reasonable evidence for a lack of induced-DNA repair following the exposure of rat primary hepatocytes to very high concentrations of glyphosate.
Overall there are a number of in vitro mammalian cell studies in which glyphosate has been reported to produce positive responses in SCE or Comet assays. Most of these positive responses have occurred at high exposures to glyphosate in the millimolar range. Although lower than the limit dose of 10 mM (appropriate for glyphosate) recommended for several in vitro mammalian cell culture assays (OECD 473, Citation1997, OECD 476, Citation1997, OECD 487, Citation2010), there have been some suggestions that lower dose levels may be more appropriate, particularly because of concerns about relevance of positive in vitro findings observed at higher dose levels (ICHS2(R1), Citation2011; Morita et al., 2012; Parry et al., Citation2010). In addition, many of the studies have functional limitations such as the lack of pH control and no explicit statement regarding the coding of slides for visual scoring.
Concerns over the possibility of effects induced by toxicity have led to several suggestions for experimental and interpretive criteria to distinguish between genotoxic DNA-reactive mechanisms for induction of comet effects and cytotoxic or apoptotic mechanisms. One recommendation for the in vitro Comet assay is to limit the toxicity to no more than a 30% reduction in viability compared to controls (Henderson et al., Citation1998; Storer et al., Citation1996; Tice et al., Citation2000). Importantly, dye exclusion measurements of cell membrane integrity, such as those reported in some of the above publications, may significantly underestimate cytotoxicity that could lead to comet effects (Storer et al., Citation1996). Other recommendations include conducting neutral diffusion experiments to determine if apoptotic processes might be responsible for comet effects (Tice et al., Citation2000).
In contrast to the SCE and comet endpoints, two independent studies of technical glyphosate in the primary rat hepatocyte UDS assay have both been negative. These results provide evidence that this endpoint is not affected by glyphosate at high concentrations in cell lines with endogenous mammalian metabolic activation capability.
Glyphosate-based formulations
Some positive results for glyphosate or GBFs in the SCE endpoint were reported in cultured human and bovine lymphocytes in the earlier review (Williams et al., Citation2000). These results tended to be weak, inconsistent and with limited evidence for dose–response.
Subsequent publications of DNA damage assays of GBFs in in vitro mammalian cell assays are presented in . Positive SCE results were observed for the uncharacterized herbazed GBF in mouse spleen cells (Amer et al., Citation2006). Limitations of this study are in common to those described above (see the section “In vitro mammalian cell assays”) for the chromosomal aberration endpoint portion of the study. The magnitudes of the increases in SCE/cell were less than two-fold of the control value which may not be considered biologically significant. Given these limitations, and the fact that the mechanism(s) by which SCE are induced is not understood, these positive findings should be viewed with caution. Koller et al. (Citation2012) reported positive Comet results for human epithelial cells exposed to Roundup™ UltraMax formulation. Statistically significant effects on comet tail intensity were observed from exposure to 20–200 mg/L of glyphosate (≈0.12–1.18 mM) for 20 min.
There were no regulatory DNA damage studies of GBFs in in vitro mammalian systems. The Amer et al. (Citation2006) report of a positive result for an uncharacterized GBF in the SCE endpoint agrees with other positive findings for this GBF in this publication but because of the discussed limitations does not add significantly to an evaluation of general genotoxic properties for GBFs. Similarly, the single observation of comet effects for a different GBF in an in vitro cellular assay is of limited value for assessing general GBF properties.
In vivo mammalian assays
Glyphosate and glyphosate salts
In the earlier review (Williams et al., Citation2000), positive results for DNA strand breakage were reported in kidney and liver tissue of mice treated by the i.p. route with glyphosate. The earlier review also noted reports of the absence of DNA adducts in mice treated by the i.p. route with the isopropylamine salt of glyphosate and a possible increase in 8-hydroxydeoxyguanosine (8-OHdG) in DNA of mice treated with technical glyphosate.
No new in vivo mammalian studies of DNA damage or DNA-reactivity of glyphosate were encountered in publications since 2000 and there were no regulatory studies of this category.
Glyphosate-based formulations
In the earlier review of Williams et al. (Citation2000), positive results for DNA adducts (32P-postlabeling) and DNA strand breakage were reported for mice treated by the i.p. route with Roundup™ GBF. For a number of reasons these observations were not considered to be clear evidence for DNA-reactive genotoxicity of the Roundup™ GBF.
Only one in vivo mammalian DNA damage study of a GBF has since been reported. This publication indicated an increase in SCE frequency in bone marrow cells of mice treated with uncharacterized herbazed GBF (; Amer et al., Citation2006). Statistically significant positive effects were only observed at the highest dose level tested (200 mg/kg body weight glyphosate administered p.o.) and were less than two-fold of the control value. As noted above, since the mechanism(s) by which SCEs are induced is not understood, this report for one GBF does not add significantly to an evaluation of general genotoxic potential for GBFs.
In a follow-up to 32P-postlabeling, DNA strand breakage and 8-OHdG studies cited in Williams et al. (Citation2000). Heydens et al. (Citation2008) reported on studies in mice to further investigate toxic effects and 8-OHdG levels associated with the routes, vehicles and dose levels of the earlier studies. The Heydens et al. (Citation2008) publication reported significant GBF-induced liver and kidney toxicity for high i.p. doses but no liver or kidney toxicity for comparable oral doses. Statistically significant increases in 8-OHdG were not observed in the latter study under the same conditions as employed by the earlier study. The DMSO/olive oil vehicle dramatically enhanced the toxicity of GBF administered by the i.p. route and the toxicity was also observed for formulation components without glyphosate. These results indicated that the effects reported in the earlier studies were associated with high liver and kidney toxicity that was primarily due to the non-glyphosate components of the formulation when administered at very high doses via the i.p. route of exposure. The toxicity enhancement by the unusual DMSO/olive oil dosing vehicle further calls into question whether the 32P-postlabeling finding represented effects associated with unusual toxicity rather than being indicative of adducts formed from glyphosate or glyphosate formulation components.
Non-mammalian assays
Glyphosate and glyphosate salts
The Williams et al. (Citation2000) review noted a negative result for glyphosate in the B. subtilis H17/M45 rec bacterial differential killing assay.
As presented in , two subsequent publications reported positive Comet results for glyphosate on Tradescantia flowers and nuclei (Alvarez-Moya et al., Citation2011) and negative Comet results for oyster sperm cells exposed to glyphosate (Akcha et al., Citation2012). The latter study employed a very low maximum exposure of 5 µg/L (≈0.03 µM).
Table 7. DNA damage assays of glyphosate, glyphosate and GBF’s in non-mammalian systems.
There was one regulatory study of technical glyphosate (95.68%) in the B. subtilis H17/M45 differential DNA damage (rec) assay ( and “online supplementary material”; Akanuma, Citation1995a). This study employed multiple levels of glyphosate on paper disks (up to 240 µg/disk) and measured zones of inhibition. No differential toxicity was observed indicating a lack of genotoxicity in this assay system. This result is in agreement with the earlier reported negative result for this assay by Williams et al. (Citation2000).
Glyphosate-based formulations
In the earlier review of Williams et al. (Citation2000), positive results were reported for DNA strand breakage in mouse tissues and for the comet endpoint in tadpoles of the frog Rana catesbiana exposed to a GBF.
There have been several subsequent publications of results for GBFs in a variety of non-mammalian DNA damage assay systems (). Two published DNA damage assays in vitro reported a positive result for a GBF in the E. coli SOS DNA damage test (Raipulis, Citation2009) and a negative Comet result for oyster sperm cells exposed to a very low (5 µg/L glyphosate, ≈0.03 µM glyphosate) concentration of a Roundup™-branded GBF (Akcha et al., Citation2012).
