Abstract
Over the last couple of decades, the awareness of the potential health impacts associated with early-life exposures has increased. Global regulatory approaches to chemical risk assessment are intended to be protective for the diverse human population including all life stages. However, questions persist as to whether the current testing approaches and risk assessment methodologies are adequately protective for infants and children. Here, we review physiological and developmental differences that may result in differential sensitivity associated with early-life exposures. It is clear that sensitivity to chemical exposures during early-life can be similar, higher, or lower than that of adults, and can change quickly within a short developmental timeframe. Moreover, age-related exposure differences provide an important consideration for overall susceptibility. Differential sensitivity associated with a life stage can reflect the toxicokinetic handling of a xenobiotic exposure, the toxicodynamic response, or both. Each of these is illustrated with chemical-specific examples. The adequacy of current testing protocols, proposed new tools, and risk assessment methods for systemic noncancer endpoints are reviewed in light of the potential for differential risk to infants and young children.
| Abbreviations | ||
| ADH | = | Alcohol Dehydrogenase |
| ADHD | = | Aldehyde Dehydrogenase |
| ADI | = | Acceptable Daily Intake |
| ADME | = | Absorption, Distribution, Metabolism, Excretion |
| BMD | = | Benchmark Dos |
| CHAP | = | Chronic Hazard Advisory Panel |
| CNS | = | Central Nervous System |
| CPSIA | = | Consumer Product Safety Improvement Act |
| EFSA | = | European Food Safety Authority |
| ILSI | = | International Life Sciences Institute |
| LO(A)EL | = | Lowest Observed (Adverse) Effect Level |
| NMDR | = | Non-Monotonic Dose Response |
| NO(A)EL | = | No Observed (Adverse) Effect Level |
| NoG | = | Notes of Guidance |
| NRC | = | National Research Council |
| OECD | = | Organization for Economic Co-operation and Development |
| PBPK | = | Physiologically-Based Pharmacokinetic |
| PK | = | Pharmacokinetic |
| POD | = | Point of Departure |
| QRA | = | Quantitative Risk Assessment |
| R/B | = | Risk/Benefit |
| RfC | = | Reference Concentration |
| RfD | = | Reference Dose |
| RIVM | = | Dutch National Institute for Public Health and the Environment |
| SCCS | = | Scientific Committee on Consumer Safety |
| TD | = | Toxicodynamics |
| TEWL | = | Transepidermal Water Loss |
| TH | = | Thyroid Hormone |
| TK | = | Toxicokinetics |
| UF | = | Uncertainty Factor |
| TTC | = | Threshold of Toxicological Concern |
| US EPA | = | US Environmental Protection Agency |
| US FDA | = | US Food and Drug Administration |
| WHO | = | World Health Organization |
Introduction, purpose, and scope
The period of infancy and early childhood encompasses a special life stage of rapid growth, development, and maturation that has received less attention with regard to sensitivity to environmental chemical exposures than the in utero exposure window. Organ systems undergo rapid development during gestation and are still developing in infants and young children, resulting in the potential for different sensitivities to chemical exposures, compared to adults. Early adverse effects may have lifelong consequences; chronic diseases, including neurological conditions and immune-related diseases, often begin in infancy or childhood. The possible relationship of early onset diseases with exposures to chemicals and drugs is largely unknown; epidemiological studies to date have not established cause and effect, or have been equivocal or inconclusive. While there has been a significant focus on understanding effects after in utero exposures through testing in laboratory animals, fewer studies are available to evaluate effects after early-life exposures. In laboratory animals, chemical exposure during the comparable period of infancy and early childhood and the effects on the development of functional endpoints related to, for example, the immune and neural systems, are largely lacking. Innovations to the current testing paradigm, especially to the functional development of organ systems such as the nervous and immune systems, may be needed to ensure the identification of hazards after pre- and perinatal life stage exposures to chemicals.
Over the last 70 years, methods for quantitative risk assessment (QRA) have evolved and converged to provide a consistent process for establishing an exposure limit below which no appreciable risk for adverse human health effects are expected, including sensitive populations and different life stages. Although some methodological differences exist among different regions and regulatory bodies, a default 10-fold uncertainty factor (UF), to account for the heterogeneity of the human population, has been used consistently. As knowledge of the mechanistic bases for human heterogeneity and the potential for differential sensitivity associated with early-life stage exposure increases, questions remain about whether these methods are adequate to protect infants and young children. Here, we review factors that need to be considered; these include an understanding of the testing protocols and other tools available to evaluate the potential for a chemical to pose an increased risk to infants and children, as well as physiological and maturational differences associated with infancy and early childhood that might result in increased sensitivity during these early-life stages. In light of these considerations, we specifically evaluate whether the default intraspecies10-fold UF used in QRA is sufficient to protect infants and children.
The focus of this review is on the postnatal period of exposure. While in utero exposures must be included in the full consideration of protection of infants and young children, the rationale for focusing on early-life exposure is that chemical-specific toxicity data available for risk assessment do not always (or only partially) cover postnatal and early-life periods. The most commonly available data are from repeat-dose toxicity studies in rodents that generally start when the animals are about 6 weeks old. Developmental toxicity studies include exposures during highly sensitive periods in utero, but do not include postnatal exposures; multi-generational studies and directly dosed juvenile animal studies are lacking for most chemicals. Given the significant growth and development that continues past birth and through early childhood, it is important to re-evaluate our risk assessment methods, to ensure they are adequately protective for early-life exposures.
The scope of this review includes only full term infants. Available data suggest that birth-related events play a role in initiating the maturation of many physiological processes such as the surge of glucuronidation activity and glutathione levels (CitationCuzzolin 2013) and the significant increase in CYP2E1 expression associated with birth (CitationJohnsrud et al. 2003). These data are consistent with a number of other maturational processes that are initiated by birth. However, assessments for premature infants vary among individual ages and circumstances, and are most appropriately considered on a case-by-case basis. A complete evaluation of early-life exposures should also include latent effects. For example, carcinogenicity is a well-recognized effect with a potentially long latency period, but is beyond the scope of this review. We have focused this review on threshold-based noncancer risk assessment approaches for systemic endpoints.
Terminology and definitions
Terms used throughout this manuscript are defined here to ensure common understanding.
Life stages
The focus of this review is on exposures associated with the early-life period that is often missing in a toxicological dataset for chemicals, corresponding to the period from birth up to ∼ 6–7 years of age. There are no universally accepted definitions for the terms commonly used to refer to early-life stages. Further, it is recognized that the choice of how one defines a specific age group is often a reflection of a developmental (life) stage rather than a strict chronological age. For purposes of this review, we use the definition of life stage provided by CitationFirestone et al. (2007) as “a distinguishable timeframe in an individual's life, characterized by unique and relatively stable behavioral and/or physiological characteristics that are associated with development and growth.”
From a physiological perspective, there is the potential for differential sensitivity related to organ growth and maturation. The most dramatic changes, particularly with regard to TK handling of xenobiotic exposures, generally occur within the first 4 to 6 months. We consider the term “infant” to refer to the first 12 months of life. We refer to infants from birth to 1 month of age as neonates, infants from 1 to ∼6 months of age as “young infants”, and infants from 6 to 12 months as “older infants”; the term “child” refers to the ages of 1 to about 6 to 7 years.
Sensitivity, susceptibility, and vulnerability
There are many factors that contribute to the likelihood of an adverse effect after chemical exposure. The following definitions from the glossary in the US EPA's Risk Assessment PortalFootnote1 are used in this review:
Sensitivity
Differences in toxic response resulting from toxicodynamic differences and/or toxicokinetic differences. These differences can arise due to numerous biological factors such as life stage (windows of enhanced sensitivity), genetic polymorphisms, gender, disease status, nutritional status, etc.
Susceptibility
Differences in risk resulting from variation in both toxicity response (sensitivity) and exposure (as a result of gender, life stage, and behavior).
Vulnerability
Differences in risk resulting from the combination of both intrinsic differences in susceptibility and extrinsic social stress factors such as low socioeconomic status, crime and violence, lack of community resources, crowding, access to health care, education, poverty, segregation, geography, etc.
Policies and initiatives relevant to infants’ and children's risk assessment
A number of major events have shaped the discussion and the current regulatory focus on infants and children as a potentially more susceptible life stage.
WHO/IPCS (1986)
The first publication from a major international agency to focus specifically on infants and children as a special subpopulation (life stage) was the 1986 WHO/IPCS Environmental Health Criteria (EHC), “Principles for Evaluating Health Risks from Chemicals During Infancy and Early Childhood: The Need for a Special Approach” (CitationWHO 1986). The EHC summarized a number of physiological and exposure differences between infants/children and adults, including: small size and large surface area in relation to weight; a higher metabolic rate; rapid growth; different body composition; and functional immaturity of the organs and other systems of the body. The report also emphasized the need for revised testing protocols to be able to better predict effects on developmental endpoints, but did not recommend specific changes to existing QRA methodologies.
ILSI/EPA 1990
In November 1990, a conference was co-sponsored by the International Life Sciences Institute (ILSI) Risk Science Institute, the ILSI-Nutrition Foundation,Footnote2 and the US EPA, entitled, “Similarities and Differences between Children and Adults: Implications for Risk Assessment” (CitationGuzelian et al. 1992). An oft-cited conclusion from this workshop is that “children are not simply small adults but rather are a unique population for health risk assessment.” Factors contributing to these differences include physiological factors, metabolism, pharmacokinetics, diet, and the physical environment (e.g. as it impacts exposure). It is emphasized that these factors could render an infant or child more or less susceptible than an adult, with many examples provided for both scenarios.
CitationNRC (1993)
Some of the earliest concerns for early-life exposure are centered on dietary pesticides. In 1993, the National Research Council (NRC) of the National Academy of Sciences (NAS) released its influential report on “Pesticides in the Diets of Infants and Children.” Key conclusions from this report are summarized as follows:
Infants and children differ both qualitatively and quantitatively from adults in their exposure to pesticide residues in foods. This was generally found to be a more important source of differences in risk associated with dietary pesticides than were age-related differences in toxicologic sensitivity.
Both quantitative and occasionally qualitative differences exist in the toxicity of pesticides between children and adults. Children may be more sensitive or less sensitive than adults, depending on the pesticide, and there is no simple way to predict the relative sensitivity in the absence of data.
Quantitative differences in toxicity between children and adults are usually less than a factor of 10-fold. Because newborns are the group most different from adults anatomically and physiologically, they can have the most significant differences in sensitivity.
The default 10X UF for intrahuman variability “generally provides adequate protection for infants and children”; however, the report also stated that “this population subgroup may be uniquely susceptible to chemical exposures at particularly sensitive stages of development.”
A lack of data led the NRC to recommend expanding testing protocols to cover early-life stages.
The report recommended that in the absence of data to the contrary, there should be a presumption of greater toxicity to infants and children. Accordingly, they recommended that “an UF up to the 10- fold factor traditionally used by EPA and FDA for fetal developmental toxicity should also be considered when there is evidence of postnatal developmental toxicity and when data from toxicity testing relative to children are incomplete.”
With regard to this last bullet, the NRC report referred several times to the US EPA's use of an additional 10X UF for developmental toxicants. However, this is not standard practice for the US EPA, according to the Agency's risk assessment guidelines (CitationUS EPA 1987, Citation1996). It is noted however, that the Agency does have examples where an additional 10X UF is applied when there is a lack of information on reproductive or developmental toxicity (i.e. the database UF). The conclusion by the CitationNRC (1993) that in some cases, infants and children might not be adequately protected by current regulatory policies, provided the impetus for what would become an international focus on infants and children as a special subpopulation (life stage) requiring further evaluation.
1995 US EPA Policy on Evaluating Health Risks to Children
In 1995, the US EPA issued a policy statement that the Agency would, “consider the risks to infants and children consistently and explicitly as a part of risk assessments generated during its decision making process, including the setting of standards to protect public health and the environment” (CitationUS EPA 1995). This policy was reaffirmed in 2013 (CitationMcCarthy 2013).
1996 Food Quality Protection Act
In 1996, The Food Quality Protection Act (FQPA) was passed into law in the US (CitationUS EPA 1996), largely as a consequence of the CitationNRC (1993) report on Pesticides in the Diets of Infants and Children. One of the focal areas was to provide increased health protection for infants and children, requiring the US EPA to apply an additional 10X factor in setting pesticide tolerances to protect infants and children, unless it could be demonstrated that it was not needed based on existing data. To date, this additional factor applies only to pesticide residues, and the EPA has not moved to extend the mandate of the FQPA to other environmental risk assessments.
ILSI, 2001
In 2001, the ILSI Risk Science Institute (RSI) held a workshop to develop a conceptual framework for assessing risks to children from exposure to environmental agents (CitationOlin and Sonawane 2003, CitationDaston et al. 2004). It was emphasized that life stage-linked exposure assessment is a critical component of any children's risk assessment. The TK considerations should include both chemical- and life stage/age-specific factors, and the TD considerations should include an understanding of the mechanisms(s) of action in identifying critical target organs and how these might translate into differential toxicity in infants and children.
National Children's StudyFootnote3
The National Children's Study (NCS) is planned as a large, longitudinal study to examine the effects of environmental factors, defined broadly to include chemical, genetic, and psychological factors on the health and development of children in the U.S., by following more than 100,000 children from before birth until age 21. The goal of the NCS is to improve our understanding of the role of environmental factors on development and disease. It is being implemented in two phases: the Vanguard Study, a pilot study from 2009 to 2010, and the subsequent Main Study, for which initiation is currently on hold due to feasibility issues raised by an NAS review (CitationNAS 2014).
Exposure differences
It is well recognized that compared to adults, infants and children can have different exposures to chemicals in the environment, drinking water, food, pharmaceuticals and/or consumer products. This can be the result of (i) greater intake on a body weight basis; and/or (ii) unique behaviors such as breast- feeding, crawling, and hand/object to mouth behavior. Exposure differences and how they impact differential response to a chemical by life stage are not the main focus of this review; however, it is important to recognize that this can be a major contributor to differential susceptibility by life stage. In many cases, an overall higher susceptibility associated with early-life exposures can be driven primarily by exposure (versus an inherently higher sensitivity), which must be considered in a comprehensive risk assessment. An example of unique exposures to infants was recently highlighted by an expert panel convened by the US FDA to evaluate potential exposure and safety issues related to chemical migration from packaging into infant formula and breast milk bags (CitationNeal-Kluever et al. 2014). Recommendations from the panel included changing the expression of toxicological testing tiers in FDA guidance documents from units of parts per billion and microgram (μg)/person/day to units of μg/kg body weight/day, to account for exposure differences between general and infant populations.
In recognition of the importance of evaluating life stage-specific exposures, the US EPA developed a Child Specific Exposure Factors Handbook in 2008 (CitationUS EPA 2008), which was subsequently updated within the current Exposure Factors Handbook (CitationUS EPA 2011a). Additional child-specific exposure factors have been reviewed by CitationMoya and Phillips (2014). It is also recognized that exposure can change quickly in a relatively short period of time; this has been highlighted in a guidance document by the CitationUS EPA (2005), which recommends age bins to use when evaluating exposure. The age bins are narrow in young infants where rapid developmental changes occur and broader as children grow. Recently, a consolidation of some of the age groups was recommended in a review by the World Health Organization (WHO) to reduce the burden of developing age-specific exposure factors for different regions (CitationCohen Hubal et al. 2014).
Quantitative risk assessment: Current approaches
Quantitative risk assessment (QRA) dates back 70 years, originating with the protection of the public food supply by US FDA (CitationLehman and Fitzhugh 1954). In 1983, the NAS formalized what has widely become known as the 4-step risk assessment paradigm of Hazard Identification, Dose-Response Assessment, Exposure Assessment, and Risk Characterization (CitationNRC 1983). While it has been modified over the years and updated in 2009 (CitationNRC 2009), the basic tenets of QRA remain the same, and global regulatory agencies use very similar approaches for establishing acceptable exposure limits for chemicals. With very few exceptions, QRAs for noncancer (systemic) endpoints are based on an assumption of a threshold, below which it is expected that there is no (or negligible) health risk. For example, the US EPA's definition of a reference dose (RfD)Footnote4 is as follows:
“An estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime“.
Risk values generated by other regulatory agencies (e.g. an Acceptable Daily Intake, Minimal Risk Level, Derived No Effect Level) have similar definitions.
Hazard identification
The first step in the traditional risk assessment paradigm is to determine what hazard is posed by a chemical, and to identify the “critical effect’, which is defined as the adverse effect seen at the lowest exposure. To assess safety for systemic endpoints following chemical exposure, all sources of relevant information should be considered. This includes information on chemical structure (including comparison with structurally similar chemicals), physical-chemical properties, in vitro and in vivo screening studies, mechanistic data, relevant human data, and laboratory animal toxicity studies. While epidemiological data are preferred for use in risk assessment, such human data are often not available, and rarely provide insight into the potential for differential toxicity associated with early-life exposures.
