Immune homeostasis is preserved by a balance between the effector and regulatory immune mechanisms. During cancer progression this balance is skewed toward immune tolerization, facilitating the tumor cells to escape the antitumor immune responses of the host. It is suggested that this immune evasion can be effectively reversed by immunomodulatory agents (IMiDs) such as lenalidomide [Citation1]. Lenalidomide has antitumor activity in various hematologic cancers (including multiple myeloma, chronic lymphocytic leukemia, lymphomas and myelodysplastic syndromes) independent of the type or origin of the malignant cell [Citation2]. Preclinical evaluations identified its ability to modulate the immunological microenvironment both via cellular activation of the effector T and natural killer (NK) cells and by down-regulation of critical tumor supporting and immune suppressive cytokines (interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor) in the tumor microenvironment [Citation3]. In addition, it affects antigen-presenting cell function and the humoral immune response mediated through antibody-dependent cell-mediated cytotoxicity. The immune modulatory actions of lenalidomide aptly include both the effector T cells and regulatory T cells (Tregs). While the action of lenalidomide on effector T cell function is unequivocally documented both in vitro and in vivo [Citation4], its role in the modulation of Tregs remains unresolved.
Tregs are a subset of T cells with a critical role in the maintenance of tolerance and immune homeostasis due to their ability to suppress the immune responses [Citation5]. Based on cell surface markers or cytokine secretion profiles, Tregs can be grouped into two categories: natural Tregs (nTregs) and induced Tregs (iTregs). The natural CD4+ Tregs (nTregs) are of thymic origin with an immunophenotype consisting of CD25, FOXP3, CTLA-4, LAG3 and GITR. These cells suppress innate and adaptive immune cells. Conventional T cells in the presence of exogenous transforming growth factor-β (TGF-β) are transformed into iTregs and acquire immunosuppressive properties. Expression and secretion of TGF-β and interleukin-10 (IL-10) are substantially increased in iTregs. The exact mechanism of the immunosuppressive function of Tregs is not known. It is proposed that in cancer, Tregs suppress immune responses and induce immune tolerance through multiple mechanisms, including cell contact-dependent and -independent mechanisms. Also progressive immune failure is associated with an increasing percentage of Tregs in cancer. Thus, a therapeutic regimen that includes depletion of Tregs is being tested in cancer therapy and, in particular, immunotherapy. Conversely, the effect of immunotherapy on the fate of Tregs is also being debated.
Lenalidomide was shown to inhibit the proliferation and function of Tregs in vitro [Citation6]. Idler et al. studied the changes in Treg population in patients with chronic lymphocytic leukemia (CLL) treated with lenalidomide over a prolonged period of time, and showed that lenalidomide decreased the percentage as well as the absolute number of Tregs [Citation7]. However, a recent report showed that lenalidomide has a biphasic effect on Tregs in CLL. Treatment with lenalidomide for three cycles (28 days) increased the Tregs (percentage and absolute number), whereas a prolonged treatment for 15 cycles with progressively increasing doses of lenalidomide (increased by 5 mg/day after every two cycles of 28 days each) brought the numbers to a lower level, equivalent to that of healthy donors [Citation8].
Lenalidomide is highly effective in treating newly diagnosed as well as relapsed/refractory multiple myeloma (MM) and is now being evaluated as maintenance therapy, as preventive therapy, and in combination with other new agents. In particular, the combination of dexamethasone, an immunosuppressant, and lenalidomide resulted in an impressive and long-lasting remission in patients with relapsed and/or refractory MM [Citation9]. The reason for this paradoxical but remarkable clinical response is not known. Clinical data indicate that lenalidomide in combination with dexamethasone is highly effective in relapsed/refractory MM following allogeneic stem cell transplant, which is associated with an increase in the number of Tregs [Citation10]. Consistent with these studies in CLL, Raja et al. have shown that the combination of lenalidomide and dexamethasone increases Tregs among patients with previously untreated MM [Citation11]. The data suggest that in spite of a positive antitumor immune response in patients treated with lenalidomide in combination with dexamethasone, Treg numbers are increased. The data also show a significant increase in Tregs at the end of cycles 3 and 4, suggesting that the change in Tregs is a late event, as compared to the positive immune response.
