Abstract
Omacetaxine mepesuccinate (Synribo®) is an inhibitor of protein synthesis indicated for the treatment of patients with chronic- or accelerated-phase chronic myeloid leukemia (CML) with resistance and/or intolerance to two or more tyrosine kinase inhibitors. Myelosuppression is the most common and clinically significant toxicity experienced by patients treated with omacetaxine. Here, we further examine the patterns of hematologic toxicity observed in clinical trials and describe the approach to management as well as resolution of events. Omacetaxine-related myelosuppression typically occurs more frequently during induction cycles. In general, the myelosuppression observed with omacetaxine treatment is manageable and reversible, and long-term administration is feasible. Careful monitoring, dose delays and reduction in administration days, and appropriate supportive care are critical for successful management of hematologic toxicity. Concerns regarding myelosuppression, observed with many cancer treatments, should not prevent eligible patients from receiving omacetaxine, particularly CML patients with unsatisfactory responses to multiple lines of prior treatment.
Introduction
Chronic myeloid leukemia (CML) is distinguished by the presence of the BCR-ABL hybrid oncogene and expression of Bcr-Abl oncoprotein, which mediates the activation of signaling pathways leading to leukemogenesis [Citation1]. Tyrosine kinase inhibitors (TKIs) that target Bcr-Abl represent the mainstay of CML treatment; however, for patients who develop resistance or intolerance to multiple TKIs, effective therapy remains a major unmet need.
Omacetaxine mepesuccinate (omacetaxine – a semi-synthetic form of homoharringtonine) is indicated for adult patients with chronic-phase CML (CML-CP) or accelerated-phase CML (CML-AP) with resistance and/or intolerance to two or more TKIs [Citation2]. Omacetaxine is a transient inhibitor of protein synthesis and most profoundly impacts levels of short-lived proteins [Citation3–6]. In preclinical studies, omacetaxine reduced levels of several oncoproteins, including Bcr-Abl and Mcl-1, and induced apoptosis in leukemic cells [Citation7–10]. In clinical studies, omacetaxine produced durable hematologic and cytogenetic responses in patients with CML-CP and CML-AP, regardless of mutational status [Citation11–15].
Among patients with CML-CP or CML-AP with resistance and/or intolerance to two or more TKIs, myelosuppression is the primary hematologic toxicity observed with omacetaxine treatment; in clinical studies, 85% of patients with CML-CP experienced grade ≥3 thrombocytopenia at any time during treatment, 81% experienced grade ≥3 neutropenia, and 62% experienced grade ≥3 anemia [Citation2]. The majority of patients who received more than one cycle of treatment required at least one treatment delay, mainly due to myelosuppression. In spite of this toxicity, myelosuppression is typically manageable with a combination of treatment delays, reduced dosing days per cycle, supportive care, and patient education that allows for continuation of treatment. With the exception of myelosuppression-related events such as infection, grade 3/4 nonhematologic adverse events (AEs) were infrequent; most notably, grade 3 or 4 hyperglycemia was reported in 11% of patients [Citation2,Citation14,Citation15]. Here, we further examine the hematologic toxicity in the safety population composed of all CML-CP and CML-AP patients with any prior TKI treatment and who received omacetaxine in the two omacetaxine clinical trials, and describe the monitoring, management, and outcome of these events to prescribing practitioners and healthcare professionals.
Methods
Patients
In a post hoc analysis, data were pooled from two multicenter, open-label, single-arm phase 2 studies (CML-202 and CML-203) in CML patients previously treated with TKIs [Citation11,Citation13,Citation16]; data were included from all patients with CML-CP and CML-AP who participated in the two trials. Safety data were also included for four additional patients with CML-AP who were enrolled in a pilot study (CML-04.2/04.3) and were refractory to or had relapsed on imatinib (at a minimum).
Treatment
Patients were treated with subcutaneous omacetaxine at 1.25 mg/m2 twice daily for up to 14 consecutive days per cycle. In the clinical trials (CML-202 and CML-203), patients received treatment for 14 consecutive days during induction (days 1–14 every 28 days until hematologic response), followed by maintenance omacetaxine given at 1.25 mg/m2 twice daily for 7 consecutive days every 28 days. If clinically indicated, hydroxyurea could be administered immediately before and during the first two cycles of treatment in patients with rapidly proliferating disease.