In the absence of human data, laboratory animal studies commonly serve as the primary source of data for use in QRA. Studies that have most commonly been used include repeated-dose, reproductive and developmental, and multi-generational toxicity studies. Internationally agreed upon test methods for these studies have been published by the OECD (see CitationOECD 2013 for a complete list of guidelines); a concise summary of these protocols has been provided by CitationNeal-Kluever et al. (2014). Additional guidance documents with similar protocols have been developed by the US EPA, US FDA, and the ICH (International Conference on Harmonization) for pharmaceuticals. While these studies look broadly across possible endpoints, other studies are focused on specific endpoints, such as developmental neurotoxicity (DNT) or developmental immunotoxicity (DIT) (CitationDeWitt et al. 2012). For pharmaceuticals, the lack of data for early-life exposures is addressed with an additional toxicity study when data are insufficient to support clinical trials in pediatric patients. A juvenile animal toxicity study is designed on a case-by-case basis, taking into account the existing toxicological profile and other factors such as the age of the intended pediatric population, with direct dosing to juvenile animals (CitationCappon et al. 2009).
Concordance of developmental timelines across species
A comparison of developmental timelines (in utero and postnatal) across species is helpful in determining windows of potential sensitivity among animal models and to address questions about the human relevance of findings associated with early-life exposure. However, there is no simple temporal comparison across species, as this varies depending on the organ system. A series of papers was published in Birth Defects Research, which describe timelines for specific organs including the female reproductive system (CitationBeckman and Feuston 2003); male reproductive system (CitationMarty et al. 2003); immune system (CitationHolsapple et al. 2003); central nervous system (CitationWood et al. 2003); bone (CitationZoetis et al. 2003); kidney (CitationZoetis and Hurtt 2003a); lung (CitationZoetis and Hurtt 2003b); and the gastrointestinal system (CitationWalthall et al. 2005).
For each of these organ systems, different mammalian species follow similar anatomical and functional developmental pathways but with differing timeframes; a comparison across species does not support a conclusion that any one laboratory species is most similar to humans. Species differences in anatomical and functional maturation are most apparent in the neonatal stage. For example, the development of the human kidney is similar to that of the mouse, with anatomical development (nephrogenesis) being complete prior to birth; this is in contrast to the rat, in which nephrogenesis occurs at a rapid rate in the first couple of weeks postnatally. Functional maturation for all species continues for some time after birth, being complete in the post-weaning rat, about 3 weeks of age, and reaching adult levels in the human by 1 to 2 years of age.
While our understanding of cross-species development continues to grow, the inherent complexity of organ and system development makes it difficult to draw conclusions about the relative sensitivity of a target organ based on anatomical or functional immaturity. For example, reduced renal function in a neonate could render an infant either more or less sensitive to a given toxicant. While reduced renal function could result in a longer half-life and hence increased systemic level of a toxicant, it could also render the kidney less sensitive because of decreased systemic exposure to the kidney in early postnatal life. It is also noted that while infants and newborns have an intact urine diluting ability, their ability to concentrate urine is limited (CitationBaum 2009). Interestingly, studies on melamine and cyanuric acid, which have been associated with kidney toxicity in infants exposed to contaminated formula, have found that neonatal rats are less sensitive than adults; it has been hypothesized that this is because of a decreased ability to concentrate urine (CitationGamboa da Costa et al. 2013).
Dose-response assessment
After the critical effect (hazard) for a chemical has been identified, the next step in the risk assessment paradigm is to quantify the potency through dose-response analysis to determine an exposure limit that is considered to pose little risk to humans. With very few exceptions, QRAs for noncancer (systemic) endpoints are based on an assumption of a threshold, below which it is expected that there is no (or negligible) health risk. A point of departure (POD) is identified, which is typically a No/Lowest Observed Adverse Effect Level (NOAEL/LOAEL), or Benchmark dose (BMD) expressed in units of mg/kg bw/day. The POD is most commonly identified or calculated from data on the critical effect identified in a laboratory animal study. From there, various UFs are applied to the POD to extrapolate to an exposure level that is considered to pose a negligible risk to humans, including sensitive subpopulations and life stages. With regard to the protection against early-life exposures, the question is whether this approach is adequately protective when toxicity data covering this life stage are lacking.
Uncertainty factors
The use of UFs in QRA date back to the publication of CitationLehman and Fitzhugh (1954), in which a NOAEL from a chronic rodent study was identified, and divided by a composite ‘safety factor’ of 100X to determine an Acceptable Daily Intake (ADI). The 100X factor was comprised of a 10X factor to account for species differences, and a 10X factor to account for the heterogeneity of the human population, including age, gender, underlying disease, and other factors impacting sensitivity. In the years following, this method was adopted by other agencies (e.g. CitationWHO 1974, CitationBarnes and Dourson 1988) and over time, the 100X factor became widely accepted as a conservative default to cover both cross-species extrapolation and heterogeneity of the human population (often called “intraspecies” or “inter-individual extrapolation”). Additional factors were later introduced to account for other areas of extrapolation and/or uncertainty such as extrapolation from subchronic to chronic exposure, lack of a NOAEL, and an incomplete database (CitationDourson et al. 1996).
Central to the concern of adequacy of QRA methods for assessing early-life exposures is the fact that risk assessments are typically based on data from repeated-dose toxicity studies conducted in rodents. Although a complete toxicity dataset will include dosing during all life stages, many chemicals have limited toxicity data and often are missing data from early-life exposures (i.e. less than 6 weeks old in the rodent). Studies involving direct pre-weaning exposure are often lacking and must be inferred based on maternal exposure (with the assumption that the chemical passes into breast milk). This review considers the potential for early-life sensitivity, against the backdrop of current regulatory practices and approaches designed to protect the safety of infants and children. The following discussion is focused on those UFs associated with protection against early-life exposures.
Interspecies UF: TK and TD components
The interspecies UF is used to extrapolate from the test animal species to humans, and accounts for differences in the TK and TD handling of a chemical. While this UF does not directly address human variability, it is predicated on a basic assumption that animal toxicity data can be used to extrapolate findings to humans, and thus is an important part of the overall risk assessment paradigm when we consider whether current methods are adequate to answer questions about early-life exposures. Historically, a default value of 10X has been used for interspecies extrapolation; more recently, this has been subdivided into factors for TK and TD, as has the intraspecies UF (described below).
There are relatively few data to support a comparison across species for the potency of chemical exposures during early postnatal life. However, investigators have examined the literature on developmental toxicants to compare hazard and potency across species (CitationKimmel et al. 1984, CitationSchardein et al. 1985; and CitationSchardein and Keller 1989). While limited, these comparisons support the appropriateness of studies in rodents and other commonly used laboratory animal species (the rabbit, for developmental toxicity), to predict and provide some quantitatively meaningful information on developmental toxicity in humans. In a limited review of 10 known teratogens, CitationKimmel et al. (1984) reported that there were no unique qualitative differences (based on yes/no for effects) between species, with the exception of thalidomide. The difference between the LOAEL in the most sensitive animal species tested and the level known to cause adverse effects in humans was in the range of 10- to 100-fold, leading the authors to conclude that the available data, although limited, supported the traditional UFs used in QRA. CitationSchardein et al. (1985) provided an extensive review of animal models used to identify and characterize developmental toxicants, as well as their predictability for effects on humans. This review considered 48 human developmental toxicants, and evaluated the predictive potential for different types of effects in 11 different species. Ratios of threshold doses in the most sensitive animals to those in humans were found to range from 1.2–200; the review referenced work by others that suggested an even tighter range of 1.8–50. Taken together, these analyses also support the adequacy of the traditional 100X UF to extrapolate across species and account for human heterogeneity. As these studies evaluate effects during the most rapid period of growth and development in utero, it is reasonable to consider that these findings would also be relevant to exposure during postnatal growth and development.
Intraspecies UF (Human variability): TK and TD components
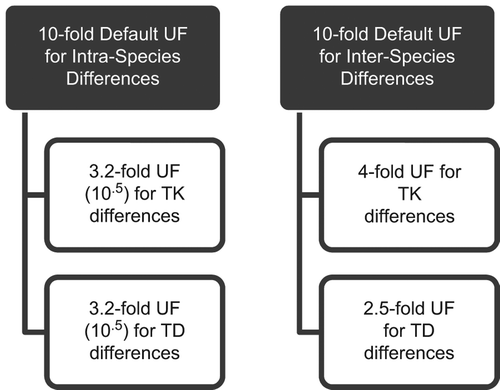
The intraspecies (also called inter-individual) UF used in QRA is intended to cover the heterogeneity (e.g. related to gender, ethnicity, genetic polymorphisms, pre-existing diseases, or age) of the human population, including sensitive subpopulations and life stages (CitationAldridge et al. 2003). Historically, a default factor of 10X for noncancer health effects has been used by all regulatory agencies to account for human variability in the absence of chemical-specific data (reviewed in CitationRenwick and Lazarus 1998). To provide an opportunity to refine this factor when data are available, CitationRenwick (1993) proposed dividing each of the 10X default factors for inter- and intraspecies extrapolations into sub factors for TK and TD. Based on an analysis of data on the variability in each of these factors, Renwick suggested an even subdivision of the 10X factor into 100.5 (3.16) for kinetics, and 100.5 (3.16) for dynamics. This allows for the incorporation of quantitative chemical-specific data, relating to either TK or TD, to replace part of the usual 10X factor for interspecies extrapolation and/or intraspecies variability, but reverts back to the 10X factor in the absence of sufficient data. The scheme proposed by CitationRenwick (1993) was subsequently modified by an international review group such that the sub factors were weighted more heavily toward TK for the interspecies factor, but weighted equally for the intraspecies UF (CitationWHO 1994, Citation2001) (see ). Thus, the question of whether the 10X default factor for intrahuman variability is sufficient to provide protection for infants and children, can further be refined by asking whether the sub factors of 3.2X (rounded from 3.16) are sufficient to address the TK and TD variability associated with early-life exposures in cases where we have sufficient data.
Figure 1. Subdivision of the default 10-fold UFs for inter- and intraspecies Differences (adapted from CitationRenwick 1993, IPCS 1994).
Database UF
Another source of uncertainty in risk assessment can be the lack of a complete database. For example, there are many cases where the dataset includes studies of repeat dose toxicity, but lacks reproductive and/or developmental toxicity studies. If there are insufficient data to judge whether the missing endpoints might represent the critical effect for a chemical, one way to address this in risk assessment is through the application of a database UF. This UF is used by the US EPA; a review of the Agency's IRIS databaseFootnote5 shows that a value of 3X is applied most commonly. The default 10X factor, applied under the 1996 Food Quality Protection Act to pesticides lacking DNT data, is also a form of a database UF. It is recognized that the FQPA 10X factor represents a default regulatory decision. In fact, in a review of the FQPA conducted in 2006, the US EPA determined that it was not necessary to apply the additional 10X safety factor in 48 of 59 cases for pesticide risk assessments (reviewed in CitationNRC 2009).
QRA for pharmaceuticals
The risk assessment approach for pharmaceuticals is a bit different. Human pharmaceuticals are developed and designed to treat a particular disease or condition, so the approach is not based on risk assessment for inadvertent exposure but is done in the context of a risk/benefit (R/B) analysis. Uncertainty factors are not applied per se, but the same considerations are incorporated into the R/B assessment. The risks for reproductive and developmental toxicities are characterized using the same toxicological data as described above. Importantly, for fetal and neonatal development, initial risk assessment relies almost entirely on data generated in animal studies. As outlined in the Guidance for Industry, Reproductive and Developmental Toxicities – Integrating Study Results to Assess Concerns (CitationUS FDA 2011), data from the reproductive and developmental studies, general toxicity studies, human adult or developmental data (when available), and pharmacokinetic (PK) and TK data are considered, to determine the risk for adverse developmental outcomes. The totality of the available data determines the level of concern for the likelihood of risk to humans and forms the basis of the initial labeling for the pharmaceutical. As human data are gained (e.g. pregnancy registries and conduct of pediatric clinical trials), R/B assessments are reviewed and the risk summary found in labeling is modified to reflect refinement of the assessment.
Regulatory positions on the adequacy of QRA methods for early-life exposure
A number of regulatory agencies have reviewed their methods in terms of adequacy to provide protection for early-life exposure. Key reviews are summarized below.
US EPA
The EPA convened a technical panel of the Agency's senior scientists to evaluate the RfD/RfC process, including how well children and other potentially sensitive subpopulations/life stages are protected (CitationUS EPA 2002). The panel concluded that in some cases, the default 10X factor used in risk assessment for intrahuman variability may be large, and in other cases too small, but that “often there are insufficient data to support a factor other than the default.” They advocated for continued research and future re-evaluations of the protectiveness of the 10-fold default UF, particularly for children and the elderly.
As described earlier, the US EPA has a legal mandate under the FQPA to apply an additional UF for food-use pesticides when adequate data on early-life exposure are lacking. A consensus group concluded that this additional factor should be regarded as a policy decision, to encourage the generation of additional data rather than a science-based UF (CitationDaston et al. 2004). Although this mandate applies only to pesticide regulations, the US EPA's risk assessment methods generally require consideration of developmental and early-life toxicity, and the Agency will commonly employ a database UF to provide additional conservatism if it is judged that data are inadequate to evaluate all life stages.
Consumer Products Safety Commission
The Consumer Products Safety Commission (CPSC) conducts a limited number of risk assessments for chemicals in consumer products. Chemical hazard statutes administered by the CPSC include the Consumer Product Safety Act, Federal Hazardous Substances Act, Poison Prevention Packaging Act, and the 2008 Consumer Product Safety Improvement Act (CPSIA). Similar to the assessments by the US FDA and US EPA, risk-based decisions about chemicals are made, but there are some minor differences in the process (reviewed in CitationBabich 1998). Some recent CPSC efforts, including risk assessments for phthalates in children's products (CitationBabich et al. 2004, CitationCPSC 2014) and metals in children's jewelry (CitationHillyer et al. 2014), highlight the Agency's focus on chemical safety for children.
European Union (EU) SCCNFP/SCCS
In Europe, questions about the adequacy of risk assessment methods to provide protection for children were first addressed by the Scientific Committee on Cosmetic Products and Non-Food Products (SCCNFP) in reference to consumer products with dermal exposure. The question posed was whether the default 100X safety factorFootnote6 should be adjusted in an assessment for children by multiplying this factor by the difference in Skin Surface Area-to-Body Weight ratio (SSA/BW) between adults and children. This factor is estimated to be 2.3X at birth, 1.8X at 6 months, 1.6X at 12 months, 1.5X at 5 years, and 1.3X at 10 years (CitationRenwick 1998). The CitationSCCNFP (2002) issued an opinion concluding that these differences are well within a factor of 3.2 (half-log of 10X intraspecies factor) which is considered to account for the intrahuman variability in TK, and therefore there is no need for an additional factor for children when intact skin is involved. It is noted that the difference in SSA/BW actually relates to exposure and not the TK, but the point remains that the difference is quantitatively small and changes quickly as the infant grows.
The most recent EU Scientific Committee on Consumer Safety (SCCS) Notes of Guidance for the Testing of Cosmetic Ingredients for their Safety Evaluation (NoG) is the 8th revision (SCCS/1501/12), published in December 2012 (CitationSCCS 2012). The guidance concludes that an additional safety factor is not needed for assessments of infants and children involving exposure to intact skin. It was suggested that a specific risk assessment is appropriate for cosmetic products used in the “nappy zone” (diapered area) because of factors that may increase risk relative to the rest of baby skin (e.g. the presence of urine and/or feces, diaper rash, a more occluded environment); however, no details were provided. As with the earlier SCCNFP guidance, the considerations described in the NoG are related to factors that might have the potential to impact exposure, as opposed to being related to a difference in inherent susceptibility.
European Food Safety Authority
The difference in exposure as a driver that can make children more susceptible was highlighted in a 2011 European Food Safety Authority (EFSA) report on the procedures currently used for the assessment of dietary exposure to different chemical substances (CitationEFSA 2011). In this report, the EFSA emphasizes the importance of considering children, especially toddlers, since they are often at the “high end” when calculating exposure. There were no recommendations to change current QRA methods.
Dutch National Institute for Public Health and the Environment
The Dutch National Institute for Public Health and the Environment (RIVM) issued a report in 2007 entitled, “Guidance for assessment of chemical risks for children” (CitationWolterink et al. 2007). The report addresses child-specific exposure, TK, and TD considerations, as well as the adequacy and data gaps in current risk assessment methods. The report acknowledges the potential for differential TK between children and adults, and concludes that children can be potentially more or less sensitive to the effects of a chemical. With regard to QRA, RIVM states that these differences (as well as those associated with other potentially sensitive groups) “are assumed to be accounted for by the [10X] intraspecies safety factor”, and that the use of an additional safety factor would need to be justified. The RIVM report also notes that many exposures in young children are limited to a relatively short period of life (e.g. as in the case of diapering). When performing a risk assessment for children, this short-term exposure is often compared with limit values based on chronic toxicity data (e.g. an ADI), resulting in a conservative risk assessment.
