Waldenström macroglobulinemia (WM, also known as lymphoplasmacytic lymphoma) is an indolent and incurable lymphoproliferative neoplasm affecting B-cells. The bone marrow is infiltrated by a population ranging from small B-cells to differentiated plasma cells. A unique characteristic of the disease is that the involved B-cells produce excess amounts of immunoglobulin (IgM). The disease, named after the Swedish oncologist Jan Waldenström, was first described in 1944. WM is a rare disease, with an incidence of about one case per 100 000 per year. The median age of onset of WM is between 60 and 65 years.
WM is characterized by an uncontrolled clonal proliferation of terminally differentiated B-lymphocytes. It is assumed that WM cells are derived from post-germinal center B-lymphocytes that have undergone extensive somatic hypermutation and are hypersecreting monoclonal IgM. While the IGH (immunoglobulin heavy chain gene) variable region is generally mutated, intraclonal variation is usually absent and IGH switching does not occur [Citation1].
The underlying etiology of the disease is not yet known. For many years, WM was considered to be related to multiple myeloma, due to the presence of monoclonal gammopathy and infiltration of the bone marrow and other organs by plasmacytoid lymphocytes. The latest World Health Organization (WHO) classification, however, places WM under the category of lymphoplasmacytic lymphoma, itself a subcategory of the indolent (i.e. low-grade) non-Hodgkin lymphomas.
While the disease is incurable given current therapy, it is treatable. However, there is no single accepted treatment for WM, and hence there is a marked variation in clinical outcome. Objective response rates are high, but complete response rates are low. In recent years there have been advances in understanding the biology of WM and the applied treatment protocols [Citation1].
Overall the pathogenesis of this disease remains poorly understood, notably at the molecular level. Although individual cell line models may prove useful tools in such cases, larger panels are demanded to fully exploit the systems biology approach allowed by current high-throughput array-based methods to reveal any underlying heterogeneities, data informing disease stratification [Citation2]. Cell line panels subjected to array-based investigation often flag up “bad apples” in the form of misclassified cell lines. The lack of validated WM cell models is a chronic problem. Indeed, even in the heyday of cell line establishment during the 1980s and 1990s when some 30–50 new hematopoietic cell lines were reported each year, the absence of WM examples was conspicuous [Citation3].
The first cell line derived from a patient with WM (named WM1) was published in 1982 [Citation4]. However, this cell line was not well characterized. Given its reportedly normal karyotype, authentication, i.e. derivation from the index patient, was not confirmable by cytogenetics (the only practical method available at the time). Neither was its clonal origin from the founding tumor verified, to provide unequivocal proof of malignant origin. Based on the available data, it remains unclear whether WM1 represents the WM tumor clone itself, or merely bystander B-cells immortalized by Epstein–Barr virus (EBV) transformation [Citation5].
The second putative WM cell line, WSU-WM, showed a karyotype and morphology more reminiscent of Burkitt lymphoma than of WM [Citation6]. For this cell line, informative authentication and confirmation of its neoplastic derivation were also missing [Citation5].
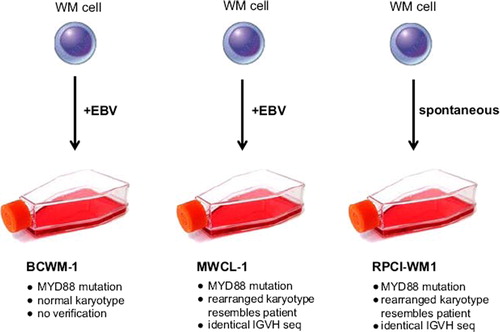
After these two faltering efforts to establish WM cell lines, the pace of progress has quickened over the last 5 years with the publication of three promising new candidates: BCWM-1 in 2007, MWCL-1 in 2011 and now RPCI-WM1 in this issue [Citation7–9] (). In addition there seems to be a welcome improvement in reports regarding characterization, authentication and verification.
While BCWM-1 retains biologic properties of the initial tumor cells and has frequently been used as a disease model, its authentication and verification is the weakest of the triad [Citation5], prompting doubts whether these cells adequately represent WM [Citation10].