The omacetaxine dosing schedule was modified in the event of hematologic toxicity. For patients with grade 4 neutropenia (absolute neutrophil count [ANC] ≤ 0.5 × 109/L and/or grade 3 thrombocytopenia (platelet count ≤50 × 109/L), induction dosing was delayed until recovery to ANC ≥ 1.0 × 109/L and platelet count ≥50 × 109/L. Upon recovery, the number of dosing days per cycle was reduced by 2 days. Further reduction in the number of administration days per cycle (in decrements of 2 days) was allowed for patients with recurrent myelosuppression. Additionally, omacetaxine could be discontinued in patients with moderate to severe AEs considered possibly or probably related to treatment based on clinical investigator assessment. Administration of hematopoietic growth factors was allowed in the event of febrile neutropenia and use of erythropoietin or darbepoetin alfa was permitted for treatment of anemia. Patients did not receive prophylactic antibiotics.
Assessments
The objective of this post hoc analysis was to characterize the incidence and duration of myelosuppression as well as its management in patients with CML-CP and CML-AP receiving omacetaxine therapy. In each of the clinical trials included, patients were assessed for safety and tolerability every 7 days during induction cycles and every 14 days during maintenance. The severity of myelosuppression events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Hematologic nadirs were calculated as mean days to the lowest value in a cycle. To evaluate recovery from nadir, recovery thresholds were defined as platelets ≥50 × 109/L, neutrophils ≥ 0.5 × 109/L, and hemoglobin ≥80 g/L. In patients with nadirs below the threshold, time to recovery was defined as the number of days from nadir to recovery above threshold; patients without recovery were censored at the start of the next treatment cycle, or treatment discontinuation, whichever occurred first.
For patients with CML-CP, complete hematologic response (CHR) was defined as white blood cell count <10 × 109/L, platelets <450 × 109/L, myelocytes + metamyelocytes <5% in blood, no blasts or promyelocytes in blood, <20% basophils in peripheral blood, no extramedullary disease, and lasting ≥8 weeks. For CML-AP, CHR was defined as ANC ≥1.5 × 109/L, platelets ≥100 × 109/L, no blood blasts, bone marrow blasts <5%, no extramedullary disease, and lasting ≥4 weeks.
Statistics
Descriptive statistics (number of patients, mean, median, range and standard deviation) were used to summarize continuous variables. For categorical variables, the number and percentage of total were tabulated. Median time to recovery and 95% confidence interval (CI) were obtained using a Kaplan–Meier estimate.
Role of the funding source
The original research was sponsored by ChemGenex Pharmaceuticals Limited, Menlo Park, CA, USA (now a wholly owned subsidiary of Teva Branded Pharmaceutical Products R&D, Inc. Frazer, PA, USA). Teva Branded Pharmaceutical Products R&D provided financial support for medical writing and editorial review in preparation for submission for publication.
Results
Patients
A total of 108 patients with CML-CP and 55 patients with CML-AP were treated with subcutaneous omacetaxine at 1.25 mg/m2 twice daily. Patient demographics and baseline characteristics, including the incidence of myelosuppression at baseline are described in . The median time from CML diagnosis to omacetaxine treatment was 63 months (range 8–234 months) for patients with CML-CP and 91 months (range 20–286 months) for those with CML-AP. Most patients had received two or more prior TKIs; 36 (33%) patients with CML-CP and 24 (44%) with CML-AP demonstrated resistance or intolerance to three TKIs (imatinib, dasatinib, and nilotinib). Twenty-four (22%) CML-CP and 14 (25%) CML-AP patients had received only one prior TKI (imatinib); these patients had a history of T315I.
Table I. Patient baseline characteristics and demographics.
Exposure
A median of two (range 1–6) omacetaxine induction cycles were administered in both the CML-CP and CML-AP groups []. Patients with CML-CP received a median of eight (range 1–55) maintenance cycles and patients with CML-AP received a median of four (range 1–24) maintenance treatment cycles.
Table II. Exposure and dose delays.
Ten patients (six CML-CP, four CML-AP; 8.6%) discontinued treatment due to hematologic toxicity: thrombocytopenia (two CML-CP, three CML-AP), pancytopenia (two CML-CP, one CML-AP), and aplasia and bone marrow necrosis (one CML-CP each). Most of these discontinuations occurred during early treatment cycles (cycle 1: n = 7; cycle 2: n = 4; cycle 3: n = 1; cycle 7: n = 2). In addition, one CML-CP patient and two CML-AP patients discontinued treatment due to infection.