Sensitivity of infants and children: Review of the literature
There is an extensive body of literature reviewing the physiological and metabolic differences between infants, children, and adults (e.g. CitationRenwick 1998, CitationGinsberg et al. 2002, CitationScheuplein et al. 2002, CitationDourson et al. 2002, CitationWolterink et al. 2002, CitationAlcorn and McNamara 2003, Citationde Zwart et al. 2004, CitationDorne et al. 2005, CitationStrolin Benedetti et al. 2005). Others have focused more specifically on characterizing what is known specifically about infants and children (e.g. CitationBjorkman 2004, CitationEdginton et al. 2006, CitationCuzzolin 2013). These studies have generally been conducted either for the purpose of understanding dosimetric adjustments for pharmaceuticals, or for evaluating the sufficiency of current QRA methods and the default UF used for intrahuman variability to protect infants and children.
Toxicokinetics
Toxicokinetics (TK) is broadly considered to include four components: absorption, distribution, metabolism, and excretion (ADME). A number of differences between infants, children, and adults have been documented in the TK handling of xenobiotics. Several reviews have been published, providing a broad overview of these life stage TK differences (e.g. CitationScheuplein et al. 2002, CitationStrolin Benedetti et al. 2005). Key factors in the TK differences that can influence the relative sensitivity of infants and children are described below, and are summarized in .
Table 1. Comparison of key physiological parameters resulting in TK differences between infants and adults (summarized from CitationAlcorn and McNamara 2003, CitationDorne et al. 2005, Strolin Bendetti 2005, CitationDeWoskin and Thompson 2008, CitationQuigley 2012, CitationCuzzolin 2013, CitationHines 2013).
Absorption
The first step in determining systemic exposure to a chemical is absorption at the portal of entry, typically the GI tract, skin, or lungs. The following describes maturational differences in the GI tract and skin as they pertain to early-life sensitivity; the lung is not covered here as inhalation is beyond the scope of this review.
Gastrointestinal
There are a number of age-dependent anatomical/physiological factors that can influence the rate and/or extent of gastrointestinal (GI) absorption (reviewed by CitationEdginton and Fotaki 2010, CitationMakri et al. 2004, CitationAlcorn and McNamara 2003). These include gastric pH, gastric emptying time, intestinal surface area, intestinal transit time, pancreatic and biliary function, bacterial flora, and enzyme transporter activity. GI absorption is generally lower in newborns and younger infants and higher in older infants. However, it is also acknowledged that this can vary, depending on the nature of the chemical. For example, the high gastric pH of a newborn and young infant can lead to lower bioavailability of weakly acidic compounds such as phenobarbital but increased bioavailability of weakly basic compounds such as ampicillin and penicillin G (CitationAlcorn and McNamara 2003). Other physiological differences can exert influences that can have opposing effects. For example, newborns and young infants have reduced gastric emptying and GI motility compared to adults. While the lower rate of gastric emptying can have the effect of reducing the rate of drug absorption, which occurs primarily in the small intestine, the decreased GI motility could increase overall absorption due to longer retention time in the small intestine.
Dermal
By the third trimester in utero, fetal skin has developed all of the layers found in mature skin, and at birth, the stratum corneum is completely keratinized and similar to that seen in adults (CitationAfsar 2009, CitationFluhr et al. 2012). While there are some differences in the early neonatal period (e.g. fewer cell layers in the stratum corneum, higher pH, and greater hydration), these are relatively short-lived and are not expected to have a significant impact on skin integrity or the absorption of xenobiotics across the skin. Importantly, newborn skin has been found to exhibit good barrier function, as measured by transepidermal water loss (TEWL) (CitationChiou and Blume-Peytavi 2004, CitationFluhr et al. 2012, CitationLudriksone et al. 2014). This supports a conclusion that the differences between full-term infant skin and that of older children and adults is not expected to contribute significantly to differential absorption that would impact an exposure assessment for chemicals in contact with the skin. The one caution is that the SSA/BW ratio is higher in infants compared to adults, such that a whole body dermal exposure can lead to a greater systemic exposure to the infant; this should be considered when conducting the exposure assessment.
Distribution
The volume of distribution for water-soluble compounds is generally highest in newborns and infants, as they have a higher percent of total body water and a correspondingly lower fat content compared to older children and adults. The effect of this is that, given the same exposure in units of mg/kg of body weight, an infant will have a lower concentration of a circulating water-soluble toxicant than an adult. Quantitatively, this difference has been estimated to be between 2 to 4-fold for water-soluble drugs (CitationStrolin Benedetti et al. 2005).
Distribution of toxicants is also influenced by the extent of protein binding, which is generally lower in neonates as a result of both lower levels of binding proteins, and often, lower binding capacities compared to adults (CitationStrolin Benedetti et al. 2005). While this can result in a higher fraction of unbound chemical circulating in the blood, this also means that a larger fraction can be subject to enhanced renal clearance by glomerular filtration. The net effect is that, as with other physiological differences, the higher volume of distribution in an infant can contribute to either increased or decreased sensitivity.
An age-related difference in transporters is also recognized as a factor that can influence systemic distribution and ultimately sensitivity. CitationMooij et al. (2014) recently evaluated the ontogeny of human hepatic and intestinal transporter gene expression in autopsy samples from fetuses, neonates, infants, children, and adults. For a series of transporters with well- defined roles in drug PK, they found that for some hepatic transporters, levels were lower in neonates and infants compared to adults, while for some intestinal transporters it was similar, and in the case of one intestinal transporter, expression was the highest in neonates. While our understanding in this area is growing, little is known about the overall quantitative impact of differential levels of transporters in age-related sensitivity.
Metabolism
Metabolism is broadly classified into phase I (oxidation, reduction, and hydrolysis) reactions and phase II (conjugation) reactions. These reactions serve to make a compound more hydrophilic so that it can be more easily excreted in urine. The metabolites of phase I reactions can be more or less toxic than the parent compound, whereas products of phase II reactions are generally less toxic (with some notable exceptions). The liver is the most metabolically active organ in the body, although skin and other organs also have some metabolic activity. There can be significant intraindividual differences in metabolism, which can lead to differences in sensitivity following exposure to toxicants. These can be related to age, gender, ethnicity, or a number of other factors, including co-exposure to drugs or other toxicants. However, the largest differences are often a reflection of individual genetic make-up, with well-known polymorphisms driving the most significant differences in metabolism. These differences, lasting an entire lifespan, can be a greater source of differential sensitivity than the short time period during early infancy when metabolic capacity is not fully mature.
It is well established that while most phase I and phase II pathways are functional at birth, they are not fully mature (recent reviews include CitationCuzzolin 2013, CitationHines 2013). CitationHines (2008, Citation2013) evaluated the temporal development of drug-metabolizing enzymes in infants and young children. He described three classes of hepatic enzymes: Class 1 enzymes, which are highly expressed in the fetus, with levels significantly reduced after birth; Class 2 enzymes, which are expressed at relatively constant levels throughout gestation and into adulthood; and Class 3 enzymes, which are at very low levels in the fetus and begin to increase either late in gestation or soon after birth. While not fully understood, the differences in these developmental timelines are thought to reflect physiological needs in utero and postnatally. Of the enzymes that have been characterized, the majority (19/31) are in Class 3. While activity is generally low at birth, levels approaching those of maturity may be achieved within a few weeks (e.g. CYP2D6), within 1 to 2 years (e.g. CYP1A2, CYP3A4), or may take up to several years to reach full maturity (e.g. FMO3, CYP2C9). However, CitationHines (2013) also points out that the ratio of liver mass to body mass is much greater in infants and young children compared to adults, and this somewhat offsets the lower level of hepatic enzymes, when expressed on a mg/g liver basis.
The implication of decreased metabolic capacity in neonates and infants for risk assessment has been the topic of many workshops and reviews. Dorne and colleagues published a series of papers investigating the human variability in TK associated with phase I metabolism (specifically: CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, hydrolysis, alcohol dehydrogenase); phase II metabolism (N-acetyltransferases, glucuronidation, glycine conjugation, sulfation); and renal excretion (CitationDorne et al. 2001a,Citationb, Citation2002, Citation2003a,Citationb, Citation2004a,Citationb). This work was summarized in a review in which they concluded that the default 3.16X UF for human variability in TK is quite conservative when considering the healthy adult population, and can often be refined with chemical-specific data (CitationDorne et al. 2005). Data for other life stages including neonates and children were also evaluated; data on kinetic variability in children were available for ten pathways, and in neonates for five pathways: CYP1A2, CYP3A4, glucuronidation, glycine conjugation, and renal excretion. provides a compilation of the analyses presented in CitationDorne et al. (2005) comparing neonates, children, and adults, and the pathway-related UF that would be needed to address the variability between these life stages. Importantly, the data on neonates came from pharmaceutical studies involving chronic intravenous dosing, whereas the data on children and adults came from chronic oral exposures, significantly impacting the dosing kinetics. The authors concluded that the majority of pathway-related UFs for children were below the 3.16X default for intrahuman variability, but were higher than this for neonates.
Table 2. Pathway-related uncertainty factor for neonates and children compared to adults (data from CitationDorne et al. 2005).
The 95th percentile for pathway-related UFs for neonates ranged from 2.8 (renal excretion) to a high value of 25 for glycine conjugation. However, glycine conjugation has been recognized to be mature in neonates, and the data from an intravenous exposure may not be relevant to oral kinetics in neonates because it is a highly saturable pathway (CitationDorne et al. 2005). The other three values ranged from 8.1 to 11X, ∼2.5–3.5X higher than the default 3.16X UF. However, the differences in oxidative metabolic capacity (e.g. cytochrome P450s) do not necessarily imply a higher degree of sensitivity, as this pathway can either increase or decrease toxicity of a compound. Redundancy of metabolic pathways can also mean that differences in the activity of a single CYP enzyme will not translate directly to an effect on overall sensitivity; this was recently demonstrated by CitationWetmore et al. (2014) who showed in a PK model that chemicals cleared by multiple enzymes generally displayed the least variability across life stages and ethnic populations.
It is important to recognize that these differences, particularly for neonates, are only significant for a relatively short duration of time, as metabolic capacity matures rapidly during the first year. It is noted that for all 5 of the factors for which data are available in neonates, the data in children show that an UF of 1.2 to 1.5 would cover 95% of this life stage, well below the 3.16X UF. Data were available for an additional four pathways in children that were not available in neonates (CYP2C19, CYP2D6, hydrolysis, and N-actetyltransferase); for these pathways, UFs ranged from 1.5–22 (summarized in ). The factor of 22 was reported for CYP2D6, which is known to be significantly influenced by genetic polymorphisms. Data in healthy adults for three genetic variations of CYP2D6 indicated that the UF for this pathway would range from 3.0 to 21 in adults, depending on whether the individual was a poor or extensive metabolizer by this pathway; this is generally considered to be a more important determinant than age (CitationWolf and Smith 1999). CYP2D6 is responsible for the metabolism of a wide variety of clinically important pharmaceuticals, and is an important determinant in establishing the appropriate dose for many pharmaceuticals, especially in infants and children. However, it is not associated with metabolism of most environmental chemicals or chemicals associated with consumer products used by infants and children. The only other value above the default UF of 3.1 for children was for CYP2C19, which is also recognized as having significant genetic variation (the range in healthy adults for this CYP was reported to be from 2.0 to 45).
The issue of population variability, including that associated with life stages, was recently addressed by CitationWetmore et al. (2014) who estimated oral equivalent doses in various life stages and other subpopulations that were needed to achieve steady-state blood concentrations (Css) equivalent to media concentrations that have a defined effect in an in vitro high throughput assay (note that these were effect levels and not representative of human exposure levels). Hepatic clearance rates of nine ToxCastFootnote7 chemicals were measured in vitro for 13 cytochrome P450 and UGT enzymes; these were then incorporated into an in vitro-to-in vivo (IVIVE) model that includes known differences in enzyme expression across several life stages and other subpopulations. The most sensitive group for the majority of chemicals was that of infants under 6 months old. The ratio of the median Css for a healthy adult population against the median Css for the most sensitive population ranged from 1.3 to 4.3; the ratio for the 95th percentile ranged from 3.1 to 13.1.
Excretion
The major route of excretion for toxicants and/or their metabolites is via the kidneys into urine (reviewed in CitationStrolin Benedetti et al. 2005). Elimination of toxicants via the kidney is dependent first on renal blood flow, which increases in the weeks following birth, as a function of increased cardiac output and a decrease in renal resistance. Although nephrogenesis is complete by the time of birth, functional changes associated with maturation continue throughout infancy (reviewed in CitationDeWoskin and Thompson 2008, CitationQuigley 2012, CitationSulemanji and Vakili 2013). Kidney function itself involves three main processes: glomerular filtration, secretion, and reabsorption:
Glomerular filtration (GFR) is a passive process that serves as a nonselective filter permeable to all the compounds with a molecular weight lower than ∼ 65,000. GFR is reduced in the neonate (about 40 ml/min/1.73 m2), but rises quickly to 66 ml/min/1.73 m2 by 2 weeks of age (CitationSulemanji and Vakili 2013). The literature provides varied reports on the age at which GFR reaches adult values (100–120 ml/min/1.73 m2), ranging from 3 months to 2 years (CitationRoutledge 1994, CitationAlcorn and McNamara 2003, CitationAnderson 2010, CitationSulemanji and Vakili 2013). The GFR is also influenced by differences in plasma protein binding, as only unbound chemical can be removed by this process. Because neonates and infants have reduced levels of plasma protein binding, this can offset the effect of reduced GFR for protein-bound chemicals.
Tubular secretion is an active process that is important for organic acids and bases. It is also reduced at birth, but the timeline for maturation exhibits more variability during the first year (Dorne et al. 2004). In general, the rate of tubular secretion reaches adult levels at about 7 to 12 months of age. In children, starting around the age of 3 years, the tubular secretion capacity is often greater than that of adults.
Tubular reabsorption is generally a passive process, although there is also active reabsorption of endogenous compounds such as sodium, potassium, glucose, and amino acids. The maturation time frame of tubular reabsorption is not well characterized. It is generally more important for compounds that are liposoluble and not metabolized.
While full maturation of all kidney processes might not be achieved for a couple of years, overall kidney function is considered to be reasonably mature by about 6 months of age, and generally at its greatest during childhood. This is supported by dosing recommendations for pharmaceuticals that are excreted predominantly unchanged by the kidneys. CitationAnderson (2010) reports that for these drugs, the dose (weight-normalized) needs to be reduced only for neonates and young infants, and that by 1 year of age, dosing based on body weight is similar to that for adults. The overall rate of elimination of a toxicant via urine is dependent on renal blood flow and one or more of the three functions described above, which can vary significantly depending on the chemical. If there is no tubular resorption, then renal clearance can range from the GFR to the renal blood flow, depending on which is the rate-limiting process. For chemicals excreted via tubular secretion or reabsorbed in the tubules, urinalysis data are needed to quantify differences between infants, children, and adults. Because of this, it is not possible to develop generic guidelines to predict age-specific differences in renal excretion; rather, one would need to know how the chemical is handled by the kidney in order to make a chemical-specific prediction.
CitationDorne et al. (2005) reviewed the human variability, including for neonates and children, in TK associated with phase I and phase II metabolism, and renal excretion. For renal excretion, they concluded that a pathway-related UF of 1.2 would cover 95% of children. For neonates, a factor of 2.8 would be required to cover 95% (and a factor of 3.4 would cover 99%), within the 3.16X factor for human variability associated with differences in TK handling of a chemical.
CitationStrolin Benedetti et al. (2005) have described the immaturity of kidney function during the neonatal period and early infancy, but noted that in contrast, a similar or greater rate of renal elimination compared to adults is seen in late infancy and childhood. In part because of the greater rate of elimination, there are many drugs for which children require higher doses (per kg of bw) than adults to achieve the same plasma concentrations.
Clinical data on pharmacokinetics and/or toxicokinetics
Hattis and colleagues have developed a database of kinetic parameters for 44 pharmaceuticals.Footnote8 CitationGinsberg et al. (2002, Citation2004a,Citationb) used this database to conduct a number of analyses examining the variability of half-life or clearance with age and by metabolic enzyme class. They reported that the average half-life for the chemicals in this database was elevated for full-term neonates, but that this difference disappears by 2 to 6 months of age. Beyond 6 months, the half-life was often shorter than that in adults. While CitationGinsberg et al. (2002) indicated that the range of neonate/adult half-life ratios exceeds the 3.16X factor for intrahuman variability in TK, the authors also stress that this “may not be the major determinant of whether a separate children's PK factor is warranted.” They emphasize that the differences between neonates and adults are most important when the endpoint of concern can result from short-term exposures, especially if there is a critical window of sensitivity that overlaps with the time period associated with significant PK differences, such as with lead or mercury-induced neurotoxicity. The authors go on to state that, “Such differences may have less impact on RfDs/RfCs that are set based upon chronic effects that require years of cumulative exposure and toxicity, since altered dosimetry in early-life would be a short-term factor in the exposure assessment.”
