In this issue of Leukemia and Lymphoma, Auner and colleagues report the effectiveness of a second autologous stem cell transplant at relapse for patients with multiple myeloma who previously underwent a stem cell transplant as part of planned induction therapy. These 83 patients had a median overall survival of 31.5 months. Chemosensitivity, as has been reported for transplant in large cell lymphoma, predicts a superior outcome, and the progression-free survival after the first autologous stem cell transplant also predicted a favorable outcome. Moreover, in this highly diverse population, health disparities were not recognized [Citation1].
One important aspect of this approach was that these patients had the requisite stem cells collected prior to the first transplant, and the stem cells were then cryopreserved for the possibility that they would be used as part of salvage chemotherapy. This approach incurred the costs of cryopreservation for up to 93 months, but the ongoing costs of maintaining the stem cells are less than the cost of a single course of novel agent-based chemotherapy.
Others have also reported favorable outcomes with durable responses using a salvage second autologous (auto) transplant. Investigators at the M. D. Anderson Cancer Center reported 44 patients who were transplanted, with a treatment-related mortality of 2% and a median overall survival from the salvage auto transplant of 31.7 months, and, as reported in the current paper, time to progression after the first auto stem cell transplant was predictive of outcome following a second course of high-dose therapy. In the M. D. Anderson study, unlike the Auner study, black patients had a shorter survival [Citation2]. A Swedish team reported 66 patients who received melphalan conditioning at 100 mg/m2, achieving a 62% response rate, no treatment-related mortality and a median overall survival of 24 months. The Swedish team concluded that a durable response after the first stem cell transplant justified consideration of a second stem cell transplant at the time of progression [Citation3].
It is common for transplant centers to collect sufficient stem cells for more than one transplant. However, often, the two transplants are done in tandem, based on the randomized phase III study that demonstrated a survival advantage for tandem auto transplant [Citation4]. Centers in the United States are moving away from the tandem approach. When sufficient stem cells are collected for two transplants, one is done as part of planned induction, and the other collection is cryopreserved for salvage. All centers are not willing to cryopreserve stem cells for protracted periods of time, and sometimes third-party payers in the United States refuse to carry the costs of indefinite cryopreservation. With the increasing costs of new anti-myeloma therapy, this may be a short-sighted approach.
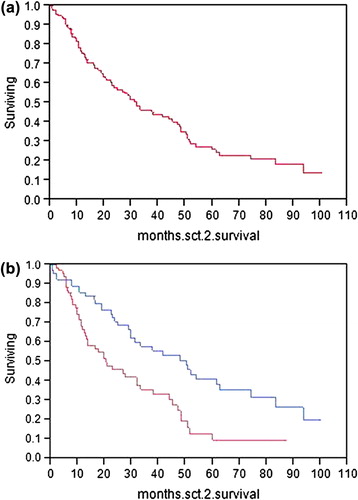
We have recently reported our experience with a salvage auto stem cell transplant in 98 patients. The median overall survival was 33 months, and salvage was most effective when the first transplant had a time to progression of at least 12 months [Citation5]. For the purpose of this Commentary, we update our results. We have now performed 143 second auto stem cell transplants, and the time between the transplants ranges from 210 to 4246 days (median 981 days). The time between documentation of relapse and the second stem cell transplant ranges from 10 to 2933 days (median 482 days). The 143 patients had a response duration following their first transplant of 3.6–123.3 months (median 25.5 months). In this cohort, second transplant all-cause mortality at day 100 was 5%, which is substantially higher than at first auto transplant (0.5%). The mortality risk is part of the discussion before a patient selects this option. Median survival following the second auto transplant was 31.7 months, virtually identical to the Auner report. When our patients were analyzed based on the median duration of response after the first stem cell transplant of greater than or less than 25.5 months, the 71 patients whose response was > 25.5 months had a median overall survival following their second transplant of 50.1 months. Those with a shorter first post-transplant response had a median survival, following the second transplant, of 20.4 months (p = 0.001) [ and ].