Incidence of myelosuppression
The most frequent hematologic events were thrombocytopenia and anemia, and most events were grade ≥ 3 in severity []. The incidence of grade 3 or 4 hematologic toxicity (anemia, neutropenia, and thrombocytopenia) was highest within the first five cycles of treatment in patients with CML-CP []. Starting counts and nadirs for platelets and neutrophils in CML-CP patients were lowest in cycles 3–6 []. Platelet values recovered in a median of 13–28 days, neutrophil values 6–8 days, and hemoglobin values 4–13 days, during the first six cycles []. For patients with CML-AP, median times to recovery of platelets, neutrophils, and hemoglobin were 2–7 days, 7–8 days, and 7–14 days, respectively, during the first four cycles. Rates of hematologic toxicity tended to be similar among CML-AP patients with or without major hematologic response (MHR; n = 16 and 35, respectively), and were slightly higher in CML-CP patients with major cytogenetic response (MCyR; n = 24) or MHR (n = 79) vs. non-responders []. The incidence of myelosuppression was not higher in more heavily pretreated patients; in cycle 1 of treatment, for example, rates of grade 3/4 myelosuppression were 67%, 63%, and 47% in CP patients and 80%, 71%, and 79% in AP patients with 1, 2 or ≥ 3 prior TKIs, respectively.
Figure 1. Proportion of chronic-phase patients (A) and accelerated-phase patients (B) experiencing grade 3/4 hematologic toxicity (anemia, neutropenia, and thrombocytopenia) by cycle.
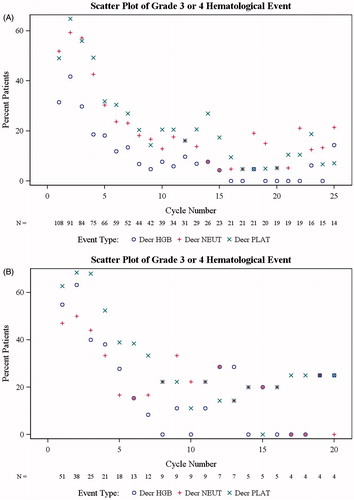
Table III. Hematologic treatment-emergent adverse events occurring in ≥5% of patients with CML-CP and CML-AP.
Table IV. Hematologic parameters by cycle (1–10) in patients with CML-CP: starting and nadirTable Footnote* values, time to nadir, and time to recoveryTable Footnote†,Table Footnote‡.
Table V. Rates of myelosuppression in CML-CP and CML-AP patients with and without major hematologic response (MHR), and major cytogenetic response (MCyR) to omacetaxine.
Myelosuppression-related events
Thirty-six CML-CP and 17 CML-AP patients experienced at least one serious myelosuppressive AE. Serious myelosuppression-related events included thrombocytopenia (10% CML-CP, 9% CML-AP patients), febrile neutropenia (6% CML-CP, 18% CML-AP), and anemia (7% CML-AP). In patients with CML-CP and CML-AP, eight and six events, respectively, of grade 3 or 4 hemorrhage occurred. Grade ≥ 3 bleeding events occurring in more than one patient included fatal cerebral hemorrhage in CML-CP and CML-AP patients (two each), and gastrointestinal hemorrhage in three CML-CP patients. Infection was reported in 49% and 56% of patients with CML-CP and CML-AP, respectively. Eleven grade 3/4 infections considered to be related to omacetaxine occurred in six CP patients (four pneumonias and one [each] extremity abscess, fungal infection, influenza, injection site abscess, sepsis, and urinary tract infection) and one AP patient (one pneumonia).
Five patients (5%) died from events related to myelosuppression that were considered to be related to omacetaxine by the investigator. The deaths of three CML-CP patients were attributed to sepsis (n = 2) and pancytopenia (n = 1). In two CML-AP patients, death was related to pancytopenia (n = 1) and febrile neutropenia (n = 1).
Monitoring and management
Blood counts were typically obtained on a weekly basis during induction cycles; over 75% of patients in both disease phases underwent three or more scheduled blood draws per cycle in the first two cycles. During maintenance, monitoring was performed less frequently in many patients. Approximately 55% of patients with CML-CP underwent two or fewer scheduled blood draws per cycle after cycle 2. The proportion of patients with CML-AP who underwent three or more blood draws decreased from over 80% in cycles 1 and 2 to 56% and 67% in cycles 3 and 4, respectively. After cycle 4, 50% or more patients with CML-AP received two or fewer blood draws per cycle.