Several recent publications report Comet results for GBFs in aquatic species and a reptile (). Negative Comet results were reported in cells of freshwater mussel larvae exposed to a Roundup™-branded GBF at 5 mg/L (glyphosate a.i.) in water for 24 h (Conners & Black, Citation2004). This concentration was reported to be one-half of a no observable effect concentration and the 24-h LC50 for this GBF was reported to be 18.3 mg/L in parallel experiments. Four publications reported positive Comet results in aquatic vertebrates exposed to Roundup™-branded GBFs in water. These publications have a common feature that Comet results were reported as categories of visually damaged cells. In one publication, increases in nuclei exhibiting comet visual damage effects were observed in erythrocytes and gill cells of the tropical fish Prochilodus lineatus exposed to 10 mg/L of a Roundup™-branded GBF in water (Cavalcante et al., Citation2008). Measurement of erythrocyte micronucleus frequency and nuclear abnormalities did not show statistically significant increases in these endpoints. A second publication reported positive Comet results in erythrocytes of the goldfish, Carassius auratus, exposed to up to 15 ppm glyphosate concentration of a Roundup™-branded GBF for 2, 4 or 6 d (Cavas & Konen, Citation2007). Positive comet results were also reported in erythrocytes and liver and gill cells of the European eel, Anguilla anguilla, exposed to 0.058 and 0.116 µg/mL of a Roundup™-branded GBF in water for 1 or 3 d (Guilherme et al., Citation2010; Guilherme et al., 2012). Positive comet effects were also observed in liver and blood cells isolated from the fish species Corydoras paleatus exposed to 0.067 µg/mL of Roundup™-branded GBF for 3, 6 or 9 days (de Castilhos Ghisi & Cestari, Citation2012). No toxicity data other than the absence of mortality were presented but results were negative for the piscine micronucleus endpoint in this study. Two publications previously discussed reported positive erythrocyte Comet results in caiman hatchlings from eggs exposed to Roundup™ Full II GBF (Poletta et al., 2009; Poletta et al., 2011).
Significance of DNA damage endpoint results
DNA damage endpoints such as SCE or comets are generally regarded as supplementary to the gene mutation and chromosomal damage endpoint categories. They are considered indirect measures of genotoxicity. As mentioned above, the precise mechanism(s) behind SCE induction are not understood. DNA damage as measured by Comet assays does not provide information on the consequences of that damage (e.g. repair, mutation or cell death) and such endpoints, therefore do not directly measure effects on heritable mutations or events closely associated with chromosomal mutations. It is widely recognized that in vitro DNA damage endpoints such as the SCE or Comet assay can be induced by cytotoxicity and cell death processes rather than from DNA-reactive mechanisms, as discussed below.
There are numerous examples of SCE positive responses which are unique compared to other genotoxic endpoints, are not concordant with carcinogenicity, or which are induced by oxidant stress (Benigni, Citation1989; Bradley et al., Citation1979; Decuyper-Debergh et al., Citation1989; Djelic et al., Citation2006; Eckl et al., Citation1993; Speit, Citation1986; Tayama and Nakagawa, Citation1994; Zeiger et al., Citation1990). These examples indicate that the SCE endpoint, particularly in in vitro assays, should not be assumed to indicate DNA-reactive genotoxicity or to have the same weight as genotoxicity assays using other endpoints such as gene mutation or chromosomal effects.
Similarly, there are abundant data supporting the concept that induction of DNA strand breakage or comet effects can be secondary to necrotic or apoptotic processes that do not involve DNA reactivity (Amin et al., Citation2000; Burlinson et al., Citation2007; Henderson et al., Citation1998; Kiffe et al., Citation2003; Storer et al., Citation1996; Tice et al., Citation2000). Several clear specific examples exist of in vitro induction of comet effects in mammalian cells by conditions which do not appear to be relevant to genotoxic potential at lower doses or which occur by mechanisms that do not involve direct interaction with DNA. These include the induction of comet effects by apoptosis inducers which inhibit topoisomerases (Boos & Stopper, Citation2000; Gieseler et al., Citation1999); cytokine treatment of cultured cells (Delaney et al., Citation1997); sodium dodecyl sulfate and potassium cyanide (Henderson et al., Citation1998); colchicine, dl-menthol and sodium acetate (Kiffe et al., Citation2003); luteolin (Michels et al., Citation2005); gossypol (Quintana et al., Citation2000), carbon tetrachloride (Sasaki et al., Citation1998) and vitamin C (Anderson et al., Citation1994). Further examples of induction of comet effects of questionable genotoxic biological significance include dietary flavonoids quercetin, myricetin and silymarin (Duthie et al., 1997); hemoglobin (Glei et al., Citation2006); olive oil extracts (Nousis et al., 2005) and capsaicin (Richeux et al., 1999).
The observation of effects of sodium dodecyl sulfate is particularly interesting because it suggests responses to surfactants, which are typically components of GBFs. As a more specific example, polyoxyethylenealkalylmine (POEA), a surfactant component of some GBFs, has been shown to elicit cytotoxic effects such as perturbation of the mitochondrial membrane and disruption of mitochondrial membrane potential in cultured mammalian cells (Levine et al., Citation2007). Surfactant effects provide a very plausible mechanism for observations of GBFs inducing DNA damage responses. Such responses would be expected to be associated with cytotoxic exposures and to exhibit a threshold.
Some data suggest better concordance of the Comet assay with other genotoxic endpoints or carcinogenicity in in vivo mammalian studies (Brendler-Schwaab et al., Citation2005; Hartmann et al., Citation2004; Kirkland & Speit, Citation2008). However, there are examples of in vivo studies of comet effects with questionable significance for genotoxicity because of negative results for other in vivo genotoxic endpoints or carcinogenicity assays, or which appear to be due to toxicity. Some examples of non-concordance between comet effects and carcinogenicity include thiabendazole, saccharine, tartrazine and ortho-phenylphenol (Brendler-Schwaab et al., Citation2005). Discordance between carcinogenicity species specificity and in vivo Comet assay results has also been observed (Sekihashi et al., Citation2002), as well as other positive results for non-carcinogens (Kirkland & Speit, Citation2008). Another example of questionable in vivo genotoxic significance is positive comet effects produced in lymphocytes of exercising humans that were not accompanied by micronucleus induction (Hartmann et al., Citation1998).
In the context of unique results for DNA damage systems, there are several specific examples of published studies considered in this review containing reported positive results for DNA damage in contrast to negative or equivocal results for chromosomal effect endpoints for glyphosate and glyphosate salts in mammalian cells in the absence of S9 (Manas et al., Citation2009; Mladinic et al., Citation2009a; Sivikova & Dianovsky, Citation2006) and GBFs in fish species (Cavalcante et al., Citation2008; de Castilhos Ghisi & Cestari, Citation2012).
Concurrent assessment of cytotoxicity is recommended in in vitro and particularly in in vivo studies to assist in the interpretation of positive results. The reported “gold standard” for cytotoxicity in in vivo studies is the histopathological evaluation of the tissues or cells being evaluated (Burlinson et al., 2007). Other measures for evaluating cytotoxicity include neutral pH SCGE to detect double strand breaks associated with apoptosis or necrosis and measurement of “hedgehogs” which are nuclei in which almost all of the DNA is in the tail (Tice et al., Citation2000). The latter are thought to represent dead or dying cells severely damaged by cytotoxicity. While “hedgehogs” are usually not included in tabulation of comet effects, they may be used as an additional measure of toxic effects (Smith et al., 2008).
As noted earlier in the section “In vitro mammalian cell assays”, several Comet studies of glyphosate and GBFs did not employ concurrent measures of cytotoxic effects that were optimally suitable for the interpretation of a relationship between comet DNA damage and cytotoxicity. Examination of different markers of toxicity in some studies indicated the possibility of association with some markers but not others. The development and routine use of cytotoxicity measurements with maximum relevance to comet effect mechanisms would greatly improve the ability to interpret the significance of this endpoint in both in vitro and in vivo mammalian systems.
Genotoxicity weight of evidence conclusions
The earlier review of Williams et al. (Citation2000) applied a weight of evidence analysis to the available genotoxicity data. Various weighted components included assay system validation, test system species, relevance of the endpoint to heritable mutation, reproducibility and consistency of effects and dose-response, and relationship of effects to toxicity (Williams et al., Citation2000). The conclusion of that analysis was that glyphosate and Roundup™-branded GBFs were not mutagenic or genotoxic as a consequence of direct chemical reaction with DNA. This was supported by a strong preponderance of results indicating no effects in in vivo mammalian assays for chromosomal effects and consistently negative results in gene mutation assays. Although some DNA damage responses were noted, these were judged likely to be secondary to toxicity rather than DNA reactivity.