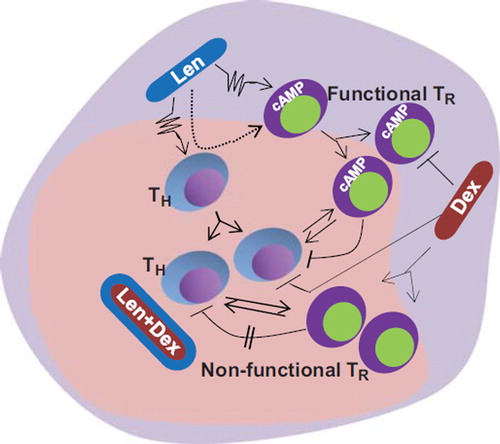
However, the conflicting but compelling data from in vitro studies that lenalidomide inhibits proliferation and the immune suppressive function of Tregs suggest that the in vivo effects of lenalidomide might be a result of the influence of the microenvironment on the immune cells. In this regard, it is important to note that T cells and in particular Tregs show plasticity as dictated by the microenvironment [Citation12]. Thus, the data may be interpreted such that once lenalidomide induces effector immune responses in vivo, there is a negative feedback induced by transformation of the T helper (TH) cells into Tregs to maintain the immune homeostasis. Inclusion of dexamethasone is reported to increase the Tregs. We suggest that dexamethasone-induced Tregs are non-functional, as it also inhibits the cyclic adenosine monophosphate (cAMP)-mediated signaling, which is very important for the suppressive function of Tregs [Citation13] (). This results in a balance between the effector T cells and non-functional Tregs, thus skewing the balance toward active immune-mediated functions elicited by lenalidomide.
Figure 1. Possible mechanisms of action of lenalidomide and dexamethasone in vivo. Lenalidomide (Len) stimulates immune effector cells that in turn transform to Tregs (TR) to secure immune homeostasis. Dexamethasone (Dex), which induces proliferation of Tregs, also blocks the cAMP signaling pathway, resulting in the production of Tregs that are non-functional as suppressors of immune function. In the presence of a combination of Len and Dex it is possible that the non-functional Tregs fail to block the immune effector functions induced by Len, thus causing a sustained antitumor immune reaction.
Supplementary Material
Download Zip (1.9 MB)Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica 2009;94:1198–1202.
- Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol 2008;26:1544–1552.
- Quach H, Ritchie D, Stewart AK, . Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010;24:22–32.
- Chanan-Khan A, Porter CW. Immunomodulating drugs for chronic lymphocytic and leukemia. Lancet Oncol 2006;7:480–488.
- Wan YY. Regulatory T cells: immune suppression and beyond. Cell Mol Immunol 2010;7:204–210.
- Galustian C, Meyer B, Labarthe MC, . The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 2009;58: 1033–1045.
- Idler I, Giannopoulos K, Zenz T, . Lenalidomide treatment of chronic lymphocytic leukaemia patients reduces regulatory T cells and induces Th17 T helper cells. Br J Haematol 2010;148:948–950.
- Lee BN, Gao H, Cohen EN, . Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer 2011;117: 3999–4008.
- Dimopoulos M, Spencer A, Attal M, ; Multiple Myeloma (010) Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007;357:2123–2132.
- Minnema MC, van der Veer MS, Aarts T, . Lenalidomide alone or in combination with dexamethasone is highly effective in patients with relapsed multiple myeloma following allogeneic stem cell transplantation and increases the frequency of CD4+ Foxp3+ T cells. Leukemia 2009;23:605–607.
- Raja KRM, Kovarova L, Hajek R. Induction by lenalidomide and dexamethasone combination increases regulatory cells of patients with previously untreated multiple myeloma. Leuk Lymphoma 2012;53: 1406–1408.
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010;327:1098–1102.
- Bopp T, Becker C, Klein M, . Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 2007;204:1303–1310.