In considering the database from which Ginsberg and colleagues conducted their analyses, it is also important to consider that the data came from pharmaceuticals, which are dosed in humans at a level intended to have a physiological effect while minimizing the potential for adverse side effects. Hence, these doses are much more likely to be in a range of exposures close to or associated with saturation of metabolic or clearance pathways, as compared to exposure to low level contaminants. This is consistent with a review conducted by the French Health Products Safety Agency (CitationAFSSAPS 2010) which concluded that as long as the exposure level remains lower than the detoxification systems’ saturation doses, children are not at any more risk than adults.
Developmental/clinical dosing recommendations
CitationBartelink et al. (2006) summarized the impact of developmental changes in ADME on general clinical dosing recommendations in the pediatric population, recognizing that these recommendations can be impacted by a number of chemical and/or age-specific considerations. In brief:
Hydrophilic drugs with a high V(d) in adults should be normalized to bodyweight in young children (age < 2 years), whereas hydrophilic drugs with a low V(d) in adults should be normalized to body surface area (BSA) in these children (resulting in a higher dose in units of mg/kg bw). This is because the higher V(d) for water-soluble compounds in infants and children compared to adults means that they will have a lower concentration of a circulating water-soluble toxicant than an adult. For drugs that are metabolized by the liver, V(d) is a factor for the first dose, and subsequent doses take into consideration the rate of hepatic clearance.
PK studies on liver and kidney function suggest that a distinction should be made between development and growth of the organs. While development is largely complete within the first 6 months of age, changes in growth and blood flow to the liver and kidney continue to change as the child grows.
For drugs that are primarily metabolized by the liver, dosing before the age of 2 months should be based on response and therapeutic drug monitoring. From 2 to 6 months, doses determined according to bodyweight are recommended. After 6 months of age, BSA is generally a good marker as a basis for drug dosing, with a couple of exceptions.
For drugs that are significantly excreted by the kidney, dosing should be based on the determination of renal function in the first 2 years of life. After maturation, the dose should be normalized to BSA.
These considerations for pharmaceutical dosing support a conclusion that in general, infants over 2 months old are not expected to be more highly sensitive than adults. For infants and children over the age of 6 months, doses should be based on BSA, which may result in a slightly higher dose in mg/kg bw for the infant compared to an adult. Similar findings have been published by CitationEdginton et al. (2006). These are general recommendations for clinical practice, and are based on PK considerations only; these should be combined with pharmacodynamic effects to achieve the most appropriate dosing selections for infants and children.
While these data come from pharmaceutical dosing, it is noted that the CitationUS EPA (2006, Citation2011b) also cites the pharmaceutical literature, concluding that (body weight)3/4 scaling, which provides a similar adjustment to BSA and is most commonly used for interspecies adjustments, may also be useful for intraspecies adjustments based on life stage, citing pharmaceutical data of TK processes in children over 2 months old. As described above, this scaling suggests that a higher dose (in mg/kg bw) in older infants and children compared to adults would be needed to achieve a comparable systemic exposure level.
Impact of organ growth
In addition to maturation in function, it is important to recognize the impact of organ growth on the potential for early-life sensitivity, and the implications of these differences when evaluating maturational differences. For example, measured levels of hepatic enzyme activity suggesting higher variability will not always translate to the same variability in actual hepatic metabolism, since the metabolism of many chemicals in the liver at environmentally relevant doses is rate-limited by blood flow (CitationAndersen 1981, CitationYoon et al. 2012). CitationNong et al. (2006) estimated the mean (range) of hepatic blood flow for different ages. For neonates, the mean blood flow normalized to either liver volume or body weight is about 1.5 to 2-fold higher than for infants, children and adults, which were all similar (see ). These investigators modeled intraindividual differences in the metabolism of toluene as a function of age, and found that only in neonates was the extent of metabolism influenced more by enzyme (CYP2E1) content; in infants, children and adults, it was influenced more by hepatic blood flow. Although neonates have a relatively large hepatic blood flow when normalized to liver volume or body weight, they also have a much lower and more highly variable level of CYP2E1. By the age of 3 months, however, the levels were found to be similar to those of children and adolescents.
Table 3. Hepatic blood flow normalized to liver volume or body weight for different life stages.a
Toxicokinetic summary and conclusion
It is well documented that metabolic and renal capacity are not fully mature at birth, such that infants, especially neonates, can demonstrate differences in the TK handling of a chemical that can exceed the 3.2X factor for intrahuman variability in kinetics. Although there are clearly examples where certain parameters will exceed the 3.2X factor, this does not necessarily raise concerns for the overall adequacy of the 10X intraspecies UF for several reasons:
Metabolic and excretory systems mature rapidly after birth, and many are reasonably mature or even reach adult values at around 6 months of age. Consistent with this, analyses of data on pharmaceuticals by several investigators have shown that the major differences in kinetic handling are seen in the first 6 months of life, and primarily in the first 2 months. This is particularly important in light of QRAs that have been established to be protective for chronic/lifetime exposures.
With regard to the data from pharmaceuticals, it is important to remember that the differences seen at higher doses associated with these agents (intended to maximize therapeutic effectiveness while minimizing the potential for toxicity) are not necessarily relevant to lower doses more reflective of actual human environmental exposures that are generally orders of magnitude below a NOAEL in a rodent study.
Maturational differences are not always the rate-limiting factor determining the disposition of a chemical. For example, hepatic blood flow often determines the rate of metabolism such that differences in hepatic enzyme levels do not necessarily translate to overall differences in TK handling. There is often a redundancy of metabolic/excretory pathways, which has the net effect of reducing quantitative differences in the capacity of an individual pathway.
Although the 3.2X factor for TK might be exceeded in some cases for a short period of time during development, the overall difference might still be less than the 10X UF for intrahuman variability.
Toxicodynamics
Compared with TK, less is known about the potential for differences in toxicodynamics (TD) between infants, children, and adults, and information is generally lacking to support a quantitative assessment of differences in TD across life stages. In reviewing differential impacts on infants and children, the relevant differential sensitivity factors based on the time of exposure (i.e. critical windows of exposure) and the potential impact on TD are considered. Target organs or endpoints for systemic toxicity for which infants are known or thought to have a higher degree of sensitivity include the skeletal, nervous, immune, and endocrine systems (reviewed in CitationHines et al. 2010, CitationNeal-Kluever et al. 2014).
Growth (e.g. skeletal maturation)
As opposed to many organ systems that are anatomically and functionally mature by early infancy, the skeletal system continues to develop throughout childhood, with some periods of rapid growth and development that may render an actively growing organism more sensitive. Bone growth and development has been shown to be similar in all mammalian species studied (CitationZoetis et al. 2003), such that findings in laboratory animal studies conducted in the appropriate life stage will be relevant to human health. For example, the structure of the epiphyseal growth plate, which is responsible for skeletal growth, is similar in the rat and human, with a period of rapid growth occurring in both species at around the time of sexual maturation. In the rat, this period of rapid growth has been reported to occur until about 8 weeks of age, which would be covered in a standard repeat-dose toxicity study (the age of rats at the start of these studies is typically 6 weeks; CitationZoetis et al. 2003).
One class of drugs known to induce toxicity in cartilage, bone, and tendons is the quinolones, a family of broad- spectrum antibacterial drugs, for which it has been suggested that children are at increased risk (CitationStahlmann 2003). Studies in rats have shown the highest sensitivity for chondrotoxicity at 3 to 6 weeks of age, with no effect on mature articular cartilage. Some studies have also suggested that the epiphyseal plate can be damaged, with subsequent effects on bone growth from exposures during early development. Finally, experimental data suggest that quinolones can be injurious to tendons with increased sensitivity in young animals, although it is noted that clinical data have not confirmed an increased sensitivity in children. Nevertheless, for chemical or pharmaceutical risk assessment, a special consideration of pediatric populations is warranted for any drug or other agent shown to have an effect on bone or connective tissue because of the sustained growth of these tissues throughout infancy and childhood.
Neurotoxicity and developmental neurotoxicity
The neurological system of infants and young children has received significant attention as a potentially sensitive target organ. Effects can result from either direct effects on the nervous system or secondary effects resulting from other pathways such as interference with endocrine systems (reviewed in CitationGiordano and Costa 2012). For neurotoxicants, it was traditionally thought that the blood brain barrier (BBB) may not be fully intact at birth, thereby leading to potentially higher exposures to the central nervous system of infants (this is actually a TK factor). This has been challenged in a recent investigation of the functioning of the BBB by CitationAFSSAPS (2010), which concluded that “for a potentially neurotoxic substance that is fat-soluble and of low molecular weight, the ability to cross the BBB is probably the same, regardless of whether the brain is mature or immature. For molecules that are not fat-soluble, the BBB seems to function differently, but is not necessarily more permeable to them.” That stated, for chemicals that do cross the BBB, it is clear that young infants and children in whom the nervous system is still developing may be more sensitive.
The US EPA released final guidelines for neurotoxicity risk assessment in 1998 (CitationUS EPA 1998a, CitationTilson 2000). The Guidelines note that in general, it is assumed that the default 10X UF for intrahuman variability will cover differences in sensitivity, including children and the elderly. However, the Guidelines also note the special sensitivity of the nervous system of infants and children, and state that an additional factor may be considered in cases where adequate data are not available to sufficiently characterize children's health risks. Testing for DNT is required by the US EPA when other information indicates the potential for developmental neurotoxicity. This information most commonly comes from adult neurotoxicity studies, standard developmental toxicity studies, or multigenerational studies. It can also be informed by epidemiology data, or information on the chemical class, structure, or activity, indicating that the nervous system may be a potential target. Where neurotoxicity has been identified as the critical effect for a toxicant, an assessment of infant exposures and the potential for increased sensitivity needs to be carefully considered.
The OECD has issued guideline studies for DNT testing (TG426) and the extended one-generation reproductive toxicity study containing a neurodevelopmental toxicity cohort (TG443); the US EPA has issued similar guidelines (CitationUS EPA 1998b). A retrospective analysis of the value of DNT testing in setting regulatory guidance was conducted by CitationMiddaugh et al. (2003). This survey of 174 chemicals (primarily pharmaceuticals) indicated that effects on neurobehavioral endpoints were not as common as on other developmental endpoints, they were never seen in isolation (i.e. the chemicals produced other manifestations of developmental toxicity), and they were the most sensitive developmental endpoint only 2.6% of the time. The number of guideline DNT studies conducted increased significantly as a result of the passage of the FQPA, which requires specific consideration of children in the evaluation of pesticides. CitationRaffaele et al. (2010) evaluated the use of DNT data in risk assessments of 69 pesticides (most of which were already identified as known or suspected to be neurotoxicants) in submissions made to the US EPA, and reported a DNT endpoint that was used as the point of departure for fifteen chemicals (mostly for acute endpoints); an additional 13 were identified as having the potential to be used. A quantitative comparison of the sensitivity of the DNT endpoint as compared to adult neurotoxicity was not included in this evaluation.
While DNT studies have been shown to identify compounds with DNT potential and utility in QRA (CitationMakris et al. 2009, CitationRaffaele et al. 2010, CitationBushnell 2014), they have also been the subject of ongoing debate about whether they are overly sensitive (CitationClaudio et al. 2000) or insufficiently sensitive, and comprehensive (CitationCory-Slechta et al. 2001). Issues related to the role of maternal toxicity and other factors influencing the interpretation of test results have also been raised (CitationKaufmann 2003, CitationLi 2005). While work has continued to evaluate and improve the protocol for DNT testing and interpretation of results, this testing is resource-intensive, and the need for alternative models that can be used for screening a larger number of chemicals is also recognized (CitationGiordano and Costa 2012).
Questions around the use of screening level neurotoxicity/DNT studies versus the need for more complex tests of cognitive function (e.g. tests involving learning or memory) have also been raised. CitationBushnell (2014) reviewed the evidence for whether tests of cognitive function are needed, looking at studies involving both adult and perinatal exposures in rodents. While complex tests did identify effects at lower doses in some cases for adult exposures, the results were not as clear after perinatal exposures, raising the question of whether the added information warrants the additional time and expense associated with conducting complex studies.
Finally, it is recognized that while most neurotoxicity resulting from early-life exposures (including in utero) is apparent shortly after exposure, concerns have also been raised about the potential for delayed effects, including effects such as attention deficit hyperactivity disorder (ADHD) that typically manifest later in childhood (CitationYolton et al. 2014), and effects that are not apparent until aging, when age-related decrements in neurological function can be exacerbated by exposures that occurred decades earlier (e.g. CitationBarone et al. 1995). While the importance and sensitivity of DNT hazard assessment has been established, further study and regulatory follow-up for environmental, chemical, and pharmaceutical safety are needed.
Immunotoxicity
The immune system is essential in maintaining overall health, with adverse outcomes associated with an underactive (e.g. reduced resistance to infection, increased risk of several cancers) or an overactive system (e.g. allergic and autoimmune conditions). Historically, risk assessment for immunotoxicity has focused on immunosuppression, although it is increasingly recognized that immune stimulation in a rodent study can also be indicative of immunotoxicity. The immune system, and the developing immune system in particular, are complex systems that are not yet fully understood in terms of sensitivity to toxicants. In developing a database of NOELs to support the Threshold of Toxicological Concern (TTC), CitationKroes et al. (2004) found that the NOELs for immunotoxicity did not differ from the NOELs for other endpoints, suggesting that this is not an inherently more sensitive endpoint. However, data on a limited number of immunotoxicants were available for this review.
During development in utero, there is active inhibition of the development of the fetal immune system to ensure that allorejection by the mother's immune system does not occur (reviewed in CitationM’Rabet et al. 2008). However, antibodies are passed from the mother through the placenta immediately after birth, so that the neonate has relatively high levels of the mother's antibodies in the bloodstream. The level of maternal antibodies decreases over time, reaching a low by the age of 2 to 3 months, when the infant's immune system begins producing its own antibodies. Infants who are breastfed also continue to receive antibodies via breast milk during this time, providing additional protection. By about 6 months of age, infants can produce antibodies at a normal rate, although immunological competency continues to develop further over time.
Specific immunotoxicity studies are not available for most chemicals, but histopathological examination of immune tissues (thymus, spleen, and lymph nodes) is often performed as part of standard toxicological studies. Total leukocyte counts and absolute differential leukocyte counts can also be used to assess immunotoxicity. Oftentimes, these are the only data from which an evaluation of immunotoxicity can be made.
In 2012, the WHO/IPCS published a Harmonization Project Document entitled, “Guidance for Immunotoxicity Risk Assessment for Chemicals”. CitationWHO (2012) describes the factors governing sensitivity, including the potential for the developing immune system in early-life to be more sensitive to chemicals than the fully mature system. In addition, the consequences of exposure to immunotoxicants during development can be more persistent than effects observed following adult exposure, which often resolve soon after exposure ends (CitationHolladay and Smialowicz 2000, CitationDietert and Dietert 2007). CitationWHO (2012) states the following with regard to QRA for immunotoxicity:
… In the absence of information on the potential variability in susceptibility among the general population to the particular type of immunotoxicity considered for a POD, a default intraspecies uncertainty factor of 10 is recommended, similar to that used for other non-cancer end-points. … Age-related physiological differences and immaturity of the developing immune system are likely to affect susceptibility to immunotoxicity. As a result, the young (in utero and postnatal exposure or children) and the elderly may be at greater risk for immunotoxicity. … The risk assessor should consider a reduction in the intraspecies uncertainty factor if data for the POD are derived from the most sensitive subpopulation of humans.
Immunotoxicity studies in adult animals are scarce, and developmental immune effects after pre- and postnatal exposure have been the subject of even less research. Nevertheless, a number of DIT studies have shown compound effects on immune parameters at doses below those causing general developmental toxicity parameters to be affected; examples include tributyltin oxide (CitationVos et al. 1990), TCDD (CitationGehrs et al. 1997), heptachlor (CitationSmialowicz et al. 2001), and ethanol (CitationTonk et al. 2013). Remarkably, the most sensitive immune parameters appeared after immune challenge, e.g. Keyhole Limpet Hemocyanine-induced or Sheep Red Blood Cell- induced increases in immunoglobulin levels. This indicates that an immune challenge may reveal compromised responsiveness of an immune system that appears normal by classical parameters such as spleen and thymus weight, cellularity and cell subpopulations, and background immunoglobulin levels. These findings provide important background information for contemplating enhancements of current test guidelines for regulatory toxicology.
In 2011, the European Teratology Society organized a workshop on “Juvenile Toxicity testing protocols for chemicals”, focusing on DNT and DIT testing, and their place in the OECD TG 443 extended one-generation reproductive toxicity study (CitationPiersma et al. 2012). The workshop concluded that “functional immune parameters largely made up the most sensitive parameter sets in all studies, demonstrating the relevance of including them by default in protocols for developmental immunotoxicity testing. …In addition, the dependence of parameter effects on the exposure period and the route of exposure was illustrated by reviewing experimental studies.” Similar conclusions were drawn in more recent workshops (CitationCollinge et al. 2012, CitationBoverhof et al. 2014). Clearly, the groundwork has been laid for establishing the importance of juvenile immunotoxicity hazard assessment, and regulatory follow-up both in the realms of chemical and pharmaceutical safety is warranted; recommendations for inclusion of functional DIT parameters in current hazard identification approaches and greater utility of relevant study protocols have recently been published by CitationHessel et al. (2014).