Since this earlier review, several genotoxicity studies of glyphosate, glyphosate salt solutions and GBFs have been published. Additionally, a large number of unpublished regulatory studies of glyphosate and GBFs were available for this review. A weight of evidence approach was applied to these data that considers the same factors used by Williams et al. (Citation2000) and which are consistent with recommendations for weight of evidence evaluations for genotoxicity data (EFSA, Citation2011; ICH S2(R1), Citation2011; UK COM, Citation2011; U.S. EPA, Citation1986; U.S. FDA, Citation2006). Additional considerations include the robustness of the experimental protocols and more recent elaborated considerations relevant to whether genotoxic effects result from direct interaction with DNA or are secondary to other processes such as cytotoxicity (Kirkland et al., Citation2007; Thybaud et al., Citation2007).
In terms of composition, the genotoxicity studies of both glyphosate and glyphosate salts can reasonably be considered together to provide an overall evaluation for the glyphosate molecule. This is especially useful when numerous consistent results are observed for a particular endpoint. The fact that glyphosate is present in all GBFs should be considered in evaluating the genotoxicity of GBFs. It is unlikely that glyphosate or glyphosate salts would contribute novel genotoxic activity (i.e. different from when tested alone) as part of a GBF. Analysis of a weight of evidence of genotoxicity of GBFs should consider the fact that different formulations have different compositions. The weight of evidence, therefore, can allow some conclusions about genotoxicity typical of GBFs but the possibility always exists that individual components could lead to different toxic and genotoxic properties.
Apart from genotoxicity, the data indicate that GBFs are more toxic to the genotoxicity test systems than glyphosate or glyphosate salts, which is consistent with findings in aquatic systems (Folmar et al., Citation1979; Perkins et al., Citation2000; Tsui & Chu, Citation2003). In many cases a reasonable explanation for this difference is that surfactants in GBFs contribute more to toxicity than glyphosate or glyphosate salts per se.
Gene mutation is one of the two primary endpoints with direct relevance to heritable mutation and is considered to be one of the key drivers in the carcinogenic process. A large number of regulatory bacterial reverse gene mutation studies provide a very consistent pattern that glyphosate, glyphosate salts and numerous GBFs are negative in well-conducted GLP regulatory assays.
Additionally, there are two regulatory in vitro mammalian cell gene mutation (mouse lymphoma tk locus) studies which gave negative results for glyphosate. As noted earlier, these mouse lymphoma tk locus studies detect large deletions as well as gene mutational events that are also detected in the CHO/HGPRT locus assay. The earlier reported negative CHO/HGRPT result (Williams et al., Citation2000) and these negative tk mutation results support the conclusion that glyphosate and glyphosate salts do not induce gene mutations in mammalian cells.
The second primary endpoint with direct relevance to heritable mutation and the carcinogenic process is chromosomal effects, such as the induction of chromosomal aberrations or micronuclei in cultured mammalian cells. The earlier review (Williams et al., Citation2000) noted mixed results for three in vitro chromosomal aberration assays for glyphosate, but concluded that the most reliable result was the negative assay. No in vitro mammalian cell chromosomal aberration reports were noted for GBFs in the Williams et al. review.
A number of in vitro chromosomal aberration and micronucleus assay results for glyphosate or glyphosate salts have been subsequently published using bovine or human lymphocytes. Some technical limitations of these assays were discussed earlier and should be considered in the weight attributed to these studies. Both positive and negative results were reported in these assays. In the absence of exogenous metabolic activation, the majority of studies were negative up to high (mM) dose levels that were toxic or close to toxic levels measured in parallel experiments. Two publications from a laboratory reported an increase in micronucleus frequencies for glyphosate in human lymphocytes in the presence of S9 mix but these studies have several limitations discussed earlier that complicate the interpretation of these effects.
A recent publication reported positive CB MN results for glyphosate in cultured human epithelial cells in the absence of metabolic activation at very low dose levels. The dose levels and exposure time reported as producing effects were much lower than dose levels and exposure times of many published and regulatory in vitro mammalian cell genotoxicity studies using different cell types that did not produce either genotoxic or toxic effects. Thus, the results of this study, especially the quantitative aspects, are quite unusual.
Three regulatory chromosomal aberration studies, which used upper dose levels of an estimated 3 mM to around 7 mM, gave negative results in both the presence and absence of S9. These results therefore agree with the majority of negative published data in the absence of S9 and support a weight of evidence that glyphosate is not active in in vitro mammalian cell gene mutation or chromosomal aberration assays in the presence of S9.
Overall, the weight of evidence indicates that glyphosate and glyphosate salts do not typically induce chromosomal effects in vitro in mammalian cells.
Two publications subsequent to the Williams et al. (Citation2000) review reported positive results for chromosomal aberrations with two different GBFs in two different assay systems. The paucity of studies and study limitations discussed earlier precludes any general conclusion for GBFs for this endpoint. However, as discussed above, the weight of evidence is that glyphosate or glyphosate salts are not clastogenic in mammalian cells, so any positive results with GBFs do not appear to be due to glyphosate.
In vivo mammalian chromosomal effect studies are a particularly important class of studies because they are the pre-eminent core assays for in vivo mammalian genotoxicity. The Williams et al. (Citation2000) review noted a predominance of negative results for glyphosate in these types of assays with only one study exhibiting a weak positive result.
Two subsequently published studies of glyphosate or glyphosate salt solutions in mouse bone marrow micronucleus assays gave discordant results with one study reporting positive results. However, eight out of 12 regulatory bone marrow micronucleus studies (seven mouse and one rat study) of glyphosate or glyphosate salts did not yield any statistically significant increases in the frequencies of micronucleated PCEs. Three other studies did give statistical increases in MN PCE frequency for high dose levels but these were judged not to be treatment-related because they were clearly within the historical negative control range. A fourth study exhibited a statistically significant increase in MN PCE only in females. This study had high vehicle control MN PCE frequencies and no historical control data were presented. In addition to the micronucleus results, a mouse bone marrow chromosomal aberration study was also negative. There did not appear to be any data to suggest that, in the minority of studies that exhibited some statistical increases in MN PCE frequencies, the effects might be due to factors such as gender, route of exposure or dose level. The clearly negative results from the vast majority of studies, including a large number of robust regulatory studies conducted in accordance with good laboratory practices, indicate that, on weight of evidence, glyphosate and glyphosate salts are not genotoxic in rodent bone marrow micronucleus or chromosomal aberration studies.
A preponderance (4/5) of mouse bone marrow micronucleus assays on GBFs were indicated as negative in the earlier Williams et al. (Citation2000) review. Mixed results were observed in subsequent published rodent bone marrow micronucleus or chromosomal aberration studies with a majority (4/6) being negative including 3/4 studies of Roundup™-branded GBFs. One rabbit drinking water study of a Roundup™-branded GBF was positive but there were some significant limitations of this study, and this is an unusual test model with little or no background data. Another GBF study reported positive results in spermatocytes with extended oral or i.p. treatments. No clear explanation exists for the discordant published mouse bone marrow results such as unique routes or dramatically different maximum dose levels.
The majority of regulatory rodent bone marrow micronucleus studies (11 mouse and one rat study) of various GBFs gave clearly negative results and the two that had statistical increases were also considered negative because the increases were well within historical control values.
The large number of negative regulatory studies, in combination with a majority of negative published studies, indicate that GBFs are generally negative for this important in vivo endpoint. The preponderance of negative results for GBFs is also consistent with a weight of evidence that glyphosate or glyphosate salt solutions are negative for chromosomal effects and suggests that formulation surfactant components are also negative for chromosomal effects in vivo.
The micronucleus test detects aneugenic as well as clastogenic (chromosomal breakage) events. The negative results for the large number of in vivo rodent micronucleus studies therefore support the conclusion that glyphosate, glyphosate salts and GBFs do not induce aneuploidy.
In addition to the rodent bone marrow studies, one regulatory rat dominant lethal study of glyphosate, albeit with some limitations, appears to confirm the earlier negative result for this type of assay, and reinforces the conclusion that glyphosate is not genotoxic for mammalian germ cells.
Although generally consistent negative results were observed for rodent micronucleus or chromosomal aberration assays of GBFs, discordant results were observed in in vivo erythrocyte micronucleus studies of fish, amphibians and reptiles. In addition to some technical limitations there is considerably less experience with these assay systems, and consequently these should have less influence in evaluating overall weight of evidence for chromosomal effects.
In general, induction of DNA damage is considered supplementary to induction of gene mutations and chromosomal effects because it does not directly measure heritable events or effects closely associated with heritable events. Regulatory genotoxicity testing focuses on gene mutation and chromosomal effects for initial in vitro core testing (Cimino, Citation2006; Eastmond et al., Citation2009; EFSA, 2011; ICHS2(R1), 2011; UK COM, 2011).