In general, hematologic toxicity was managed by treatment delays, reduction in the number of dosing days per cycle, and supportive care. The majority of CML-CP patients (87%) required at least one cycle delay, with a median of three cycle delays per patient (range 0–39 cycles) []. In CML-CP patients who received at least two cycles, the need for cycle delay days was greatest for cycle 2 (54% of patients experienced a median delay of 17 days [range 2–109]), cycle 3 (67%; median delay 25 days [range 2–184]), and cycle 4 (71%; median delay 13 days [range 1–81]). Fewer CML-AP patients required cycle delays (68%), with a median of one cycle delay per patient (range 0–19 cycles). In AP patients, the cycle delay days were greatest for cycle 3 (44% of patients experienced a median delay of 31 days [range 4–75]), cycle 4 (67%; median delay 14 days [range 1–74]), and cycle 5 (44%; median delay 17 days [range 3–46]). In both CP and AP patients, thrombocytopenia was the most frequent reason for cycle delay, followed by neutropenia and pancytopenia. Approximately half of CML-CP (56%) and CML-AP (47%) patients required a reduction in the number of dosing days per cycle (for any reason) during induction; during maintenance cycles, 75% and 64%, respectively, required a reduced number of dosing days [].
The majority of patients in both disease phases received concomitant hematologic supportive care, consisting of transfusions and growth factors. Red blood cell transfusions were administered in 49% and 43% of CML-CP and CML-AP patients, respectively; platelets were administered in 56% and 51%. The median number of transfusions administered in CML-CP patients (among patients who received transfusions) was four (range 1–19) for platelets and three (range 1–55) for red blood cells; in CML-AP patients the median number of transfusions per patient was three (range 1–40) for platelets and four (range 1–18) for red blood cells. In the CML-CP population, use of transfusions was highest in patients with grade 3/4 myelosuppression and within the first five cycles of treatment []; a similar trend was observed in CML-AP patients (data not shown).
Figure 2. Transfusions (A) and growth factor use (B) by treatment cycle in CML-CP patients (n = 108) with or without grade 3/4 myelosuppression. Percentages are based on the number of patients in each category. Grade 3 or 4 myelosuppression is defined as any grade 3 or 4 neutropenia, thrombocytopenia, and/or anemia detected by laboratory tests. Patients are counted once in a given cycle if multiple myelosuppression events occurred.
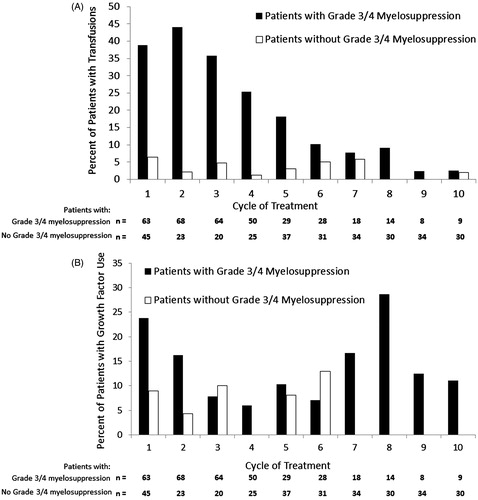
Twenty-five percent of patients with CML-CP and 10% with CML-AP received concomitant granulocyte colony-stimulating factors. Erythropoiesis-stimulating agents were administered to 22% of CML-CP and 14% of CML-AP patients. The incidence of growth factor use was generally similar in early and later cycles [].
In patients with CML-CP, the proportion of patients receiving transfusions decreased in later cycles, from 46–27% in cycles 1–4, 21–14% in cycles 5–7, and <10% in cycles 8–10. In patients with CML-AP, the proportion of patients receiving transfusions decreased in later cycles, from 63–24% in cycles 1–4, 22–8% in cycles 5–7, and approximately 11% in cycles 8–10. Rates of transfusions were slightly higher in patients with dose delays (vs. those without), and in CML-CP patients with dose reductions during induction (vs. those without) []. Having a CHR at the start of therapy with omacetaxine did not influence the need for transfusions: 76% of CML-CP patients (73% of CML-AP patients) with CHR at baseline were transfused vs. 73% of CML-CP patients (83% of CML-AP patients) without CHR at baseline [].
Table VI. Transfusion in CML-CP and CML-AP patients with or without dose delays.
Table VII. Transfusions in patients with CML-CP and CML-AP by complete hematologic response (CHR) at baseline (top), and hematologic responseTable Footnote* to omacetaxine (bottom).