Endocrine-mediated toxicity
Endocrine-mediated effects have long been a focus of concern for early-life exposure, driven by the potential for irreversible effects if perturbation of hormone levels occurs during critical periods of development. Rather than being a toxicological endpoint, endocrine disruption is more accurately viewed as a mode of action that can lead to a number of effects, most notably on reproductive organ development, reproductive function, and effects on the hypothalamic-pituitary-thyroid axis. Some investigators have asserted that these chemicals can present unique risk assessment challenges because of the potential for nonmonotonic dose-response (NMDR) relationships such that adverse effects could occur at exposure levels below the NOAELs identified in laboratory animal studies. However, others argue that while there could be adverse biological changes not captured by traditional testing protocols, these would not be expected to occur below the NOAELs or other POD used in risk assessment (e.g. CitationWillhite et al. 2008, CitationRhomberg and Goodman 2012). A critical review of the data in support of NMDR relationships and the implications for risk assessment is currently underway at the US EPA.Footnote9
In response to a congressional mandate in 1996 to investigate the potential for pesticides and drinking water contaminants to adversely affect endocrine pathways, the US EPA developed its Endocrine Disruptor Screening Program (EDSP), focused on the development and validation of screening assays for effects mediated by estrogen, androgen, and thyroid pathways. A two-tiered screening and testing process was established: Tier 1 screening assays include five in vitro and six in vivo assays aimed at determining whether chemicals have the potential to interact with the endocrine pathways; Tier 2 studies include developmental and multi-generational repeat-dose toxicity studies that could provide data useful for human health QRA as well as a number of studies designed for use in environmental risk assessment. The first phase of the EDSP has been completed; a workshop held in April 2013 reported on lessons learned, challenges, and opportunities (CitationJuberg et al. 2014). A number of concerns were highlighted including the significant use of animals (> 500 for the Tier 1 battery), a lack of consideration of human exposure potential, the potential for data from Tier 1 studies conducted at high concentrations/doses (intended for hazard screening only) to be misused, and the lack of specificity of some of the assays such that effects measured are not necessarily a result of interaction with the endocrine system. Recommendations were made for refinements to the testing tiers focused on developing a more cost/resource-effective science-based approach. This input will be considered by US EPA as it transitions to make use of newer technologies including computational toxicology and high throughput screening assays, a program being referred to as EDSP21 (EDSP for the 21st Century) (CitationUS EPA 2011c). The adequacy of current methods for identifying and assessing endocrine-mediated toxicity is a topic of ongoing debate (e.g. CitationEFSA 2013, CitationBars et al. 2012, CitationDekant and Colnot 2013, CitationLewis 2013).
Toxicodynamic summary and conclusions
With regard to TD differences, less is known in terms of quantitative differences between infants, children, and adults. As with TK, concern is highest during early infancy, when growth and the rate of maturation are at their greatest. While children can be highly resilient, for some effects, there is also a concern that exposure to an infant could have permanent adverse consequences to a developing organ, whereas the same exposure in an older child or adult would result in effects that are fully reversible. The target organs/effects for which increased sensitivity of infants has been shown most often include the skeletal, CNS/neurotoxicity, immunotoxicity, and endocrine-mediated effects. These are identified as effects for which greater scrutiny of a QRA is appropriate.
Developmental origins of adult disease
In addition to effects manifested in association with early-life exposures, effects can also manifest later in life. There are a number of examples, including reproductive effects in adulthood after early-life exposure in animal multigenerational studies, and persistent neurobehavioral effects after pre- or perinatal exposure to lead in animals and humans (CitationBellinger 2011). In addition to these effects, which reproductive toxicity and DNT test guidelines are designed to detect, there is also the possibility of latent effects on other organ systems from prenatal insult. The Barker hypothesis states that prenatal undernutrition can result in a higher risk of cardiovascular disease, metabolic syndrome and Type-2 diabetes (CitationBarker 1999). There is some evidence that the effect can be mediated via chemical-induced maternal toxicity/undernutrition (CitationRogers et al. 2014). While these kinds of effects are not evaluated in current testing guidelines, the presence of maternal toxicity, decreased fetal weight, and low birth weight are evaluated in current study designs. Further evaluation is needed to determine whether these metrics are indicative of risks beyond the juvenile period.
Examples of differential sensitivity
The relative sensitivity of infants and children to a given exposure, compared to adults, can be similar, higher, or lower, and can be defined by critical windows of development. The complexity of understanding the potential differential sensitivity notwithstanding, a number of reviews have provided multiple illustrations of both increased and decreased sensitivity (e.g. CitationCalabrese 2001, CitationRodriguez et al. 2001). While the relative frequency of higher versus lower sensitivity is not known, several examples are provided that illustrate known cases of both increased and decreased sensitivity.
Examples of increased sensitivity
There are a number of examples of increased sensitivity of infants, especially of neonates. Children can have greater functional capacity compared to adults (particularly renal), and are highly resilient with the ability to repair damage from toxicologic insults, such that it is not uncommon for children to be less sensitive than adults; however, there are also examples of increased sensitivity during this life stage.
Chloramphenicol
Chloramphenicol, a broad-spectrum antibiotic, was given intravenously to neonates to treat certain infections. In the 1950s, some treated infants developed a condition termed “gray baby syndrome”, in which they developed an ashen gray-colored skin, limp body tone, hypotension, and other symptoms. The doses given to the infants were extrapolated from adult therapeutic doses based on body weight. It was fatal in some cases, with premature infants being the most sensitive. The increased sensitivity has been attributed to a lack of fully functional liver enzymes, specifically UDP-glucuronyl transferase, resulting in higher levels of unmetabolized chloramphenicol in the blood (CitationWeiss et al. 1960).
Quinolones
As described earlier in the section on skeletal maturation, studies in rats and dogs have shown an increased sensitivity of the young animal to connective tissue toxicity induced by quinolones, which is likely a reflection of the very rapid growth of the skeletal system postnatally.
Lead
Exposure to a number of heavy metals is associated with neurotoxicity, DNT, and neurobehavioral effects, with lead being the best studied. There are several factors relating to both exposure and inherent sensitivity that render infants and children more susceptible than adults, with boys appearing to be more sensitive than girls (CitationLlop et al. 2013). Ingestion is the primary route of exposure to lead. Infants and children have a higher exposure (in mg/kg body weight) compared to adults, because of greater hand-to-mouth activity, ingestion of paint chips/dust, and greater exposure associated with living closer to the ground. They also have a much higher fractional absorption of ingested lead from the gastrointestinal tract; it has been estimated that absorption ranges from 40–70% in young children compared to 10–20% in adults (CitationATSDR 2000). While lead is toxic to many organs, the critical effect is (developmental) neurotoxicity, with the greatest concern for exposure occurring during critical periods of brain development that can result in permanent neurological impairment. This is in contrast to multiple effects in adults which can be reversible upon cessation of exposure.
Propylene glycol
Propylene glycol (PG) is a widely-used and well-studied chemical associated with very low toxicity. However, its use as a solvent for several drugs administered orally or intravenously has been associated with toxicity following large exposures (e.g. CitationZar et al. 2007, CitationZosel et al. 2010). The toxicity of high doses of PG can manifest as central nervous system (CNS) depression associated with the parent compound, similar to that caused by ethanol (but about one-third as potent). Large exposures have also been associated with metabolic acidosis associated with its metabolism through oxidation by alcohol dehydrogenase (ADH) to form lactaldehyde, and then to lactate by aldehyde dehydrogenase (ALDH). Lactate then enters into the Krebs cycle where it is ultimately metabolized to carbon dioxide and water; however, very large doses can result in excess production of lactic acid, resulting in a metabolic anion gap and ultimately metabolic acidosis. When used as a food additive, the World Health Organization (WHO) established an acceptable daily intake (ADI) for PG of 25 mg/kg/day (CitationWHO 1974). The ADI is contrasted with levels determined to be safe in pharmaceutical applications. CitationZar et al. (2007) recommended a maximum dose of 2.9 g PG/hr (69 g PG/day, or about 1000 mg/kg/day) as being safe for intravenous therapy with PG in the absence of risk factors. For patients with compromised liver and/or kidney function, CitationZar et al. (2007) recommended a 2-fold reduction in the maximum daily dose of PG.
Clinical data have shown that sensitive individuals are those with functional impairments in the liver or kidney, which may lead to increased sensitivity to toxicity from either the parent compound (PG) or its metabolites (lactic acid). CitationFowles et al. (2013) have postulated that due to the low intrinsic toxicity of PG, saturation of its metabolism typically plays a protective role in its toxicity, since the conversion of PG to the more toxic lactate would be slower. Infants and young children (up to the age of about four) have a lower level of ALDH/ADH activity compared with adults, so that high acute doses of PG could lead to an accumulation of the parent compound. This would be expected to increase the sensitivity of the young child to CNS effects mediated by high acute doses to the parent compound, but at the same time it would be expected to be protective against the development of acidosis because of the slower metabolism to lactic acid. Thus, this case study illustrates the potential for both increased and decreased sensitivity associated with early-life exposure, depending on the endpoint being evaluated.
Examples of decreased sensitivity
It is fairly common that older infants and children are less sensitive than adults. A number of case studies of chemicals that pose a greater risk to adults than to children have been described by CitationCalabrese (2001). These include several target organs that are often thought to be more highly sensitive in the young: e.g. kidney, lung, brain, and liver Many of the case studies described involve exposure in young children, infants older than 6 months, or young rodents, but not necessarily young infants or neonates. Nevertheless, this highlights the fact that even if a young infant has a slightly higher sensitivity, it is generally only for a very short time (i.e. early infancy), such that when compared to a risk value established for chronic exposure (e.g. an RfD), the overall conservatism is such that even neonates are protected by current risk assessment methods.
Organophosphate-induced delayed neurotoxicity
Organophosphates (OPs) are associated with both acute effects and a delayed neurotoxicity (OPIDN). Whereas there has been concern for increased sensitivity of infants and children to the neurotoxicity of OPs, there have been studies showing decreased sensitivity to OPIDN. Studies in a chicken model have shown that young chicks (from 10–50 days old) are resistant to the OPIDN observed in older chicks (72–100 days old) at the same dose. The same age-dependent response has been reported in cats. In humans, infants and children were less sensitive than adults to OPIDN induced by tricresyl phosphate, an OP that had contaminated cooking oil in Morocco, leading to the poisoning of over 10,000 persons (reviewed in CitationCalabrese 2001).
Aminoglycosides
Aminoglycosides (e.g. gentamicin) have long been considered to be somewhat less toxic in infants than in adults, particularly with regard to the kidney (CitationHeimann 1983, CitationRodriguez et al. 2001). The decreased sensitivity has been suggested to result from differences in renal blood flow in the young infant; once filtration begins in all nephrons of the infant, gentamicin nephrotoxicity is similar to that in adults (reviewed in CitationCalabrese 2001). Guidelines for therapeutic drug dosing and monitoring have evolved in recent years based on an increased understanding of the TK handling of aminoglycosides, including in neonates and young infants. CitationTouw et al. (2009) specifically address dosing considerations for neonates up to seven days old with normal kidney function, recognizing the large interindividual differences in PK related to developmental differences in neonates during the first few days of life. Beyond the first week after birth, dosing recommendations for infants and young children are a bit higher than for older children aged 10 to 18 years, but most of the dosing recommendations are within less than a 3-fold range.
Differential sensitivity dependent on dose
The immaturity of metabolic and excretory pathways of the very young is such that they can be saturated more easily than the same pathway in adults, rendering infants potentially more sensitive to effects resulting from relatively high doses that saturate these pathways. The same is also true of data derived from pharmaceuticals, where the dose administered is intended to have a physiological effect while minimizing adverse side effects; this is likely to involve exposures closer to (and sometimes exceeding) saturation limits for metabolism and/or excretion. While it is true that neonates can be more sensitive to high exposures of some environmental toxicants, CitationScheuplein et al. (2002) have suggested that this is not a generalization that should be applied to low-level environmental exposures to these same chemicals. A similar issue was also pointed out by CitationBruckner (2000) in his review of the NAS (1993) report on “Pesticides in the Diet of Infants and Children.” While this report detailed the sometimes greater sensitivity to pesticides, especially of infants, Bruckner reminds us that this conclusion was based primarily on mortality studies, the results of which may not be relevant to lower exposure levels.
The question of differential sensitivity being a function of dose (exposure level) was also highlighted by CitationSheets (2000), who evaluated the relative sensitivity of infants and adults to lower level exposures, such as those associated with acceptable residue limits (tolerances) for OP and pyrethroid insecticides on various food products. Sheets evaluated multigenerational studies in rats exposed to several different OPs and acute toxicity studies in rats exposed to several different pyrethroids. In the studies of the OP insecticides, the magnitude of cholinesterase inhibition (the critical effect) in pups (measured on postnatal days (PND) 4 and 21) was consistently less than that in adults. Likewise, for Type I pyrethroids, there was no evidence of increased sensitivity in pups compared to adults. In contrast, Type II pyrethroids did demonstrate a greater sensitivity in young rats, but this was found to be true at only at high (lethal) doses. Levels of deltamethrin in whole brain tissue were measured and found to support a kinetic difference as the basis for the greater sensitivity in young animals which are less capable of detoxifying a high level exposure. At lower doses, where detoxification is not saturated, there was no indication of young animals being more sensitive. Thus, it is of critical importance that toxicity studies are evaluated within the context of relevant human exposure; this point has similarly been emphasized in a systematic review of the toxicity of bisphenol A by CitationTeeguarden and Hanson-Drury (2013). While these papers highlight important considerations of the relevance of dose in drawing conclusions around the relative sensitivity of different age groups, it is important to acknowledge that for the case of deltamethrin (Type II pyrethroid) and some other pyrethroids, there are also significant species differences in metabolism that should be considered when extrapolating data from rats to humans (CitationGodin et al. 2006).
CitationSmith et al. (2014) published a life stage PBPK/PD model for chlorpyrifos, an OP insecticide for which it has been suggested that young animals have an increased sensitivity compared to adults. The toxicity of chlorpyrifos is mediated by its metabolite chlorpyrifos-oxon, which is a potent inhibitor of cholinesterases. In rat pups dosed postnatally by gavage in corn oil, there was an increased sensitivity to cholinesterase inhibition in rats administered either 1 or 10 mg/kg chlorpyrifos at PND 5; by PND 12, the increased sensitivity was only seen at the higher dose. However, the NOAEL (0.5 mg/kg) was the same for all age groups, indicating that the increased sensitivity was only seen at higher doses. Human-relevant exposures are two to three levels of magnitude lower than the NOAEL. A similar trend for age-related sensitivity was demonstrated in the life stage PBPK/PD model, which accounted for age-dependent changes in chlorpyrifos metabolism and distribution. For doses of 0.6 mg/kg and higher, 6-month old infants were predicted to have higher chlorpyrifos-oxon levels in blood compared to adults; this is a result of overwhelming the chlorpyrifos-oxon metabolism capacity of plasma, which is lower in infants than adults. However, at doses below 0.6 mg/kg, adults were predicted to have higher chlorpyrifos-oxon levels in blood compared to infants, resulting from increased metabolism of chlorpyrifos-oxon related to the larger liver size of infants as compared to adults.
Gender-specific sensitivity
Examples of gender-specific sensitivity associated with early-life exposure are relatively rare, other than the obvious differences associated with male and female reproductive systems. However, they do occur, and a recent example was published by CitationSaghir et al. (2013) for life stage-, sex-, and dose-dependent dietary TK of 2,4-dichlorophenoxyacetic acid (2,4-D), as they relate to toxicity. In this case study, gender differences in TK were shown to result from the approximate two-fold sex-dependent differences in 2,4-D clearance, associated with saturation of the OAT1 carrier protein responsible for active secretion of 2,4-D into urine. The difference was hypothesized to be due to a testosterone-induced increase in the number of functional transporters in the kidney for organic acid secretion. Furthermore, the authors reported on an influence of life stage: whereas the dose-dependent clearance of 2,4-D in male and female pups was similar on PND 4, 21, and 28, a difference was apparent by PND 35, when saturable renal clearance was seen in females at lower doses compared to males. The developmental delay in achieving adult levels of OAT1 transport capacity, coupled with a higher exposure (on mg/kg bw basis) to pre- and early post-weaning rats, was considered likely to have contributed to the increased blood levels of 2,4-D seen in the early postnatal life stages. Similarly, the timing of the gender-related differences in OAT1 expression was consistent with the gender-related differences reported in the 24-hr blood AUC of 2,4-D on PND 35. However, while both early-life stage and gender differences were seen, these were at doses about 4 orders of magnitude higher than human exposures that have been assessed through biomonitoring. Differences in AUC and toxicity related to saturation of excretion at high doses have limited, if any, significance to exposures several orders of magnitude lower.