The Williams et al. (Citation2000) review noted negative DNA damage results for technical glyphosate in the B. subtilis rec assay and the primary hepatocyte UDS assay, but noted positive or equivocal results for SCE assays in vitro in human or bovine lymphocytes. The negative results for the B. subtilis rec and primary hepatocyte UDS assays have been confirmed in subsequent regulatory studies. The UDS result provides information on the lack of in vitro genotoxic activity when mammalian metabolic activation other than S9 is employed.
Subsequent literature publications indicated several positive responses for in vitro mammalian DNA damage endpoint assays of glyphosate or glyphosate salts. These include an SCE response in bovine lymphocytes and four positive Comet results in cultured mammalian cell lines or human lymphocytes. The positive Comet results were observed in the absence of mammalian metabolic activation and generally at concentrations in the mM range but one publication found positive results at much lower dose levels in human epithelial cells. As noted earlier, observations of differential responses in Comet and chromosomal aberration assays for some of these studies provide some support for the conclusion that the SCE or Comet responses observed may not be predictive of effects on other more relevant endpoints.
The Williams et al. (Citation2000) review noted some equivocal or positive Roundup™-branded GBF results for the SCE endpoint in human lymphocytes and reports of DNA strand breakage in mouse tissues and induction of comets in tadpoles. An observation of mouse liver DNA adducts for a GBF were considered to be of questionable significance. Subsequent literature results for DNA damage in mammalian systems included induction of SCE in cultured mammalian cells and in mouse bone marrow for the uncharacterized herbazed formulation and induction of comets in cultured mammalian cells with a Roundup™ UltraMax formulation. There were a number of Comet assay reports for GBFs in a variety of aquatic organisms with a preponderance of positive results.
The fact that DNA damage is usually only seen at high, toxic concentrations in vitro (e.g. in the 1–10 mM concentration range) or in vivo where tissue damage might be induced, suggests that cytotoxic effects rather than DNA interaction may be responsible for the DNA damage reported for glyphosate, glyphosate salts and GBFs. In many Comet assay publications parallel data on toxic effects most directly relevant to comet mechanisms are lacking, and, in addition, many of the positive DNA damage results have been observed for GBFs in non-standard test systems. It is hoped that clarification of the mechanism and significance of comet effects can be improved by the more routine use of relevant markers such as quantitation of double-strand breaks and hedgehogs and histopathology, as appropriate, for in vivo studies. Studies with protocols for specifically identifying surfactant effects would also be useful in clarifying the significance of DNA damage effects of GBFs. However, it seems reasonably clear that GBFs are more toxic than the a.i. and a reasonable conclusion is that consistency of observations of DNA damage, particularly comets, with GBFs might be secondary to the toxicity of GBF surfactants.
As discussed extensively in the section “DNA damage” there are both general and specific reasons to consider DNA damage assays as subordinate in a weight of evidence for genotoxic risk, especially when they may arise from mechanisms secondary to toxicity. Whatever the precise causes of these DNA damage effects, they do not translate into gene mutations or chromosomal damage as demonstrated by the large preponderance of negative results for glyphosate, glyphosate salts and GBFs in well-conducted bacterial reversion and in vivo rodent bone marrow micronucleus assays.
In addition to considering the results relevant to genotoxicity hazard assessment, an important additional perspective on risk can be provided by comparing levels used in experimental studies with expected human levels. For example, estimated margins of exposure between the in vivo genotoxicity test systems (e.g. 1000 mg/kg body weight exposure) and calculated systemic doses from an exposure study of farmers (Acquavella et al., Citation2004; 0.004 mg/kg maximum systemic exposure; 0.0001 mg/kg geometric mean systemic exposure) are in the range of 250 000 for maximum systemic exposure and 10 million for geometric mean systemic exposure. The margins of exposure compared to in vitro mammalian cell exposures are also quite large. Assuming uniform distribution, the estimated systemic concentration of glyphosate from the Acquavella et al. (Citation2004) farmer biomonitoring study would be of the order of 24 nM for the maximum and 0.59 nM for the geometric mean exposure. A typical maximum in vitro mammalian exposure of 5 mM represents margins of exposure of 208 000 for the maximum farmer systemic exposure and 8.5 million for the geometric mean farmer systemic exposure. Similarly, exposure levels evaluated in several published DNA damage and micronucleus assays in non-mammalian species were conducted at much higher glyphosate concentrations than anticipated under typical environmental conditions. Relevant environmental concentrations representing biologically available glyphosate are not equivalent to application rates. Sorption to soil and sediment occurs following glyphosate applications, significantly diminishing or eliminating glyphosate and POEA surfactant bioavailability to environmental species (Giesy, Citation2000).
This evaluation of the large volume of genotoxicity data available presents a convincing weight of evidence supporting the lack of genotoxic potential for both glyphosate and typical GBFs in core gene mutation and chromosomal effect endpoints. Given this conclusion, and for other reasons discussed, the observation of DNA damage effects seems likely to be secondary to cytotoxic effects. The lack of genotoxic hazard potential evidenced by core gene mutation and chromosomal effect studies, coupled with the very low human and environmental species systemic exposure potential discussed above, indicate that glyphosate and typical GBFs present negligible genotoxicity risk.
Declaration of interest
Larry Kier and David Kirkland were paid consultants of the Glyphosate Task Force for the preparation of this review. The Glyphosate Task Force is a consortium of 25 European glyphosate registrants, listed on http://www.glyphosatetaskforce.org/. Larry Kier is also a past employee of Monsanto Company. Monsanto Company was the original producer and marketer of glyphosate formulations. The authors had sole responsibility for the writing and content of the paper and the interpretations and opinions expressed in the paper are those of the authors and may not necessarily be those of the member companies of the Glyphosate Task Force.
| Abbreviations | ||
| a.e., | = | acid equivalents |
| a.i., | = | active ingredient |
| CB MN, | = | cytokinesis block micronucleus |
| GBF, | = | glyphosate-based formulation |
| i.p., | = | intraperitoneal |
| MN, | = | micronucleus |
| MN PCE, | = | micronucleated polychromatic erythrocyte |
| NCE, | = | normochromatic erythrocyte |
| PCE, | = | polychromatic erythrocyte |
| p.o., | = | oral administration |
| SCE, | = | sister chromatid exchange |
| SCGE, | = | single cell gel electrophoresis (Comet assay) |
| OECD, | = | Organization for Economic Co-operation and Development |
| S9, | = | 9000×g liver homogenate supernatant |
| UDS, | = | unscheduled DNA synthesis. |
Supplementary Material
Download PDF (910.9 KB)Acknowledgements
The authors would like to thank the following individuals for their contributions to this work by providing regulatory studies and their thoughtful review of the manuscript: David Saltmiras (Monsanto Company), Christian Strupp (Feinchemie Schwebda GmbH), Terri Spanogle (Cheminova A/S), Jürgen Wenzel (HELM AG), Andrew Bond (Nufarm Limited), Sylvain Gautier (Arysta LifeScience Corporation), Simon Hill (Syngenta AG), Ganesh Shetgaonkar and B.M. Ravikumar (Excel Crop Care Ltd.). We would also like to acknowledge David Saltmiras for his invaluable service in providing coordination with individual companies and the Glyphosate Task Force.