The existence of somatic hypermutation in WM cells allows primary donor tumor cells to be matched with any putative cell line at the sequence level. IGVH sequence homologies between the primary tumor cells from the index patients and the respective cell lines have been confirmed for MWCL-1 and RPCI-WM1 [Citation8,Citation9] – cogent evidence supporting their WM tumor origin.
There remains, however, the issue of immortalization and the complicating role of EBV. Both BCWM-1 and MWCL-1 are EBV-positive, whereas RPCI-WM1 is EBV-negative (). The disease itself is not associated with EBV, and incorporation of the EBV genome probably renders the two positive cell lines less reliable preclinical models of WM than RPCI-WM1, which arose spontaneously. On the other hand, once the contribution of EBV is subtracted (assuming this is feasible), it might be argued that EBV+ WM cell lines are better than nothing if they retain disease-specific characteristics.
Unlike other major lymphoid neoplasms, no recurrent cytogenetic rearrangements targeting specific oncogenes have been identified in WM [Citation1]. Such changes illuminate the pathology and assist the search for potential therapeutic targets in neoplasms bearing such alterations. As a bonus, the presence of appropriate oncogenomic rearrangements is useful for confirming the neoplastic character of model cell lines. In the case of BCWM-1 the karyotype is reportedly normal, save deletion of the chromosome 3p14 – a rearrangement absent in the primary tumor [Citation7].
Fortunately, in both MWCL-1 and RPCI-WM1, cytogenetic analyses revealed conserved, fiduciary rearrangements matching cell lines to donor tumors. In the case of MWCL-1, array comparative genomic hybridization (CGH) analysis revealed three such changes – del(2)(p21), del(17)(p11p13), add(17)(q25) – together with identical TP53 mutations rendered hemizygous by the chromosomal deletion of 17p13 [Citation8]. An additional three rearrangements were found in the cell line only. Whether their formation underlies immortalization remains to be investigated.
At the cytogenetic level, the third member of the triad, RPCI-WM1, is the best investigated WM cell line described to date. Spectral karyotyping performed on the cell line revealed del(3), der(6)t(3;6), t(8;22), der(9)t(6;9), dup(11), der(14)t(3;14), t(18;19), der(18)t(3;10;14;18), der(19)t(5;19) and del(X) alterations. Fluorescence in situ hybridization (FISH) analysis using locus specific clones independently confirmed rearrangements at 6q21, 9p21 (with CDKN2A deletion) and 13q14 (RB1 deletion) in both patient and cell line, together with 14q32 (IGH amplification) and 18q21 (BCL2 deletion) in the cell line only.
In short, cytogenetic investigations performed on two of the WM cell lines show their close genetic relationship with their donor tumor cells. It is to be hoped that further molecular cytogenetic work will reveal the putative gene targets of at least some of the key rearrangements in MWCL-1 and RPCI-WM1.
A point often neglected is the stability, or otherwise, of cancer cell lines. Cytogenetic analysis of the WM cell line triad shows these to be genomically stable, the sometimes fugitive presence of multiple clones at immortalization explaining the few discrepancies observed between donor cells and cell lines.
There appears to be a strong familial component in WM, manifesting as a 10-fold increased risk of developing this or a related B-cell neoplasm among first-degree relatives. Although the putative underlying genetic factor(s) has/have yet to be identified, a candidate in the form of hyperphosphorylated paratarg-7 (STOML2) has been described recently [Citation11]. The protein encoded by this gene is ubiquitously expressed and its hyperphosphorylated form inherited as a dominant trait.