A potential for gender-based differences in early-life sensitivity to neurotoxicants has also been recognized, related to anatomical differences in the CNS and differences stemming from the influence of certain genes and hormones, particularly estrogen and thyroid hormones, on brain development (CitationLlop et al. 2013). Estrogens play a key role in the regulation of neuronal structure and brain function, and it has been postulated that the higher density of estrogen receptors in the CNS of females may confer greater protective effects from exposure to neurotoxic agents (CitationVahter et al. 2007). Thyroid hormones (TH) are essential for normal mammalian development, including brain development, with deficiency during the pre- and early postnatal periods resulting in abnormalities in brain structure and function. The expression of brain-specific genes involved in development are known to be regulated through TH receptors; gender differences in the regulation of TH-dependent gene expression have been suggested to contribute to differential sensitivity to metals in the developing brain (CitationIshitobi et al. 2007).
Sensitivity of infants and children: Conclusions
It is clear that no single conclusion can be drawn regarding the relative sensitivity of infants and children compared to adults with regard to toxicant exposures; rather, there are well-documented cases in which infants and children are more sensitive, cases in which they are less sensitive, and cases where no indication of differential sensitivity is observed. However, for most chemicals, sufficient data to draw a conclusion regarding relative sensitivity are lacking, and the reality is likely more complex than a simple determination of higher/lower sensitivity. Windows of differential sensitivity can be short, sensitivity can be dose-dependent, and competing factors can both increase and decrease sensitivity. These findings are not surprising, and it is clear that none of these situations will be a rare exception. In the face of this complexity, and generally limited information, it is important to re-evaluate over time what is known about life stage-specific sensitivity, to confirm whether the historically accepted 10X default UF for intrahuman variability is sufficiently protective for infants and children. The data available to address this question come from what is known about physiological differences, studies in laboratory animals, and some clinical data from pharmaceuticals. Rarely are there relevant epidemiological data on the relative sensitivity of infants and children to low level environmental contaminants. Data on physiological differences include structural and functional maturity of the major metabolic and excretory pathways. While it is well-recognized that some age-related physiological differences might have the potential to render infants more or less sensitive to a toxicant, there are often other factors that can minimize the impact of those differences. The complexity of interacting systems makes it very difficult, with the tools available today, to develop a generic approach to predict the relative sensitivity of infants or children in the absence of chemical-specific data. The data on pharmaceuticals confirms what is expected based on physiological differences – i.e. that neonatal infants generally exhibit the largest difference in sensitivity to toxicity compared to adults. However, the data at these doses are not necessarily relevant to lower environmental exposures where metabolic and/or excretory pathways are not near saturation levels. Both human data and laboratory animal studies involving early-life exposure have highlighted specific organ systems (i.e. skeletal, neurological, immunological, and endocrine), that warrant specific consideration when evaluating early-life exposures.
Synthesis and integration into quantitative risk assessment
Lifetime exposures
Historically, risk assessments for lifetime exposures have not been modified to account for differential sensitivity associated with early-life exposure; rather, they have been based on the critical effect, regardless of which life stage the effect is associated with. The US EPA's Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants (CitationUS EPA 2005) describes how a time-weighted average risk assessment can be done across life stages to account for different exposure scenarios and/or differential sensitivity associated with different life stages. Similarly, the CitationUS EPA's 2005 Guidance for Early-life Exposure to Carcinogens (US EPA 2005b) recommends applying an age-dependent adjustment factor (ADAF) to account for the potentially increased sensitivity associated with early-life exposures to carcinogens with a mutagenic mode of action. This guidance recommends summing exposure (or possible risk) across relevant life stages rather than assuming 70 years of exposure to a lifetime average daily adult exposure.
Exposures unique to infants and children
For exposures unique (qualitatively or quantitatively) to infants or children, both the exposure and risk assessment should be targeted to the problem and the population of concern. The exposure assessment should be based on relevant assumptions for infant or child-specific parameters. The appropriate risk value should be chosen based on all exposure considerations. An example provided by the US EPA includes methods to derive short-term drinking water standards that can be used in cases where there is a short-term excursion above standards established for lifetime exposure (e.g. in the case of a spill). Because infants and children have a higher drinking water consumption per kg bodyweight the US EPA's short-term standards are based on default assumptions for a 10 kg child drinking 1 L of water/day (vs the lifetime default assumptions of 70 kg bw and 2 L of water/day) (CitationUS EPA 2012). The risk values used for these assessments are generally based on subchronic toxicity studies, without the need for a duration UF that would be applied to a chronic risk assessment. An example of a subchronic reference dose on the US EPA's IRIS database is provided for 1,1,2,2-tetrachloroethane.Footnote10
Adequacy of current methods
In evaluating whether current risk assessment approaches are adequately protective for early-life exposures, we need to consider both the adequacy of current testing protocols and the adequacy of risk assessment methods. Specifically, do testing protocols identify all of the effects of concern for early-life exposure, and do risk assessment methods provide adequate protection, especially in cases where the available toxicity data do not include early-life dosing? Both of these have evolved, and continue to evolve over time, as human variability is better understood and the factors that might render different groups/life stages become more sensitive. The following provides perspective on the adequacy of testing protocols and current QRA methods, recognizing opportunities for improvement and refinement in both areas.
Testing protocols
Taken as a whole, a complete dataset based on current protocols is considered to provide the essential data needed to evaluate potential hazards to all life stages. However, the majority of chemicals have limited datasets and/or the available data are based on old studies that are not as robust as contemporary studies. Uncertainties associated with an incomplete dataset, including a lack of data covering early-life exposure, are addressed in the risk assessment process. Additionally, there are endpoints for which our understanding and ability to extrapolate from rodents or other laboratory species is still limited, including effects manifested later in life for which there is a developmental origin.
Toxicity testing protocols have expanded over the years to focus on specific areas of concern (e.g. increased focus on endocrine-active substances, neurotoxicity, and immunotoxicity). For example, the advent of the OECD TG 443 extended one-generation reproductive toxicity protocol provides opportunities to collect information on DNT and DIT. However, this test is generally only required for chemicals at high tonnage levels in the EU, and thus is not available for most chemicals. Given the complexity and cost of the TG 443, it should be considered whether a simplified pre- and perinatal or juvenile exposure protocol including functional parameters for neural and immune function, such as has been proposed for pharmaceuticals (CitationCappon et al. 2009), might be feasible for chemicals at lower tonnage levels. For pharmaceutical compounds, the Segment 3 pre- and postnatal dosing study in rodents is carried out as part of the reproductive and developmental testing. This study provides data on the effects of prenatal exposure and exposure via milk during the lactation period, but does not include the assessment of effects that may occur from direct dosing of the young offspring or effects that may occur only later in life. When needed, studies with direct dosing in juvenile animals may be carried out to support pediatric clinical trials. For chemicals it may be possible to identify triggers from other studies, including developmental toxicity studies that would indicate that studies on functional developmental effects are warranted.
Such triggers already exist for the endpoints of primary interest. For DNT, triggers that have been used include effects on structural nervous system development in prenatal developmental toxicity studies, or neuropathology or altered neurological function in subchronic studies. For DIT, these include alterations in thymus or spleen weight or histological changes in these organs or other tissues with immunological function in subchronic studies. For reproductive function, triggers include positive effects in Tier 1 endocrine screening, or changes in estrous cyclicity or reproductive organ weight, or histology in a subchronic study. Such a targeted approach is more pragmatic than a blanket testing policy; however, the absence of a trigger in a repeated-dose study conducted in mature animals should not be taken as confirmation of a lack of potential effects from early-life exposure. In addition to triggers from toxicity studies, it will be possible in the near future to use mode of action-based triggers from either high-throughput screening or toxicogenomic studies, to provide a more solid scientific basis for conducting tests for these endpoints.
Because of the significant resources required to generate a complete database that covers early-life exposure and special endpoints of concern, a number of first-tier screening in vitro and ex vivo studies have been developed and validated to identify chemicals that might pose a concern for developmental toxicity, with a focus on the potential for adverse impact during organogenesis of the embryo. These include studies in whole embryo cultures, cellular models using primary cultures, and permanent cell lines such as the mouse embryonic stem cell assay and the human pluripotent stem cell test (reviewed in CitationSpielmann 2005, CitationKameoka et al. 2014). A chemical that raises potential concerns will either be dropped from further development, or will be further studied to better characterize the actual potential for an in vivo response at a relevant exposure level. A number of other screening level studies have been developed to evaluate the potential for a chemical to interact with hormone receptors, thus having the potential to perturb hormonal feedback systems.
Additional opportunities for advances in testing protocols will result from the ongoing movement away from traditional laboratory animal studies to an approach based on perturbation of cellular responses measured in in vitro, high, and medium throughput assays. This is the direction of future research recommended by the U.S. NRC report, Toxicity Testing in the 21st Century: a Vision and a Strategy (CitationNRC 2007). These assays are designed to evaluate the potential for a chemical to interact with and/or perturb 'toxicity pathways” (normal cellular-response pathways that, when sufficiently perturbed, can lead to adverse health outcomes), and to determine the concentration ranges in vitro where those perturbations are considered to be of potential concern. Further work is then needed to translate these findings into relevant in vivo exposures (e.g. through biological-based modeling of toxicity-pathway circuitry and human TK). An example of how these newer technologies can be used in risk assessment has recently been published by CitationAdeleye et al. (2014). While these emerging technologies have not yet been used to specifically address potential issues associated with early-life exposure, it is anticipated that further research will lead to new tools for future generations of risk assessment that will enable us to more explicitly evaluate differential sensitivity across subpopulations and life stages. Some examples of this may be the incorporation of metabolizing systems that are more similar to those present during early-life, and the development of computational models that take into account physiological differences such as changing GFR that characterize infancy and early childhood.
QRA methods
Global regulatory guidelines for QRA require consideration of all life stages, as well as other factors (e.g. gender, genetics, and underlying disease) that might render an individual more highly sensitive to a chemical exposure. Various approaches have been used to ensure adequate protection across a diverse human population. At the core of all published regulatory approaches, with the exception of pharmaceuticals, is the use of a default 10X UF to account for human heterogeneity. This review has explored and confirmed the general adequacy of the 10X default factor to be protective for early-life exposures.
As described above, a common challenge for risk assessors is the lack of a complete toxicity database. In the current European legislation for chemical safety, for example, the information level requirements underlying hazard and risk assessment are dependent on production tonnage. As a consequence, reproductive and developmental toxicity testing is required for no more than 10–20% of chemicals. As these tests are the only ones including the pre- and postnatal phase, for the vast majority of chemicals, no information is available on possible life stage-specific sensitivity. Strategies for addressing this lack of chemical-specific data in risk assessment include use of read-across in cases where data are available on a chemical judged to be a suitable analog (e.g. CitationWu et al. 2010), or possibly the use of an additional (database) UF.
There are also increasing opportunities to refine current QRA methods so that we are not reliant on the use of default UFs. Physiologically-based pharmacokinetic (PBPK) modeling is often used to refine a risk assessment by replacing conservative default assumptions with actual data. While PBPK modeling has more commonly been used to replace default assumptions for cross-species extrapolation, it is increasingly being considered as a tool to address differences associated with life stage. For example, using moxifloxacin as a model, CitationEdginton (2011) described the use of PBPK to guide the selection of dosing recommendations to support a pediatric clinical trial. Using age-specific physiological and ontogenic parameters for clearance pathways, she estimated that recommended starting doses for infants and toddlers (4 months to 2 years old) would be about 80% higher, and for preschool children (2 to 6 years old), it would be about 25% higher than recommended doses for adults, on a mg/kg bw basis, to achieve an equivalent exposure. Of course, for other drugs, modeling might show that a lower dose compared to that of adults would be recommended, reaffirming the common theme that infants might be more or less sensitive than older children and adults.
PBPK modeling has also been suggested as one way to gain further insight into questions around the adequacy of the default value of 3.2X used in QRA for TK variability in the human population, specifically with regard to infants and children. CitationClewell et al. (2004) undertook such an exercise, using six environmental contaminants to provide a proof- of-principal demonstration of the potential for PBPK modeling to evaluate age-specific differences in dosimetry resulting from differences in TK handling. As expected, the most important contributor to age-specific differences in neonates was the potential for decreased clearance of a toxic chemical due to the immaturity of many metabolic enzymes, although it was noted that this same factor could also result in decreased toxicity in cases where a metabolite is the toxic moiety. Compared to an adult, the greatest departures in internal dose metrics tended to occur in infants under 6 months old, but these factors were still generally small (the highest calculated value was 3.4-fold higher in an infant under 6 months for nicotine), and it was emphasized that this difference was for a short duration of the overall lifetime.
PBPK models clearly offer advantages in our ability to evaluate risk across different life stages, and data on many physiological parameters for infants and children are available in the literature (e.g. CitationPrice et al. 2003, CitationBjorkman 2004). Data are still lacking on some infant and/or child-specific parameters but the usefulness of these models and our understanding of the time course of physiological maturation is advancing. This is an especially important tool in pharmaceutical research, where physiological differences impacting drug disposition must be carefully considered. CitationAbjuljalil et al. (2014) demonstrate the importance, particularly for neonates, in understanding the timeline for maturation of hepatic enzymes responsible for clearance of different drugs. This was illustrated for CYP3A4, the level of which changes very quickly after birth. For a PBPK model of a drug cleared by CYP3A4, it was found that this parameter would need to be updated hourly for infants up to 3.5 days, and then every 6 h for ages 3.5–6.5 days. For enzymes that mature more slowly, such as CYP1A2, fewer adjustments to the model are needed. While understanding these differences in enzyme-specific ontogeny can be critical for determining an appropriate drug dose for different ages, it is less critical for application of PBPK modeling to chemicals for which exposure limits are set using traditional risk assessment methods that incorporate UFs to account for population variability. Nonetheless, it is expected that PBPK models will find increasing applications for addressing questions around safety involving early-life exposures.
Summary and conclusions
Global regulatory approaches to chemical risk assessment are intended to be protective for a diverse human population, including all life stages. It is well recognized that unique sensitivities can be associated with early-life, and questions persist as to whether current testing approaches and risk assessment methodologies are adequately protective. Traditional toxicity testing in laboratory animals often covers the period in utero as well as the life stages starting with early adolescence; however, relatively few chemicals have toxicity testing that includes early-life exposures equivalent to infancy and early childhood. Risk assessment methods generally address this and other areas of uncertainty and extrapolation with UFs.
This document reviewed the adequacy of the default 10X UF and what is known about early-life development and sensitivity, including an overview of physiological and maturational differences between infants, children, and adults that might contribute to differential sensitivity. With regard to TK, the biggest differences compared with adults are seen in infants less than 6 months of age related to the immaturity of metabolic and excretory capacities. However, these systems develop very quickly, with most major metabolic and excretory pathways being fully competent by about 6 months of age (although some pathways continue to develop further well beyond that time). Even though this is a very short period of time considering that most QRA approaches are based on an assumption of lifetime exposure, this period may be critical if persistent health effects are caused. With regard to TD differences, less is known in terms of quantitative differences between infants, children and adults, and differences in organ sensitivity may result in different sensitivities among life stages even at exposures where TK differences are minimal. As with TK, the biggest concern for TD differences is generally during the first 6 months of age, when both growth and the rate of maturation are at their highest. For some effects, there is also a concern that exposure in a young infant could have permanent adverse consequences on the development of an organ whereas the same exposure in an older child or adult might result in effects that are fully reversible. The target organs/effects for which increased sensitivity of infants has been shown most often include the CNS/neurotoxicity, immunotoxicity, skeletal, and endocrine-mediated effects. These are identified as effects for which greater scrutiny of a QRA is appropriate, but available data do not currently support a change in current default QRA methods. We conclude that in the absence of specific toxicological data during early-life, current risk assessment methods, including the use of a default 10X UF to account for human heterogeneity, are generally appropriate and protective for all age groups, including infants.
Finally, risk assessment methods continue to evolve and a number of research priorities emerge, particularly in the areas of DNT and DIT. We continue to learn more about human heterogeneity and sensitivity associated with early-life exposures. Testing protocols also continue to evolve, including an increased focus on medium- and high throughput assays focused on biological pathways for which we will eventually be able to generate data more directly relevant to human subpopulations and life stages. PBPK modeling is expected to see increased utility to address questions around potential differences in sensitivity associated with different life stages. With more knowledge about life stage-dependent physiology and better tools for life stage-specific hazard assessment, future risk assessments will be associated with fewer default assumptions.
Acknowledgments
The authors appreciate the thoughtful and constructive comments provided by the anonymous reviewers assigned by the Journal Editor. Their comments assisted in improving the quality and readability of the paper.