References
- Aaron CS, Bolcsfoldi G, Glatt HR, et al. (1994). Mammalian cell gene mutation assays working group report. Mutat Res, 312, 235–9
- Acquavella JF, Alexander BH, Mandel JS, et al. (2004). Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Persp, 112, 321–6
- Akanuma M. (1995a). HR-001: DNA Repair Test (Rec-Assay). Unpublished Regulatory Study. Report Identification Number: IET 94-0141
- Akanuma M. (1995b). HR-001 reverse mutation test. Unpublished Regulatory Study. Report Identification Number: IET 94-0142
- Akcha F, Spagnol C, Rouxel J. (2012). Genotoxicity of diuron and glyphosate in oyster spermatozoa and embryos. Aquat Toxicol, 106–107, 104–13
- Alvarez-Moya C, Silva MR, Arambula AMV, et al. (2011). Evaluation of genetic damage induced by glyphosate isopropylamine salt using Tradescantia bioassays. Genet Mol Biol, 34, 127–30
- Amer SM, Aly FAE, Farghaly AA, Ibrahim AAE. (2006). In vitro and in vivo evaluation of the genotoxicity of the herbicide glyphosate in mice. Bull Nat Res Centre (Egypt), 31, 427–46
- Amin F, Bowen ID, Szegedi Z, et al. (2000). Apoptotic and non-apoptotic modes of programmed cell death in MCF-7 human breast carcinoma cells. Cell Biol Int, 24, 253–60
- Anadon A, Martinez-Larranaga MR, Martinez MA, et al. (2009). Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol Lett, 190, 91–5
- Anderson D, Yu TW, Phillips BJ, Schmezer P. (1994). The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat Res, 307, 261–71
- BBA (Federal Biological Center for Agriculture and Forestry – Germany). (1998–2000). Monograph on the active substance glyphosate and its IPA-, Na- and NH4-salts. Annex to the European Commission report for the active substance glyphosate
- Benigni R. (1989). Analysis of the National Toxicology Program data on in vitro genetic toxicity tests using multivariate statistical methods. Mutagenesis, 4, 412–19
- Boos G, Stopper H. (2000). Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicol Lett, 116, 7–16
- Bosch B, Manas F, Gorla N, Aiassa D. (2011). Micronucleus test in post metamorphic Odontophrynus cordobae and Rhinella arenarum (Amphibia: Anura) for environmental monitoring. J Tox Env Health Sci, 3, 155–63
- Bradley MO, Hsu IC, Harris CC. (1979). Relationship between sister chromatid exchange and mutagenicity, toxicity and DNA damage. Nature, 282, 318–20
- Brendler-Schwaab S, Hartmann A, Pfuhler S, Speit G. (2005). The in vivo comet assay: use and status in genotoxicity testing. Mutagenesis, 20, 245–54
- Brewster DW, Warren J, Hopkins 2nd WE. (1991). Metabolism of glyphosate in Sprague–Dawley rats: tissue distribution, identification, and quantitation of glyphosate-derived materials following a single oral dose. Fundam Appl Toxicol, 17, 43–51
- Burlinson B, Tice RR, Speit G, et al. (2007). Fourth International Workshop on Genotoxicity Testing: Results of the in vivo comet assay workgroup. Mutat Res, 627, 31–5
- Callander RD. (1996). Glyphosate acid: an evaluation of mutagenic potential using S. Typhimurium and E. Coli. Unpublished Regulatory Study. Report Identification Number: CTL/P/4874
- Callander RD. (1999). Potassium salt of glyphosate: bacterial mutation assay in S. Typhimurium and E. Coli. Unpublished Regulatory Study. Report Identification Number: CTL/P/6184
- Camolesi MR. (2009). [Glyphosate 480 g/l liquid formulation] Bacterial Reverse Mutation Test (Ames Test). Unpublished Regulatory Study. Report Identification Number: RL8563/2008 6.0AM-B
- Camolesi MR. (2010). MON 77280 Bacterial Reverse Mutation Test (Ames Test). Unpublished Regulatory Study. Report Identification Number: XX-2010-501
- Catoyra JM. (2009). [Reverse Mutation Assay of Roundup Full II M in Salmonella typhimurium]. Unpublished Regulatory Study. Report Identification Number: Study Number XX-2011-0622
- Cavalcante DG, Martinez CB, Sofia SH. (2008). Genotoxic effects of Roundup on the fish Prochilodus lineatus. Mutat Res, 655, 41–6
- Cavas T, Konen S. (2007). Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis, 22, 263–8
- Chruscielska K, Graffstein B, Szarapinska-Kwaszewska J, et al. (2000). Glyphosate evaluation of chronic activity and possible far-reaching effects. Part 2. Studies on mutagenic activity. Pestcydy, 2000, 21–5
- Cifone MA, Myhr B, Eiche A, Bolcsfoldi G. (1987). Effect of pH shifts on the mutant frequency at the thymidine kinase locus in mouse lymphoma L5178Y TK+/− cells. Mutat Res, 189, 39–46
- Cimino MC. (2006). Comparative overview of current international strategies and guidelines for genetic toxicology testing for regulatory purposes. Environ Mol Mutagen, 47, 362–90
- Clay P. (1996). Glyphosate acid: L5178Y TK+/– mouse lymphoma gene mutation assay. Unpublished Regulatory Study. Report Identification Number: CTL/P/4991
- Conners DE, Black MC. (2004). Evaluation of lethality and genotoxicity in the freshwater mussel Utterbackia imbecillis (Bivalvia: Unionidae) exposed singly and in combination to chemicals used in lawn care. Arch Environ Contam Toxicol, 46, 362–71
- Costa KC. (2008). Evaluation of the mutagenic potential of GLYPHOSATE TECHNICAL by micronucleus assay in mice. Unpublished Regulatory Study. Report Identification Number: 3996.402.395.07
- Coutinho do Nascimento A, Grisolia C. (2000). Comparative analysis between micronuclei tests in mice and in peripheral erythrocytes of Oreochromis niloticus in evaluation of mutagenic potential of the agrotoxins deltamethrin, dicofol, glyphosate, and imazapyr. Pesticides: R Ecotoxicol E Meio Ambiente, Curitiba, 10, 41–8
- de Castilhos Ghisi N, Cestari, MM. (2012). Genotoxic effects of the herbicide Roundup® in the fish Corydoras paleatus (Jenyns 1842) after short-term, environmentally low concentration exposure. Environ Monit Assess. doi: 10.1007/s10661-012-2783-x
- Decuyper-Debergh D, Piette J, Laurent C, van de Vorst A. (1989). Cytotoxic and genotoxic effects of extracellular generated singlet oxygen in human lymphocytes in vitro. Mutat Res, 225, 11–14
- Delaney CA, Pavlovic D, Hoorens A, et al. (1997). Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology, 138, 2610–14
- Dimitrov BD, Gadeva PG, Benova DK, Bineva MV. (2006). Comparative genotoxicity of the herbicides Roundup, Stomp and Reglone in plant and mammalian test systems. Mutagenesis, 21, 375–82
- Djelic N, Spremo-Potparevic B, Bajic V, Djelic D. (2006). Sister chromatid exchange and micronuclei in human peripheral blood lymphocytes treated with thyroxine in vitro. Mutat Res, 604, 1–7
- Durward R. (2006). Glyphosate technical: micronucleus test in the mouse. Unpublished Regulatory Study. Report Identification Number: 2060/014
- Duthie SJ, Johnson W, Dobson VL. (1997). The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimidines) and growth in human cells. Mutat Res, 390, 141–51
- Eastmond DA, Hartwig A, Anderson D, et al. (2009). Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS Harmonized Scheme. Mutagenesis, 24, 341–9
- Eckl PM, Ortner A, Esterbauer H. (1993). Genotoxic properties of 4-hydroxyalkenals and analogous aldehydes. Mutat Res, 290, 183–92
- EFSA. (2011). Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Scientific Committee. EFSA J, 9, 2379
- Erexson GL. (2003a). In vivo mouse micronucleus assay with MON 78239. Unpublished Regulatory Study. Report Identification Number: CV-2002-187
- Erexson GL. (2003b). In vivo mouse micronucleus assay with Mon 78634. Unpublished Regulatory Study. Report Identification Number: CV-2002-189
- Erexson GL. (2006). In vivo mouse bone marrow micronucleus assay. Unpublished Regulatory Study. Report Identification Number: CV-2005-120
- Flugge C. (2009a). Mutagenicity study of glyphosate TC in the Salmonella Typhimurium reverse mutation assay (in vitro). Unpublished Regulatory Study. Report Identification Number: 23916
- Flugge C. (2009b). Micronucleus test of glyphosate TC in bone marrow cells of the CD rat by oral administration. Unpublished Regulatory Study. Report Identification Number: 23917