Figure 1. (a) Overall survival from day 0 of second stem cell transplant. (b) Overall survival from day 0 of second stem cell transplant based on whether response duration to first transplant was > 25.5 months (upper line) or < 25.5 months (lower line).
In addition to being an effective regimen, transplant can be less expensive than novel agents used for salvage. Across the globe, where access to novel agents may be restricted by either lack of insurance or lack of government approval, the less resource-intensive second stem cell transplant is available in most countries. The fact that cells are already cryopreserved further reduces costs. Attempts to reduce the resource utilization associated with autologous stem cell transplant have included overnight refrigeration of the stem cells rather than cryopreservation, which eliminates the need for programmed freezing [Citation6]. Conducting autografting on an entirely outpatient basis [Citation7] and the use of short conditioning schedules delivered as outpatients [Citation8] further reduce resource consumption.
There is a lack of prospective randomized trials after first relapse in the setting of prior autologous stem cell transplant. A recent study randomizing relapsed patients to bortezomib, thalidomide, dexamethasone versus thalidomide, dexamethasone reported improved time to progression and response duration in the triplet arm. Unfortunately, overall survival was not significantly longer and neurotoxicity was greater in the triplet arm. Progression-free survival was 18.3 months with the triplet, and 13.6 months with the doublet [Citation9]. In the Auner study reported in this issue, progression-free survival following stem cell transplant was 15.5 months [Citation1].
The importance of the current study lies in bringing to the attention of oncologists in practice the possibility of a second autologous stem cell transplant for their patients as one alternative to new, highly effective drugs for the management of multiple myeloma. Auner and colleagues clearly demonstrate that, for a selected population who maintain chemosensitivity and have a durable response to their first transplant, a second transplant is feasible, less resource-intensive and capable of producing prolonged survival. Transplant centers that see patients with multiple myeloma should strongly consider the collection and cryopreservation of a double aliquot of stem cells with the plan to reserve half of the collection for salvage use. At Mayo Clinic, the median age of patients who had stem cells collected and re-transplanted was 54 years, but ranged as high as 72 years. Our current policy is to collect a minimum of 6 × 108 CD34 cells/kg to allow transplant, with a minimum of 3 × 108 in order to permit rapid engraftment following myeloablative therapy.
Supplementary Material
Download Zip (978.1 KB)Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Auner HW, Szydlo R, Rone A, et al. Salvage autologous stem cell transplant for multiple myeloma relapsing or progressing after up-front autologous transplant. Leuk Lymphoma 2013;54:2200–2204.
- Shah N, Ahmed F, Bashir Q, et al. Durable remission with salvage second autotransplants in patients with multiple myeloma. Cancer 2012;118:3549–3555.
- Blimark C, Veskovski L, Westin J, et al. Melphalan 100 mg/m2 with stem cell support as first relapse treatment is safe and effective for myeloma patients with long remission after autologous stem cell transplantation. Euro J Haematol 2011;87:117–122.
- Harousseau JL, Attal M. The role of stem cell transplantation in multiple myeloma. Blood Rev 2002;16:245–253.
- Gonsalves WI, Gertz MA, Lacy MQ, et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant 2012 Sep 24. [Epub ahead of print]
- Donmez A, Cagirgan S, Saydam G, et al. Overnight refrigerator storage of autologous peripheral progenitor stem cells without cryopreservation. Transfus Apher Sci 2007;36:313–319.
- Lopez-Otero A, Ruiz-Delgado GJ, Ruiz-Arguelles GJ. A simplified method for stem cell autografting in multiple myeloma: a single institution experience. Bone Marrow Transplant 2009;44:715–719.
- Ruiz-Delgado GJ, Ruiz-Arguelles GJ. A Mexican way to cope with stem cell grafting. Hematology 2012;17(Suppl. 1):S195–S197.
- Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2012;30:2475–2482.