Interestingly, a mutation of another gene, MYD88, leading to a leucine to proline substitution in codon 265 (L265P), was found in 90% of cases of WM and also in the three WM cell lines BCWM-1, MWCL-1 and RPCI-WM1 [Citation12,Citation13] (). If verified, this MYD88 mutation may provide a biomarker for differentiating WM from other related entities and may even serve as a potential therapeutic target [Citation1]. However, the MYD88 L265P mutation has also been reported in diffuse large B-cell lymphomas [Citation13] and in cases of chronic lymphocytic leukemia [Citation14], the latter a disease that, in gene expression profiling studies, clusters nearer to WM than to multiple myeloma [Citation15]. The presence of MYD88 L265P in all three cell lines provides molecular support for their right to serve as experimental disease models, even the weakest candidate BCWM-1, where it bolsters an otherwise tenuous claim to represent WM.
To sum up, while recent technological advances have provided new insights into the genetic basis of WM, continued advances may be hampered by the dearth of reliable cell line models. The three reviewed here, BCWM-1, MWCL-1 and RPCI-WM1, are stepwise advances toward this goal. While RPCI-WM1 is our best shot so far, in the systems biology era, as with other enigmatic cancers, additional reliable WM models are needed both to investigate the pathology of WM and to test new treatment options [Citation16].
Supplementary Material
Download Zip (2.2 MB)Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Braggio E, Philipsborn C, Novak A, . Molecular pathogenesis of Waldenström's macroglobulinemia. Haematologica 2012;97: 1281–1290.
- MacLeod RA, Nagel S, Scherr M, . Human leukemia and lymphoma cell lines as models and resources. Curr Med Chem 2008;15:339–359.
- Drexler HG. Guide to leukemia-lymphoma cell lines, 2nd ed. Braunschweig: DSMZ; 2010.
- Finerty S, Rickinson AB, Epstein MA, . Interaction of Epstein-Barr virus with leukaemic B cells in vitro. II. Cell line establishment from prolymphocytic leukaemia and from Waldenström's macroglobulinemia. Int J Cancer 1982;30:1–7.
- Drexler HG, MacLeod RAF.Malignant hematopoietic cell lines: in vitro models for the study of Waldenström's macroglobulinemia. Leuk Res 2008;32:1669–1673.
- Al-Katib A, Mohammad R, Hamdan M, . Propagation of Waldenström's macroglobulinemia cells in vitro and in severe combined immune deficient mice: utility as a preclinical drug screening model. Blood 1993;81:3034–3042.
- Ditzel Santos D, Ho AW, Tournilhac O, . Establishment of BCWM.1 cell line for Waldenström's macroglobulinemia with productive in vivo engraftment in SCID-Hu mice. Exp Hematol 2007; 35:1366–1375.
- Hodge LS, Novak AJ, Grote DM, . Establishment and characterization of a novel Waldenström macroglobulinemia cell line, MWCL-1. Blood 2011;117:e190–e197.
- Chitta KS, Paulus A, Allawadhi S, . Development and characterization of a novel human Waldenström macroglobulinemia cell line: RPCI-WM1, Roswell Park Cancer Institute – Waldenström Macroglobulinemia 1. Leuk Lymphoma 2012;53;XXX–XXX.
- Bergsagel PL, Kuehl MW.WSU-WM and BCWM.1 should not be assumed to represent Waldenström macroglobulinemia cell lines. Blood 2008;112:917.
- Grass S, Preuss KD, Thome S, . Paraproteins of familial MGUS/multiple myeloma target family-typical antigens: hyperphosphorylation of autoantigens is a consistent finding in familial and sporadic MGUS/MM. Blood 2011;118:635–637.
- Treon SP, Xu L, Zhou Y, . Whole genome sequencing reveals a widely expressed mutation (MYD88 L265P) with oncogenic activity in Waldenstrom's macroglobulinemia. Blood 2011;118 (Suppl. 1): Abstract 300.
- Ngo VN, Young RM, Schmitz R, . Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115–119.
- Puente XS, Pionyol M, Quesada V, . Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011;475:101–105.
- Chng WJ, Schop RF, Price-Troska T, . Gene-expression profiling of Waldenström macrogobulinemia reveals a phenotype more similar to chronic lymphocytic leukemia than multiple myeloma. Blood 2006;108:2755–2763.
- Macleod RA, Dirks WG, Drexler HG.Where have all the cell lines gone?Int J Cancer 2012 Jul 31. [Epub ahead of print]