- Flugge C. (2010a). Mutagenicity study of trop M (glyphosate 480) in the Salmonella Typhimurium reverse mutation assay (in vitro). Unpublished Regulatory Study. Report Identification Number: 24753
- Flugge C. (2010b). Mutagenicity study of glyphosate TC in the Salmonella Typhimurium reverse mutation assay (in vitro). Unpublished Regulatory Study. Report Identification Number: 24880
- Flugge C. (2010c). Micronucleus test of trop M (glyphosate 480) in bone marrow cells of the NMRI mouse by oral administration. Unpublished Regulatory Study. Report Identification Number: 24754
- Flugge C. (2010d). Mutagenicity study of [glyphosate 757 g/kg granular formulation] in the Salmonella Typhimurium reverse mutation assay (in vitro). Unpublished Regulatory Study. Report Identification Number: 25631
- Flugge C. (2010e). Micronucleus test of [glyphosate 757 g/kg granular formulation] in bone marrow cells of the CD rat by oral administration. Unpublished Regulatory Study. Report Identification Number: 25632
- Folmar L, Sanders H, Julin A. (1979). Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch Environ Contam Toxicol, 8, 269–78
- Fox V. (1998). Glyphosate acid: in vitro cytogenetic assay in human lymphocytes. Unpublished Regulatory Study. Report Identification Number: CTL/P/6050
- Fox V, Mackay JM. (1996). Glyphosate acid: mouse bone marrow micronucleus test. Unpublished Regulatory Study. Report Identification Number: SM0796
- Gava MA. (2000). Evaluation of the mutagenic potential of the test substance GLIFOSTO IPA TECNICO NUFARM by micronucleus assay in mice. Unpublished Regulatory Study. Report Identification Number: RF-G12.022/00
- Gieseler F, Bauer E, Nuessler V, et al. (1999). Molecular effects of topoisomerase II inhibitors in AML cell lines: correlation of apoptosis with topoisomerase II activity but not with DNA damage. Leukemia, 13, 1859–63
- Giesy JP, Dobson S, Solomon KR. (2000). Ecotoxicological risk assessment for Roundup® Herbicide. Rev Environ Contam Toxicol, 167, 35–120
- Glei M, Klenow S, Sauer J, et al. (2006). Hemoglobin and hemin induce DNA damage in human colon tumor cells HT29 clone 19A and in primary human colonocytes. Mutat Res, 594, 162–71
- Gohre K, Casida JE, Ruzo LO. (1987). N-Oxidation and cleavage of the amino acid derived herbicide glyphosate and anilino acid of the insecticide fluvalinate. J Agric Food Chem, 35, 386–92
- Grisolia CK. (2002). A comparison between mouse and fish micronucleus test using cyclophosphamide, mitomycin C and various pesticides. Mutat Res, 518, 145–50
- Guilherme S, Gaivao I, Santos MA, Pacheco M. (2010). European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup – a glyphosate-based herbicide. Mutagenesis, 25, 523–30
- Guilherme S, Gaivao I, Santos MA, Pacheco M. (2012). DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide – elucidation of organ-specificity and the role of oxidative stress. Mutat Res, 743, 1–9
- Hartmann A, Pfuhler S, Dennog C, et al. (1998). Exercise-induced DNA effects in human leukocytes are not accompanied by increased formation of 8-hydroxy-2'-deoxyguanosine or induction of micronuclei. Free Radic Biol Med, 24, 245–51
- Hartmann A, Schumacher M, Plappert-Helbig U, et al. (2004). Use of the alkaline in vivo Comet assay for mechanistic genotoxicity investigations. Mutagenesis, 19, 51–9
- Helal A, Moussa H. (2005). Chromosomal aberrations induced by glyphosate isopropylamine herbicide and trials for diminuting its toxicity using some chemical inactivators and antioxidant. Vet Med J Giza, 53, 169–87
- Henderson L, Wolfreys A, Fedyk J, et al. (1998). The ability of the Comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis, 13, 89–94
- Heydens WF, Healy CE, Hotz KJ, et al. (2008). Genotoxic potential of glyphosate formulations: mode-of-action investigations. J Agric Food Chem, 56, 1517–23
- Holeckova B. (2006). Evaluation of the in vitro effect of glyphosate-based herbicide on boving lymphocytes using chromosome painting. Bull Vet Inst Pulawy, 50, 533–6
- Honarvar N. (2005). Micronucleus assay in bone marrow cells of the mouse with glyphosate technical. Unpublished Regulatory Study. Report Identification Number: 872000
- Honarvar N. (2008). Glyphosate technical – micronucleus assay in bone marrow cells of the mouse. Unpublished Regulatory Study. Report Identification Number: 1158500
- ICH S2(R1). (2011). Guidance on genotoxicitytesting and data interpretation for pharmaceuticals intended for human use. International Conferenece on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step4/S2R1_Step4.pdf [last accessed 27 Aug 2012]
- Jensen JC. (1991a). Mutagenicity test: Ames Salmonella assay with glyphosate, batch 206-JaK-25-1. Unpublished Regulatory Study. Report Identification Number: 12323
- Jensen JC. (1991b). Mutagenicity test: in vitro mammalian cell gene mutation test with glyphosate, batch 206-JaK-25-1. Unpublished Regulatory Study. Report Identification Number: 12325
- Jensen JC. (1991c). Mutagenicity test: micronucleus test with glyphosate, batch 206-JaK-25-1. Unpublished Regulatory Study. Report Identification Number: 12324
- Jones E. (1999). Potassium salt of glyphosate: mouse bone marrow micronucleus test. Unpublished Regulatory Study. Report Identification Number: CTL/P/6242
- Kaya B, Creus A, Yanikoglu A, et al. (2000). Use of the Drosophila wing spot test in the genotoxicity testing of different herbicides. Environ Mol Mutagen, 36, 40–6
- Kier LD, Brusick DJ, Auletta AE, et al. (1986). The Salmonella typhimurium/mammalian microsomal assay. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutation Res, 168, 69–240
- Kiffe M, Christen P, Arni P. (2003). Characterization of cytotoxic and genotoxic effects of different compounds in CHO K5 cells with the comet assay (single-cell gel electrophoresis assay). Mutat Res, 537, 151–68
- Kirkland DJ, Aardema M, Banduhn N, et al. (2007). In vitro approaches to develop weight of evidence (WoE) and mode of action (MoA) discussions with positive in vitro genotoxicity results. Mutagenesis, 22, 161–75
- Kirkland D, Speit G. (2008). Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens III. Appropriate follow-up testing in vivo. Mutat Res, 654, 114–32
- Kirsch-Volders M, Sofuni T, Aardema M, et al. (2003). Report from the in vitro micronucleus assay working group. Mutation Res, 540, 153–63
- Kocsis JJ, Harkaway S, Santoyo MC, Snyder R. (1968). Dimethyl sulfoxide: interactions with aromatic hydrocarbons. Science, 160, 427–8
- Koller VJ, Furhacker M, Nersesyan A, et al. (2012). Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch Toxicol, 86, 805–13
- Levine SL, Han Z, Liu J, et al. (2007). Disrupting mitochondrial function with surfactants inhibits MA-10 Leydig cell steroidogenesis. Cell Biol Toxicol, 23, 385–400
- Lope N. (2008). Reverse mutation assay of Roundup Ultramax (MON 79672) in Salmonella typhimurium. Unpublished Regulatory Study. Report Identification Number: XX-2011-0621
- Manas F, Peralta L, Raviolo J, et al. (2009). Genotoxicity of glyphosate assessed by the comet assay and cytogenetic tests. Environ Toxicol Phar, 28, 37–41
- Marques MFC. (1999). A micronucleus study in mice for glifosate tecnico nufarm. Unpublished Regulatory Study. Report Identification Number: RF-G12.79/99
- Matsumoto K. (1995). HR-001: in vitro cytogenetics test. Unpublished Regulatory Study. Report Identification Number: IET 94-0143
- Mecchi MS. (2003a). Salmonella–Escherichia Coli/mammalian–microsome reverse mutation assay with a confirmatory assay. Unpublished Regulatory Study. Report Identification Number: CV-2002-186
- Mecchi MS. (2003b). Salmonella–Escherichia Coli/mammalian–microsome reverse mutation assay with a confirmatory assay. Unpublished Regulatory Study. Report Identification Number: CV-2002-188
- Mecchi MS. (2008a). Bacterial reverse mutation assay with a confirmatory assay. Unpublished Regulatory Study. Report Identification Number: CV-08-030