Declaration of interest
The authors’ affiliations are as shown on the cover page. Susan Felter and George Daston are employees of Procter & Gamble, a corporation producing and marketing many products used with infants and children. Aldert Piersma is affiliated to the National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands. RIVM is a government agency under the Dutch Ministry of Health, Welfare and Sports. RIVM has sponsored his contribution to the present piece of work. Susan Euling and Melissa Tassinari are employees of the US EPA and US FDA, respectively, which have regulatory authority related to a wide range of products/exposures that may impact the health of infants and children. This project was initiated by the Procter & Gamble scientists who asked the other authors to independently participate as professionals. The authors have sole responsibility for the writing and content of the article. The paper was reviewed internally at each author's home institution as is customary, however, those reviews did not influence the conclusions drawn nor the recommendations made by the authors. The design of the appraisal, the conduct of the appraisal, and the conclusions drawn in the paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency, the U.S. Food and Drug Administration, or RIVM.
Notes
1 Available online at: http://www.epa.gov/risk_assessment/glossary.htm#s
2 Now known as ILSI North America
3 In December 2014, the NCS, in its current design, was discontinued due to feasibility issues (http://www.nih.gov/about/director/12122014_statement_ACD.htm). The National Institutes of Health (NIH) is committed to developing a new approach to perform targeted research, including making the Vanguard Study data and specimens available, to address the links between the environment and child health and development (see https://www.nationalchildrensstudy.gov/Pages/default.aspx).
5 US EPA Integrated Risk Information System: www.epa.gov/iris
6 The term “safety factor” is used by many European regulatory agencies rather than “uncertainty factor”; they are used in an equivalent manner.
8 Available from: http://www2.clarku.edu/faculty/dhattis
References
- Abjuljalil K, Jamei M, Rostami-Hodjegan A, Johnson TN. (2014). Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: Introducing time-varying physiology into a paediatric PBPK model. AAAPS J, 16, 568–76.
- Adeleye Y, Andersen M, Clewell R, Davies M, Dent M, Edwards S, et al. (2014). Implementing Toxicity Testing in the 21st Century (TT21C): Making safety decisions using toxicity pathways, and progress in a prototype risk assessment. Toxicology. doi:10.1016/j.tox.2014.02.007. [Epub ahead of print].
- Afsar FS. (2009). Physiological skin conditions of preterm and term neonates. Clin Exp Dermatol, 35, 346–50.
- AFSSAPS. (2010). Assessment report on the safety of cosmetic products intended for children under three years of age. Agence Française de Sécurité Sanitaire des Produits de Santé [French Health Products Safety Agency], St Denis, France. April.
- Alcorn J, McNamara PJ. (2003). Pharmacokinetics in the newborn. Adv Drug Deliv Rev, 55, 667–86.
- Aldridge JE, Gibbons JA, Flaherty MM, Kreider ML, Romano JA, Levin ED. (2003). Heterogeneity of toxicant response: sources of human variability. Toxicol Sci, 76, 3–20.
- Andersen ME. (1981). A physiologically based toxicokinetic description of the metabolism of inhaled gases and vapors: analysis at steady state. Toxicol Appl Pharmacol, 60, 509–26.
- Anderson GD. (2010). Developmental pharmacokinetics. Semin Pediatr Neurol, 17, 208–13.
- ATSDR. (2000). Lead toxicity. Case Studies in Environmental Medicine. US Department of Health and Human Services. Agency for Toxic Substances and Disease Registry.
- Babich MA. (1998). Risk assessment of low-level chemical exposures from consumer products under the U.S. Consumer Product Safety Commission chronic hazard guidelines. Environ Health Perspect, 106, 387–90.
- Babich MA, Chen SB, Greene MA, Kiss CT, Porter WK, Smith TP, et al. (2004). Risk assessment of oral exposure to diisononyl phthalate from children's products. Regul Toxicol Pharmacol, 40, 151–67.
- Barker DJ. (1999). Fetal origins of cardiovascular disease. Ann Med, 31, 3–6.
- Barnes DG and Dourson ML. (1988). Reference dose (RfD): description and use in health risk assessments. Regul Toxicol Pharmacol, 8, 471–86.
- Barone S, Stanton ME, Mundy WR. (1995). Neurotoxic effects of neonatal triethyltin (TET) exposure are exacerbated with aging. Neurobiol Aging, 16, 723–35.
- Bars R, Fegert I, Gross M, Lewis D, Weltje L, Weyers A, et al. (2012). Workshop Report. Risk assessment of endocrine active chemicals: Identifying chemicals of regulatory concern. Regul Toxicol Pharmacol, 64, 143–54.
- Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. (2006). Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet, 45, 1077–97.
- Baum M. (2009). Postnatal developmental renal physiology: a study of historic significance. Am J Physiol, 296, F667–8.
- Beckman DA, Feuston M. (2003). Landmarks in the development of the female reproductive system. Birth Defects Res (Part B), 68, 137–43.
- Bellinger DC. (2011). The protean toxicities of lead: new chapters in a familiar story. Int J Environ Res Public Health, 8, 2593–628.
- Bjorkman S. (2004). Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol, 59, 691–704.
- Boverhof DR, Ladics G, Luebke B, Botham J, Corsini E, Evans E, et al. (2014). Approaches and considerations for the assessment of immunotoxicity for environmental chemicals: a workshop summary. Regul Toxicol Pharmacol, 68, 96–107.
- Bruckner JV. (2000). Differences in sensitivity of children and adults to chemical toxicity: the NAS panel report. Regul Toxicol Pharmacol, 31, 280–5.
- Bushnell PJ (2014). Testing for cognitive function in animals in a regulatory context. Neurotoxicol Teratol. pii:S0892-0362(14)00107-X. doi:10.1016/j.ntt.2014.04.068. [Epub ahead of print]
- Calabrese EJ. (2001). Assessing the default assumption that children are always at risk. Hum Ecol Risk Assess, 7, 37–59.
- Cappon GD, Bailey GP, Buschmann J, Feuston MH, Fisher JE, Hew KW, et al. (2009). Juvenile animal toxicity study designs to support pediatric drug development. Birth Defects Res B Dev Reprod Toxicol, 86, 463–9.
- Chiou YB, Blume-Peytavi, U. (2004). Stratum corneum maturation. A review of neonatal skin function. Skin Pharmacol Physiol, 17, 57–66.
- Claudio L, Kwa WC, Russell AL, Wallinga D. (2000). Testing methods for developmental neurotoxicity of environmental chemicals. Toxicol Appl Pharmacol, 164, 1–14.
- Clewell HJ, Gentry PR, Covington TR, Sarangapani R, Teeguarden JG. (2004). Evaluation of the potential impact of age- and gender specific pharmacokinetic differences on tissue dosimetry. Toxicol Sci, 79, 381–93.
- Cohen Hubal EA, de Wet T, Du Toit L, Firestone MP, Ruchirawat M, van Engelen J, Vickers C. (2014). Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul Toxicol Pharmacol, 69, 113–24.
- Collinge M, Burns-Naas LA, Chellman GJ, Kawabata TT, Komocsar WJ, Piccotti JR, et al. (2012). Developmental immunotoxicity (DIT) testing of pharmaceuticals: Current practices, state of the science, knowledge gaps, and recommendations. J Immunotoxicol, 9, 210–30.
- Cory-Slechta DA, Crofton KM, Foran JA, Ross JF, Sheets LP, Weiss B, Mileson B. (2001). Methods to identify and characterize developmental neurotoxicity for human health risk assessment. I: behavioral effects. Environ Health Perspect, 109, 79–91.
- CPSC (Consumer Product Safety Commission). (2014). Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report (2014). Available online: https://www.cpsc.gov/en/Regulations-Laws–Standards/Statutes/The-Consumer-Product-Safety-Improvement-Act/Phthalates/Chronic-Hazard-Advisory-Panel-CHAP-on-Phthalates/. Accessed August 2014.
- Cuzzolin L. (2013). Drug metabolizing enzymes in the perinatal and neonatal period: differences in the expression and activity. Curr Drug Metabol, 14, 167–73
- Daston G, Faustman E, Ginsberg G, Fenner-Crisp P, Olin S, Sonawane B, et al. (2004). A framework for assessing risks to children from exposure to environmental agents. Environ Health Perspect, 112, 238–56.
- Dekant W, Colnot T. (2013). Endocrine effects of chemicals: aspects of hazard identification and human health risk assessment. Toxicol Lett, 223, 280–6.
- DeWitt JC, Peden-Adams MM, Keil DE, Dietert RR. (2012). Developmental immunotoxicity (DIT): assays for evaluating effects of exogenous agents on development of the immune system. Curr Protocols Toxicol, 51, 18.15.1–18.15.14.
- DeWoskin RS, Thompson CM. (2008). Renal clearance parameters for PBPK model analysis of early lifestage differences in the disposition of environmental toxicants. Regul Toxicol Pharmacol, 51, 66–86.
- de Zwart LL, Haenen HE, Versantvoort CH, Wolterink G, van Engelen JG, Sips AJ. (2004). Role of biokinetics in risk assessment of drugs and chemicals in children. Regul Toxicol Pharmacol, 39, 282–309.
- Dietert RR, Dietert JM. (2007). Early-life immune insult and developmental immunotoxicity (DIT)-associated diseases: potential of herbal- and fungal-derived medicinals. Curr Med Chem, 14, 1075–85.
- Dorne JL, Walton K, Renwick AG. (2001a). Uncertainty factors for chemical risk assessment: human variability in the pharmacokinetics of CYP1A2 probe substrates. Food Chem Toxicol, 39, 681–96.
- Dorne JL, Walton K, Renwick AG. (2001b). Human variability in glucuronication in relation to uncertainty factors for risk assessment. Food Chem Toxicol, 43, 1153–73.
- Dorne JL, Walton K, Slob W, Renwick AG. (2002). Human variability in polymorphic CYP2D6 metabolism: is the kinetic default uncertainty factor adequate? Food Chem Toxicol, 40, 1633–56.
- Dorne JL, Walton K, Renwick AG. (2003a). Human variability in CYP3A4 metabolism and CYP3A4-related uncertainty factors for risk assessment. Food Chem Toxicol, 41, 201–24.
- Dorne JL, Walton K, Renwick AG. (2003b). Polymorphic CYP2C19 and N-acetylation: human variability in kinetics and pathway-related uncertainty factors. Food Chem Toxicol, 43, 225–45.
- Dorne JL, Walton K, Renwick AG. (2004a). Human variability in the renal elimination of foreign compounds and renal excretion-related uncertainty factors for risk assessment. Food Chem Toxicol, 42, 275–98.
- Dorne JL, Walton K, Renwick AG. (2004b). Human variability for metabolic pathways with limited data (CYP2A6, CYP2C9, CYP2E1, ADH, esterases, glycine and sulphate conjugation). Food Chem Toxicol, 42, 397–421.
- Dorne JL, Walton K, Renwick AG. (2005). Human variability in xenobiotic metabolism and pathway-related uncertainty factors for chemical risk assessment: A review. Food Chem Toxicol, 43, 203–16.
- Dourson ML, Felter SP, Robinson D. (1996). Evolution of science-based uncertainty factors in noncancer risk assessment. Regul Toxicol Pharmacol, 24, 108–20.
- Dourson ML, Charnley G, Scheuplein R. (2002). Differential sensitivity of children and adults to chemical toxicity: II. Risk and Regulation. Regul Toxicol Pharmacol, 35, 448–67.
- Edginton AN. (2011). Knowledge-driven approaches for the guidance of first-in-children dosing. Pediatr Anesth, 21, 206–13.
- Edginton AN, Fotaki N. (2010). Oral drug absorption in pediatric populations. In: Dressman J, Reppas C, Eds. Oral Drug Absorption: Prediction and Assessment, 2nd ed. New York: Informa Healthcare, pp. 108–126.
- Edginton AN, Schmitt W, Voith B, Willmann S. (2006). A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet, 45, 683–704.
- EFSA. (2011). Scientific Report of EFSA: Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. European Food Safety Authority. EFSA J, 9, 2490.
- EFSA. (2013). Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. European Food Safety Authority. EFSA J, 11, 3132.
- Firestone M, Moya J, Cohen Hubal E, Zartarian V. (2007). Identifying childhood age groups for exposure assessments and monitoring. Risk Anal, 27, 701–14.
- Fluhr JW, Darlenski R, Lachmann N, Baudouin C, Msika P, De Belilovsky C, Hachem JP. (2012). Infant epidermal skin physiology: adaptation after birth. Br J Dermatol, 166, 483–90.
- Fowles JR, Banton MI, Pottenger LH. (2013). A toxicological review of the propylene glycols. Crit Rev Toxicol, 43, 363–90.
- Gamboa da Costa G, Loukotková L, Von Tungeln LS, Olson G, Sprando RL, Hattan DG, et al. (2013). Nephrotoxicity of melamine, cyanuric acid, and their combination in newborn F344 rats. Society of Toxicology, Annual Meeting. March.
- Gehrs BC, Riddle MM, Williams WC, Smialowicz RJ. (1997). Alterations in the developing immune system of the F344 rat after perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin: II. Effects on the pup and the adult. Toxicology, 122, 229–40.
- Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, et al. (2002). Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci, 66, 185–200.
- Ginsberg G, Hattis D, Sonawane B. (2004a). Incorporating pharmacokinetic differences between children and adults in assessing children's risks to environmental toxicants. Toxicol Appl Pharmacol, 198, 164–83.
- Ginsberg G, Slikker W Jr, Bruckner J, Sonawane B. (2004b). Incorporating children's toxicokinetics into a risk framework. Environ Health Perspect, 112, 272–83.
- Giordano G, Costa LG. (2012). Review Article. Developmental Neurotoxicity: Some Old and New Issues. ISRN Toxicol. Article ID 814795, doi:10.5402/2012/814795
- Godin SJ, Scollon EJ, Hughes MF, Potter PM, DeVito MJ, Ross MK. (2006). Species differences in the in vitro metabolism of deltamethrin and esfenvalerate: Differential oxidative and hydrolytic metabolism by humans and rats. Drug Metabol Dispos, 34, 1764–71.
- Guzelian PS, Henry CJ, Olin SS. (1992). Similarities and Differences between Children and Adults: Implications for Risk Assessment. Proceedings of the ILSI Conference. Washington DC: ILSI Press.
- Heimann G. (1983). Renal toxicity of aminoglycosides in the neonatal period. Pediatr Pharmacol, 3, 251–7.
- Hessel EVS, Tonk ECM, Bos PMJ, van Loveren H, Piersma AH. (2014). Developmental immunotoxicity of chemicals in rodents and its possible regulatory impact. Crit Rev Toxicol, Early Online, 1–15, doi:10.3109/10408444.2014.959163.
- Hillyer MM, Finch LE, Cerel AS, Dattelbaum JD, Leopold MC. (2014). Multi-technique quantitative analysis and socioeconomic considerations of lead, cadmium, and arsenic in children's toys and toy jewelry. Chemosphere. Feb 18. pii: S0045–6535(14)00107-6. doi: 10.1016/j.chemosphere.2014.01.041. [Epub ahead of print]
- Hines RN. (2008). Ontogeny of drug metabolism enzymes and implications for adverse drug reactions. Pharmacol Therapeut, 118, 250–67.
- Hines RN. (2013). Developmental expression of drug metabolizing enzymes: Impact on disposition in neonates and young children. Int J Pharmaceut, 452, 3–7.
- Hines RN, Sargent D, Autrup H, Birnbaum LS, Brent RL, Doerrer NG, et al. (2010). Approaches for assessing risks to sensitive populations: Lessons learned from evaluating risks in the pediatric population. Toxicol Sci, 113, 4–26.
- Holladay SD, Smialowicz RJ. (2000). Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect, 108, 463–73.
- Holsapple MP, West LJ, Landreth KS. (2003). Species comparison of anatomical and functional immune system development. Birth Defects Res (Part B), 68, 321–34.
- Ishitobi H, Mori K, Yoshida K, Watanabe C. (2007). Effects of perinatal exposure to low-dose cadmium on thyroid hormone-related and sex hormone receptor gene expressions in brain of offspring. Neurotoxicology, 28, 790–7.
- Johnsrud EK, Koukouritaki SB, Divakaran K, Brunengraber LL, Hines RN, McCarver DG. (2003). Human hepatic CYP2E1 expression during development. J Pharmacol Exper Therapeut, 307, 402–7.
- Juberg DR, Borghoff SJ, Becker RA, Casey W, Hartung T, Holsapple MP, et al. (2014). Workshop Report: Lessons learned, challenges, and opportunities: The US Endocrine Disruptor Screening Program. ALTEX, 31, 63–78.
- Kameoka S, Babiarz J, Kolaja K, Chiao E. (2014). A high-throughput screen for teratogens using human pluripotent stem cells. Toxicol Sci, 137, 76–90.
- Kaufmann W. (2003). Current status of developmental neurotoxicity: an industry perspective. Toxicol Lett, 140–141, 161–9.
- Kimmel CA, Holson JF, Hogue CJ, Carlo GL. (1984). Reliability of experimental studies for predicting hazards to human development. Final Report for Experiment 6015. National Center for Toxicological Research. January.
- Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, et al. (2004). Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol, 42, 65–83.
- Lehman AJ, Fitzhugh OG. (1954). Ten-fold safety factor studies. Assoc Food Drug Officials of the US Quarterly Bulletin, 18, 33–5.
- Lewis RW. (2013). Risk assessment of ‘endocrine substances’: Guidance on identifying endocrine disruptors. Toxicol Lett, 223, 287–90.
- Li A. (2005). Regulatory developmental neurotoxicology testing: data evaluation for risk assessment purposes. Environ Toxicol Pharmacol, 19, 727–33.
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. (2013). Gender differences in the neurotoxicity of metals in children. Toxicology, 311, 3–12.
- Ludriksone L, Garcia Bartels N, Kanti V, Blume-Peytavi U, Kottner J. (2014). Skin barrier function in infancy: a systematic review. Arch Dermatol Res. Published online 05 March 2014. doi:10.1007/s00403-014-1458-6.
- Makri A, Goveia M, Balbus J, Parkin R. (2004). Children's susceptibility to chemicals: a review by developmental stage. J Toxicol Environ Health Part B Crit Rev, 7, 417–35.
- Makris SL, Raffaele K, Allen S, Bowers WJ, Hass U, Alleva E, et al. (2009). A Retrospective Performance Assessment of the Developmental Neurotoxicity Study in Support of OECD Test Guideline 426. Environ Health Perspect, 117, 17–25.
- Marty MS, Chapin RE, Parks LG, Thorsrud NA. (2003). Development and maturation of the male reproductive system. Birth Defects Res (Part B), 68, 125–36.
- McCarthy G. (2013). Reaffirmation of the U.S. Environmental Protection Agency's 1995 Policy on Evaluating Health Risks to Children. Memorandum from the Administrator of the US EPA. Oct 31.
- Middaugh LD, Dow-Edwards D, Li AA, Sandler JD, Seed J, Sheets LP, et al. (2003). Neurobehavioral assessment: a survey of use and value in safety assessment studies. Toxicol Sci, 76, 250–61.
- Mooij MG, Schwarz UI, de Koning BAE, Leeder JS, Gaedigk R, Samsom JN, et al. (2014). Ontogeny of human hepatic and intestinal gene expression during childhood: age matters. Drug Metab Dispos, 42, 1268–74.
- M’Rabet L, Vos AP, Boehm G, Garssen J. (2008). Breast-feeding and its role in early development of the immune system in infants: consequences for health later in life. J Nutr, 138, 1782S–90S.
- Moya J, Phillips L. (2014). A review of soil and dust ingestion studies for children. J Expo Sci Environ Epidemiol Apr 2. doi:10.1038/jes.2014.17. [Epub ahead of print]
- NAS. (2014). The National Children's Study 2014: An Assessment. National Academy of Sciences. Washington DC: The National Academies Press, Washington, DC. 182 pp.
- Neal-Kluever A, Aungst J, Gu Y, Hatwell K, Muldoon-Jacobs K, Liem A, et al. (2014). Infant toxicology: State of the science and considerations in evaluation of safety. Food Chem Toxicol, 70, 68–83.
- Nong A, McCarver DG, Hines RN, Krishnan K. (2006). Modeling interchild differences in pharmacokinetics on the basis of subject-specific data on physiology and hepatic CYP2E1 levels: a case study with toluene. Toxicol Appl Pharmacol, 214, 78–87.
- NRC. (1983). Risk Assessment in the Federal Government: Managing the Process. The National Research Council, National Academy of Sciences. Washington DC: The National Academies Press.
- NRC. (1993). Pesticides in the diets of infants and children. The National Research Council, National Academy of Sciences. Washington, DC: The National Academies Press.
- NRC (2007). Toxicity Testing in the 21st Century: A Vision and a Strategy. The National Research Council, National Academy of Sciences. Washington, DC: The National Academies Press.
- NRC. (2009). Science and Decisions: Advancing Risk Assessment. The National Research Council, National Academy of Sciences. Washington, DC: The National Academies Press.
- OECD. (2013). OECD Guidelines for Testing of Chemicals: Full List of Test Guidelines. Organisation for Economic Co-operation and Development. July. Available online: http://www.oecd.org/env/ehs/testing/TG_List_EN_Jul_2013.pdf. Accessed on 12 April 2014.
- Olin SS, Sonawane BR. (2003). Workshop to Develop a Framework for Assessing Risks to Children from Exposure to Environmental Agents. Environ Health Perspect, 111, 1524–6.
- Piersma AH, Tonk ECM, Makris SL, Crofton KM, Dietert RR, van Loveren H. (2012). Juvenile toxicity testing protocols for chemicals. Reprod Toxicol, 34, 482–6.
- Price K, Haddad S, Krishnan K. (2003). Physiological modeling of age-specific changes in the pharmacokinetics of organic chemicals in children. J Toxicol Environ Health Part A, 66, 417–33.
- Quigley R. (2012). Developmental changes in renal function. Curr Opin Pediatr, 24, 184–90.
- Raffaele KC, Rowland J, May B, Makris SL, Schumacher K, Scarano LJ. (2010). The use of developmental neurotoxicity data in pesticide risk assessments. Neurtoxicol Teratol, 32, 563–72.
- Renwick AG. (1993). Data-derived safety (uncertainty) factors for the evaluation of food additives and environmental contaminants. Food Addit Contam, 10, 275–305.
- Renwick AG. (1998). Toxicokinetics in infants and children in relation to the ADI and TDI. Food Addit Contam, 15, 17–35.
- Renwick AG, Lazarus NR. (1998). Human variability and noncancer risk assessment: an analysis of the default uncertainty factor. Regul Toxicol Pharmacol, 27, 3–20.
- Rhomberg LR, Goodman JE. (2012). Low-dose effects and nonmonotonic dose-responses of endocrine disrupting chemicals: has the case been made? Regul Toxicol Pharmacol, 64, 130–3.
- Rodriguez W, Roberts R, Murphy D. (2001). Adverse drug events in children: The US Food and Drug Administration Perspective. Curr Therap Res, 62, 711–23.
- Rogers JM, Ellis-Hutchings RG, Grey BE, Zucker RM, Norwood J Jr, Grace CE, et al. (2014). Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol Sci, 137, 436–446.
- Routledge PA. (1994). Pharmacokinetics in children. J Antimicrob Chemother, 34, 19–24.
- Saghir SA, Marty MS, Zablotny CL, Passage JK, Perala AW, Neal BH, et al. (2013). Life stage-, sex-, and dose-dependent dietary toxicokinetics and relationship to toxicity of 2,4-dichlorophenoxyacetic acid (2,4-D) in rats: implications for toxicity test dose selection, design, and interpretation. Toxicol Sci, 136, 294–307.
- SCCNFP. (2002). Position statement on the calculation of the margin of safety of ingredients incorporated in cosmetics which may be applied to the skin of children. Scientific Committee on Cosmetic Products and Non-food products intended for Consumers. Adopted by the SCCNFP during the 19th Plenary meeting of 27 February 2002.
- SCCS. (2012). The SCCS's Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 8th Revision. SCCS/1416/11. Scientific Committee on Consumer Safety. Adopted 11 December, 2012.
- Schardein JL, Keller KA. (1989). Potential human developmental toxicants and the role of animal testing in their identification and characterization. Crit Rev Toxicol, 19, 251–339.
- Schardein JL, Schwetz BA, Kenel, MF. (1985). Species sensitivities and prediction of tetratogenic potential. Environ Health Perspect, 61, 55–67.
- Scheuplein R, Charnley G, Dourson M. (2002). Differential sensitivity of children and adults to chemical toxicity. I. Biological basis. Regul Toxicol Pharmacol, 35, 429–47.
- Sheets LP. (2000). A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicol, 21, 57–64.
- Smialowicz RJ, Williams WC, Copeland CB, Harris MW, Overstreet D, Davis BJ, Chapin RE. (2001). The effects of perinatal/juvenile heptachlor exposure on adult immune and reproductive system function in rats. Toxicol Sci, 61, 164–75.
- Smith JN, Hinderliter PM, Timchalk C, Bartels MJ, Poet TS. (2014). A human life stage physiologically based pharmacokinetic and pharmacodynamic model for chlorpyrifos: Development and validation. Regul Toxicol Pharmacol, 69, 580–97.
- Spielmann H. (2005). Predicting the risk of developmental toxicity from in vitro assays. Toxicol Appl Pharmacol, 207, 375–80.
- Stahlmann R. (2003). Children as a special population at risk – quinolones as an example for xenobiotics exhibiting skeletal toxicity. Arch Toxicol, 77, 7–11.
- Strolin Benedetti M, Whomsley R, Baltes EL. (2005). Differences in absorption, distribution, metabolism and excretion of xenobiotics between the paediatric and adult populations. Expert Opin Drug Metab Toxicol, 1, 447–71.
- Sulemanji M, Vakili K. (2013). Neonatal renal physiology. Semin Pediatr Surg, 22, 195–8.
- Teeguarden JG, Hanson-Drury S. (2013). A systematic review of Bisphenol A “low dose” studies in the context of human exposure: A case for establishing standards for reporting “low-dose” effects of chemicals. Food Chem Toxicol, 62, 935–48.
- Tilson H. (2000). Neurotoxicity risk assessment guidelines: Developmental neurotoxicity. Neurotoxicology, 21, 189–94.
- Tonk EC, Verhoef A, Gremmer ER, Van Loveren H, Piersma AH. (2013). Developmental immunotoxicity in male rats after juvenile exposure to ethanol. Toxicology, 309, 91–9.
- Touw DJ, Westerman EM, Sprij AJ. (2009). Therapeutic drug monitoring of aminoglycosides in neonates. Clin Pharmacokinet, 48, 71–88.
- US EPA. (1987). The Risk Assessment Guidelines of 1986. EPA/600/8–87/045. Office of Health and Environmental Assessment, US Environmental Protection Agency. Washington DC. August 1987.
- US EPA. (1995). Policy on Evaluating Health Risks to Children. Available at http://www2.epa.gov/sites/production/files/2014-05/documents/1995_childrens_health_policy_statement.pdf. Accessed 10 October 2014.
- US EPA. (1996). Food Quality Protection Act (FQPA) of 1996. US Environmental Protection Agency. Washington DC. Available at http://www.epa.gov/opp00001/regulating/laws/fqpa/. Accessed 5 March 2014.
- US EPA. (1998a). Guidelines for Neurotoxicity Risk Assessment. 630/R-95/001F. Risk Assessment Forum, US Environmental Protection Agency. Washington, DC.
- US EPA. (1998b). Health Effects Test Guidelines, OPPTS 870.6300, Developmental Neurotoxicity Study, EPA 712-C-98–239. Office of Prevention, Pesticides, and Toxic Substances, US Environmental Protection Agency, Washington, DC.
- US EPA. (2002). Final Report: A Review of the Reference Dose and Reference Concentration Processes. EPA/630/P-02/002F. Risk Assessment Forum, US Environmental Protection Agency Washington DC. December 2002.
- US EPA. (2005). Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants. EPA/630/P-03/003F. Risk Assessment Forum, US Environmental Protection Agency Washington, DC. November 2005.
- US EPA. (2006). A framework for assessing health risks of environmental exposures to children. EPA/600/R-05/093F. US Environmental Protection Agency. National Center for Environmental Assessment. Washington, DC. September.
- US EPA. (2008). Child-Specific Exposure Factors Handbook (Final Report). EPA/600/R-06/096F. US Environmental Protection Agency. Washington, DC.
- US EPA. (2011a). Exposure Factors Handbook Edition (Final). EPA/600/R-09/052F. US Environmental Protection Agency. Washington, DC.
- US EPA. (2011b). Recommended Use of Body Weight 3/4 as the Default Method in Derivation of the Oral Reference Dose. EPA/100/R11/0001. US Environmental Protection Agency. Washington, DC.
- US EPA. (2011c). Endocrine Disruptor Screening Program for the 21st Century: (EDSP21 Work Plan). The Incorporation of In Silico Models and In Vitro High Throughput Assays in the Endocrine Disruptor Screening Program (EDSP) for Prioritization and Screening. Office of Chemical Safety and Pollution Prevention. US Environmental Protection Agency. Washington, DC. September 30.
- US EPA. (2012). 2012 Edition of the Drinking Water Standards and Health Advisories. EPA 822-S-12-001. Office of Water. US Environmental Protection Agency. Washington, DC. April.
- US FDA. (2011). Guidance for Industry Reproductive and Developmental Toxicities: Integrating Study Results to Assess Concerns. Center for Drug Evaluation and Research (CDER), US Food and Drug Administration. Washington DC. September.
- Vahter M, Akesson A, Liden C, Ceccatelli S, and Berglund M. (2007). Gender differences in the disposition and toxicity of metals. Environ Res, 104, 85–95.
- Vos JG, DeKlerk A, Krajnc EI, Van Loveren V, Rozing J. (1990). Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: Effects on thymus- dependent immunity and on nonspecific resistance following long-term exposure in young versus aged rats. Toxicol Appl Pharmacol, 105, 144–55.
- Walthall K, Cappon GD, Hurtt ME, Zoetis T. (2005). Postnatal development of the gastrointestinal system: A species comparison. Birth Defects Res (Part B), 74, 132–56.
- Weiss CF, Glazko AJ, Weston JK. (1960). Chloramphenicol in the newborn infant: a physiological explanation of its toxicity when given in excessive doses. N Engl J Med, 262, 787–94.
- Wetmore BA, Allen B, Clewell HJ III, Parker T, Wambaugh JF, Almond LM, et al. (2014). Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing. Toxiol Sci doi:10.1093/toxsci/kfu169. [Epub ahead of print].
- WHO. (1974). Toxicological evaluation of certain food additives with a review of general principles and of specifications. In: 17th Report of the Joint FAO/WHO Expert Committee on Food Additives, Report No. 539. World Health Organization. Geneva, Switzerland, June 25-July 4, 1973. Available at: http://www.inchem.org/documents/jecfa/jecmono/v05je90.htm. Accessed 5 March 2014.
- WHO. (1986). Environmental Health Criteria 59: Principles for evaluating risks from chemicals during infancy and early childhood: the need for a special approach. World Health Organization/International Programme on Chemical Safety. Geneva, Switzerland.
- WHO. (1994). Environmental Health Criteria No. 170: Assessing Human Health Risks of Chemicals: Derivation of Guidance Values for Health-based Exposure Limits. International Programme on Chemical Safety, World Health Organization. Geneva.
- WHO. (2001). Guidance Document for the Use of Data in Development of Chemical-Specific Adjustment Factors (CSAF) for Interspecies Differences and Human Variability in Dose/Concentration Response Assessment. International Programme for Chemical Safety, World Health Organization: Geneva.
- WHO. (2012). Harmonization Project Document No. 10. Guidance for Immunotoxicity Risk Assessment for Chemicals. World Health Organization/International Programme on Chemical Safety. Geneva, Switzerland.
- Willhite CC, Ball GL, McLellan CJ. (2008). Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Health B Crit Rev, 11, 69–46.
- Wolf CR, Smith G. (1999). Cytochrome P450 CYP2D6. IARC Sci Publ, 148, 209–29.
- Wolterink G, Piersma AH, van Engelen JGM. (2002). Risk assessment of chemicals: What about children?. RIVM Report 613340005/2002. Bilthoven, Netherlands.
- Wolterink G, van Engelen JGM, van Raaij MTM. (2007). Guidance for assessment of chemical risks for children. RIVM Report 320012001/2007. Bilthoven, Netherlands.
- Wood SL, Beyer BK, Cappon GD. (2003). Species comparison of postnatal CNS development: Functional measures. Birth Defects Res (Part B), 68, 391–407.
- Wu S, Blackburn K, Amburgey J, Jaworska J, Federle T. (2010). A framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regul Toxicol Pharmacol, 56, 67–81.
- Yolton K, Cornelius M, Ornoy A, McGough J, Makris S, Schantz S. (2014). Exposure to neurotoxicants and the development of attention deficit hyperactivity disorder and its related behaviors in childhood. Neurotoxico Teratol, 44, 30–45.
- Yoon M, Campbell JL, Andersen ME, Clewell HJ. (2012). Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol, 2, 633–52.
- Zar T, Graeber C, Perazella MA. (2007). Recognition, treatment, and prevention of propylene glycol toxicity. Semin Dialysis, 20, 217–9.
- Zoetis T, Tassinari MS, Bagi C, Walthall K, Hurtt ME. (2003). Species comparison of postnatal bone growth and development. Birth Defects Res (Part B), 68, 86–110.
- Zoetis T, Hurtt ME. (2003a). Species comparison of anatomical and functional renal development. Birth Defects Res (Part B), 68, 111–20.
- Zoetis T, Hurtt ME. (2003b). Species comparison of lung development. Birth Defects Res (Part B), 68, 121–4.
- Zosel A, Egelhoff E, Heard K. (2010). Severe lactic acidosis after an iatrogenic propylene glycol overdose. Pharmacotherapy, 30, 219–24.