- Mecchi MS. (2008b). Bacterial reverse mutation assay with a confirmatory assay. Unpublished Regulatory Study. Report Identification Number: CV-08-242
- Mecchi MS. (2008c). Salmonella–Escherichia Coli/mammalian–microsome reverse mutation asay with a confirmatory assay with MON 76171. Unpublished Regulatory Study. Report Identification Number: CV-2007-102
- Mecchi MS. (2009a). Salmonella–Escherichia coli/mammalian–microsome reverse mutation assay with a confirmatory assay. Unpublished Regulatory Study. Report Identification Number: CV-2007-082
- Mecchi MS. (2009b). Salmonella–Escherichia coli/mammalian–microsome reverse mutation assay with a confirmatory assay with MON 76138. Unpublished Regulatory Study. Report Identification Number: CV-2007-094
- Michels G, Watjen W, Niering P, et al. (2005). Pro-apoptotic effects of the flavonoid luteolin in rat H4IIE cells. Toxicology, 206, 337–48
- Miyaji CK. (2008). Evaluation of the mutagenic potential of the test substance glyphosate technical by reverse mutation assay in Salmonella Typhimurium (Ames test). Unpublished Regulatory Study. Report Identification Number: 3996.401.392.07
- Mladinic M, Berend S, Vrdoljak AL, et al. (2009a). Evaluation of genome damage and its relation to oxidative stress induced by glyphosate in human lymphocytes in vitro. Environ Mol Mutagen, 50, 800–7
- Mladinic M, Perkovic P, Zeljezic D. (2009b). Characterization of chromatin instabilities induced by glyphosate, terbuthylazine and carbofuran using cytome FISH assay. Toxicol Lett, 189, 130–7
- Monroy CM, Cortes AC, Sicard DM, de Restrepo HG. (2005). Cytotoxicity and genotoxicity of human cells exposed in vitro to glyphosate. Biomedica, 25, 335–45
- Morita T, Watanabe Y, Takeda K, Okumura K. (1989). Effects of pH in the in vitro chromosomal aberration test. Mutat Res, 225, 55–60
- Morita T, Honma M, Morikawa K. (2012). Effect of reducing the top concentration used in the in vitro chromosomal aberration test in CHL cells on the evaluation of industricl chemical genotoxicity. Mutat Res, 741, 52--6
- Negro Silva LF. (2009). A17035A -- mammalian erythrocyte micronucleus test. Unpublished Regulatory Study. Report Identification Number: RL7459/2008 -- 14.0MN-B
- Negro Silva LF. (2011). Glyphosate SL (A13013Z) – mammalian erythrocyte micronucleus test. Unpublished Regulatory Study. Report Identification Number: RL69575MN-B
- Nousis, L, Doulias PT, Aligiannis N, et al (2005). DNA protecting and genotoxic effects of olive oil related components in cells exposed to hydrogen peroxide. Free Rad Res, 39, 987--95
- OECD 471. (1983). Genetic toxicology: Salmonella typhimurium reverse mutation assay. OECD Guidelines for Testing of Chemicals. Available from: http://www.oecd.org/env/chemicalsafetyandbiosafety/testingofchemicals/45125466.pdf [last accessed 27 Aug 2012]
- OECD 471. (1997). Bacterial reverse mutation test. OECD guideline for testing of chemicals. Available from: http://www.oecd.org/chemicalsafety/assessmentofchemicals/1948418.pdf [last accessed 27 Aug 2012]
- OECD 472. (1983). Genetic toxicology: Escherichia coli reverse mutation assay. Guideline for testing of chemicals. Available from: http://www.oecd.org/env/chemicalsafetyandbiosafety/testingofchemicals/45125467.pdf [last accessed 27 Aug 2012]
- OECD 473. (1983). Genetic toxicology: in vitro mammalian cytogenetic test. OECD guideline for the testing of chemicals. Available from: http://www.oecd.org/env/ehs/testing/45125550.pdf [last accessed 27 Aug 2012]
- OECD 473. (1997). In vitro mammalian chromosome aberration test. OECD guideline for the testing of chemicals. Available from: http://www.oecd-ilibrary.org/environment/test-no-473-in-vitro-mammalian-chromosome-aberration-test_9789264071261-en [last accessed 27 Aug 2012]
- OECD 474. (1983). Genetic toxicology: micronucleus test. OECD guideline for testing of chemicals. Available from: http://www.oecd.org/env/ehs/testing/45125573.pdf [last accessed 27 Aug 2012]
- OECD 474. (1997). Mammalian erythrocyte micronucleus test. OECD guideline for the testing of chemicals. Available from: http://www.oecd.org/chemicalsafety/assessmentofchemicals/1948442.pdf [last accessed 27 Aug 2012]
- OECD 475. (1984). Genetic toxicology: in vivo mammalian bone marrow cytogenetic test – chromosomal analysis. OECD guideline for testing of chemicals. Available from: http://www.oecd.org/env/ehs/testing/45125582.pdf [last accessed 27 Aug 2012]
- OECD 475. (1997). Mammalian bone marrow chromosome aberration test. OECD guideline for the testing of chemicals. Available from: http://www.oecd.org/chemicalsafety/assessmentofchemicals/1948450.pdf [last accessed 27 Aug 2012]
- OECD 476. (1984). Genetic toxicology: in vitro mammalian cell gene mutation tests. OECD guideline for testing of chemicals. Available from: http://www.oecd.org/chemicalsafety/testing/45125600.pdf [last accessed 27 Aug 2012]
- OECD 476. (1997). In vitro mammalian cell gene mutation test. OECD guideline for the testing of chemicals. Available from: http://www.oecd.org/chemicalsafety/assessmentofchemicals/1948426.pdf [last accessed 27 Aug 2012]
- OECD 478. (1984). Genetic toxicology: rodent dominant lethal test. oecd guideline for testing of chemicals. Available from: http://www.oecd.org/env/chemicalsafetyandbiosafety/testingofchemicals/45125644.pdf [last accessed 27 Aug 2012]
- OECD 487. (2010). In vitro mammalian cell micronucleus test. OECD guideline for the testing of chemicals. Available from: http://www.oecd-ilibrary.org/environment/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264091016-en [last accessed 27 Aug 2012]
- OECD GLP. (1982). Good laboratory practice in the testing of chemicals. OECD monograph No. 45. OECD, Paris. ISBN 92-64-12367-9
- OECD GLP. (1997). OECD principles on good laboratory practice (as revised in 1997). OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring Number 1. Available deom: http://www.oecd.org/chemicalsafety/testing/oecdseriesonprinciplesofgoodlaboratorypracticeglpandcompliancemonitoring.htm [last accessed 11 Jan 2013]
- Parry JM, Parry E, Phrakonkham P, Corvi R. (2010). Analysis of published data for top concentration considerations in mammalian cell genotoxicity testing. Mutagenesis, 25, 531–8
- Perkins PJ, Boermans HJ, Stephenson GR. (2000). Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay – Xenopus. Environ Toxicol Chem, 19, 940–5
- Piesova E. (2004). The influence of different treatment length on the induction of micronuclei in bovine lymphocytes after exposure to glyphosate. Folia Vet Lat, 48, 130–4
- Piesova E. (2005). The effect of glyphosate on the frequency of micronuclei in bovine lymphocytes in vitro. Acta Veterinaria, 55, 101–9
- Poletta GL, Larriera A, Kleinsorge E, Mudry MD. (2009). Genotoxicity of the herbicide formulation Roundup (glyphosate) in broad-snouted caiman (Caiman latirostris) evidenced by the Comet assay and the micronucleus test. Mutat Res, 672, 95–102
- Poletta GL, Kleinsorge E, Paonessa A, et al. (2011). Genetic, enzymatic and developmental alterations observed in Caiman latirostris exposed in ovo to pesticide formulations and mixtures in an experiment simulating environmental exposure. Ecotox Environ Safe, 74, 852–9
- Prasad S, Srivastava S, Singh M, Shukla Y. (2009). Clastogenic effects of glyphosate in bone marrow cells of swiss albino mice. J Toxicol [Online]: Article ID: 308985. Available from: http://www.hindawi.com/journals/jt/2009/308985/ [last accessed 27 Aug 2012]
- Quintana PJ, de Peyster A, Klatzke S, Park HJ. (2000). Gossypol-induced DNA breaks in rat lymphocytes are secondary to cytotoxicity. Toxicol Lett, 117, 85–94
- Raipulis JTM, Balode M. (2009). Toxicity and genotoxicity testing of Roundup. Proc Latvian Acad Sci SectB, 63, 29–32
- Ranzani MRTC. (2000). Evaluation of the mutagenic potential of the test substance Glifosato IPA Tecnico Nufarm. Unpublished Regulatory Study. Report Identification Number: Study No. RF-G11.040/00
- Richeux F, Cascante M, Ennamany R, et al. (1999). Cytotoxicity and genotoxicity of capsaicin in human neuroblastoma cells SHSY-5Y. Arch Toxicol, 73, 403--9
- Ribeiro do Val R. (2007). Bacterial reverse mutation test (Ames test) for [glyphosate technical]. Unpublished Regulatory Study. Report Identification Number: RL3393/2007 - 2.0AM-B
- Rossberger S. (1994). DNA repair test with primary rat hepatocytes. Unpublished Regulatory Study. Report Identification Number: 931564
- Salamone MF, Mavournin KH. (1994). Bone marrow micronucleus assay: a review of the mouse stocks used and their published mean spontaneous micronucleus frequencies. Environ Mol Mutagen, 23, 239–73
- Sasaki YF, Saga A, Akasaka M, et al. (1998). Detection of in vivo genotoxicity of haloalkanes and haloalkenes carcinogenic to rodents by the alkaline single cell gel electrophoresis (comet) assay in multiple mouse organs. Mutat Res, 419, 13–20
- Saunders RA, Davies RR. (2005). Notes on rabbit internal medicine. Oxford: Blackwell Publishing
- Savage JR. (1976). Classification and relationships of induced chromosomal structual changes. J Med Genet, 13, 103–22
- Schreib G. (2010). Reverse mutation assay using bacteria (Salmonella Typhimurium and Escherichia Coli) with glyphosate technical. Unpublished Regulatory Study. Report Identification Number: 102025
- Scott D, Galloway SM, Marshall RR, et al. (1991). Genotoxicity under extreme culture conditions. International Commission for Protection Against Environmental Mutagens and Carcinogens. A report from ICPEMC Task Group 9. Mutat Res, 257, 147–205
- Sekihashi K, Yamamoto A, Matsumura Y, et al. (2002). Comparative investigation of multiple organs of mice and rats in the comet assay. Mutat Res, 517, 53–75
- Sivikova K, Dianovsky J. (2006). Cytogenetic effect of technical glyphosate on cultivated bovine peripheral lymphocytes. Int J Hyg Environ Health, 209, 15–20
- Smith CC, Adkins DJ, Martin EA, O'Donovan MR (2008). Recommendations for design of the rat comet assay. Mutagenesis, 23, 233--40
- Sokolowski A. (2007a). Salmonella Typhimurium and Escherichia Coli reverse mutation assay with glyphosate technical. Unpublished Regulatory Study. Report Identification Number: 1061401
- Sokolowski A. (2007b). Salmonella Typhimurium and Escherichia coli reverse mutation assay with glyphosate technical. Unpublished Regulatory Study. Report Identification Number: 1061402
- Sokolowski A. (2007c). Salmonella Typhimurium and Escherichia Coli reverse mutation assay glyphosate technical. Unpublished Regulatory Study. Report Identification Number: 1061403
- Sokolowski A. (2009a). Salmonella Typhimurium and Escherichia Coli reverse mutation assay with glyphosate technical. Unpublished Regulatory Study. Report Identification Number: 1236400
- Sokolowski A. (2009b). Glyphosate technical Salmonella Typhimurium and Escherichia Coli reverse mutation assay. Unpublished Regulatory Study. Report Identification Number: 1264500
- Speit G. (1986). The relationship between the induction of SCEs and mutations in Chinese hamster cells. I. Experiments with hydrogen peroxide and caffeine. Mutat Res, 174, 21–6
- Storer RD, McKelvey TW, Kraynak AR, et al. (1996). Revalidation of the in vitro alkaline elution/rat hepatocyte assay for DNA damage: improved criteria for assessment of cytotoxicity and genotoxicity and results for 81 compounds. Mutat Res, 368, 59–101
- Suresh TP (1992). Dominant lethal test in Wistar rats. Unpublished Regulatory Study. Report Identification Number TOXI:888-DLT
- Suresh TP. (1993a). Mutagenicity – Salmonella Typhimurium reverse mutation assay (Ames test). Unpublished Regulatory Study. Report Identification Number: TOXI: 887-MUT.AMES
- Suresh TP. (1993b). Mutagenicity–micronucleus test in Swiss albino mice. Unpublished Regulatory Study. Report Identification Number: TOXI: 889-MUT.MN
- Suresh TP. (1994). Genetic toxicology – in vivo mammalian bone marrow cytogenetic test – chromosomal analysis. Unpublished Regulatory Study. Report Identification Number: TOXI:890-MUT-CH.AB
- Tayama S, Nakagawa Y. (1994). Effect of scavengers of active oxygen species on cell damage caused in CHO-K1 cells by phenylhydroquinone, an o-phenylphenol metabolite. Mutat Res, 324, 121–31
- Thompson PW. (1996). Technical glyphosate reverse mutation assay (Ames test) using Salmonella Typhimurium and Escherichia Coli. Unpublished Regulatory Study. Report Identification Number: SPL Proj. No. 434/014
- Tice RR, Agurell E, Anderson D, et al. (2000). Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen, 35, 206–21
- Thybaud V, Aardema M, Clements J, et al. (2007). Strategy for genotoxicity testing: hazard identification and risk assessment in relation to in vitro testing. Mutat Res, 627, 41-58.
- Tsui MT, Chu LM. (2003). Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere, 52, 1189–97
- Uhde H. (2004). Mutagenicity study of FSG 03090 H-1 in the Salmonella Typhimurium reverse mutaiton assay (in vitro). Unpublished Regulatory Study. Report Identification Number: 18487/04
- UK COM (2011). Guidance on a strategy for genotoxicity testing of chemical substances. UK Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment. Available from: http://www.iacom.org.uk/guidstate/documents/COMGuidanceFINAL2.pdf/ [last accessed 17 Sep 2012]
- U.S. EPA. (1986). Guidelines for mutagenicity risk assessment. Fed. Register 51. 34006-34012. Available from: http://www.epa.gov/raf/publications/guidelines-mutagenicityl-risk-assessment.htm [last accessed 1 Jan 2013]
- U.S. FDA (2006). Guidance for industry and review staff: recommended approaches to integration of genetic toxicology study results. Center for Drug Evaluation and Research. U.S. Food and Drug Administration. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079257.pdf [last accessed 11 Jan 2013]
- Wallner B. (2010). Reverse mutation assay using bacteria (Salmonella Typhimurium) with glyphosate TC. Unpublished Regulatory Study. Report Identification Number: 101268
- Williams GM, Kroes R, Munro IC. (2000). Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol, 31, 117–65
- Wolf T, Niehaus-Rolf C, Banduhn N, et al. (2008). The hen's egg test for micronucleus induction (HET-MN): novel analyses with a series of well-characterized substances support the further evaluation of the test system. Mutat Res, 650, 150–64
- Wright NP. (1996). Technical glyphosate: chromosome aberration test in CHL cells in vitro. Unpublished Regulatory Study. Report Identification Number: 434/015
- Xu Y. (2006). Salmonella–Escherichia Coli/mammalian–microsome reverse mutation assay with a confirmatory assay with MON 78910. Unpublished Regulatory Study. Report Identification Number: CV-2005-119
- Xu Y. (2008a). In vivo mouse bone marrow micronucleus assay. Unpublished Regulatory Study. Report Identification Number: CV-08-031
- Xu Y. (2008b). In vivo mouse bone marrow micronucleus assay with MON 76171. Unpublished Regulatory Study. Report Identification Number: CV-2007-103
- Xu Y. (2009a). In vivo mouse bone marrow micronucleus assay with MON 79991. Unpublished Regulatory Study. Report Identification Number: CV-2007-083
- Xu Y. (2009b). In vivo mouse bone marrow micronucleus assay with MON 76138. Unpublished Regulatory Study. Report Identification Number: CV-2007-095
- Xu Y. (2009c). In vivo mouse bone marrow micronucleus assay. Unpublished Regulatory Study. Report Identification Number: CV-08-243
- Zeiger E, Haseman JK, Shelby MD, et al. (1990). Evaluation of four in vitro genetic toxicity tests for predicting rodent carcinogenicity: confirmation of earlier results with 41 additional chemicals. Environ Mol Mutagen, 16, 1–14
- Zoriki Hosomi R. (2007). Mammalian erythrocyte micronucleus test for [glyphosate technical]. Unpublished Regulatory Study. Report Identification Number: 3393/2007-3.0MN