Resolution of events
Among patients with CML-CP, most grade 3/4 thrombocytopenia (91%), neutropenia (96%), and anemia (91%) resolved with supportive care []. Notably, neutrophil and hemoglobin levels reached recovery threshold levels (neutrophils ≥ 0.5 × 109/L, and hemoglobin ≥ 80 g/L) at approximately 2 weeks, while recovery of platelet levels (platelets ≥ 50 × 109/L) occurred at approximately 3 weeks. Similarly, among patients with CML-AP, nearly all grade 3/4 thrombocytopenia (88%), neutropenia (100%), and anemia (93%) resolved, but recovery of neutrophils (approximately 2 weeks) lagged behind platelet and hemoglobin recovery (approximately 1 week) [].
Table VIII. Reversibility of hematologic treatment-emergent adverse events (by preferred term) occurring in ≥10% of patients with CML-CP or CML-AP.
Discussion
Myelosuppression is among the most clinically important toxicities associated with the treatment of CML patients demonstrating treatment resistance or intolerance. For instance, among patients treated with the TKI bosutinib in the third-line setting, 41% experienced grade 3 or 4 hematologic toxicity (26% thrombocytopenia, 15% neutropenia, 7% anemia), leading to treatment interruption in 46% of affected patients and dose reduction in 32% [Citation17]. Among patients treated with ponatinib (over 90% of whom had received at least two prior TKIs), 37% experienced any grade thrombocytopenia, 19% experienced neutropenia, and 9% experienced anemia; the majority of events were grade 3 or 4 [Citation18].
Myelosuppression occurs in the majority of omacetaxine-treated patients and at a slightly higher rate among CML-CP patients with cytogenetic or hematologic response vs. non-responders []. Despite this significant toxicity, omacetaxine treatment is feasible in many CML-CP and CML-AP patients with CML, inducing hematologic and cytogenetic responses in some patients who are able to continue treatment. In a post hoc analysis of the efficacy population from the two phase 2 studies, 11 of 50 patients (22%) with CML-CP who completed more than three cycles of omacetaxine achieved MCyR; three of these patients maintained a response for at least 12 months [Citation15]. Rate of CHR was 94% in this subgroup and the response was durable (≥12 months) in 26% [Citation15]. Progression-free survival (PFS) and overall survival (OS) of 9.9 months and 49.3 months, respectively, were associated with patients who received more than three cycles of treatment. In the efficacy population (n = 76), PFS and OS were 9.6 months and 40.3 months, respectively. Among 14 patients with CML-AP who received more than three cycles of omacetaxine treatment, MHR was achieved in four patients (29%). PFS and OS were 7 months and 24.6 months, respectively, in patients who received more than three cycles of treatment, and 3.6 months and 14.3 months in the overall population (n = 35) [Citation15]. While this subanalysis showed that the majority of responses in patients with CML-CP occurred within the first three cycles, two patients achieved MCyR after cycle 3 and complete cytogenetic response may occur as late as 8.5 months [Citation15]. Thus, effective management of myelosuppression, including withholding treatment and reducing the number of days dosed per cycle, and early use of growth factors and transfusions, is essential to allow continuation of treatment; this is particularly important during the early induction cycles (cycles 1–4), in which the rates of grade 3/4 hematologic toxicities were highest.
Delay of treatment until recovery of blood counts is imperative in patients with myelosuppression; once recovery has occurred, a reduction in the number of dosing days in subsequent cycles will often allow continued treatment. Both dose delays and reductions are encouraged if warranted at any point throughout the treatment period. In addition, avoidance of anticoagulants, aspirin, and nonsteroidal anti-inflammatory drugs when the platelet count is <50,000/μL is necessary as this may increase the risk of bleeding. In this clinical trial experience, the need for supportive care, particularly transfusions, may be highest in early cycles (cycles 1–4), with fewer than 10% of patients requiring transfusions in maintenance cycles.
Careful monitoring of patients for myelosuppression is necessary. Complete blood counts should be performed weekly during induction and initial maintenance cycles and every 2 weeks during later maintenance cycles, as clinically indicated. Patients with treatment-related neutropenia should be watched for signs of infection (e.g. fever) and be instructed to contact their physician immediately in case of signs or symptoms of infection. Patients should be educated and given home training materials by their treating physician or oncology nurse regarding important signs and symptoms suggestive of hemorrhage (unusual bleeding, easy bruising, or blood in urine or stool; confusion, slurred speech, or altered vision) or to bring to the physician’s attention if they plan to have any dental or surgical procedures [Citation19,Citation20]. Equally important is to be aware of any concomitant medications that may increase the risk of bleeding. Working closely with oncology providers such as the oncology nurse can minimize patient- and environment-related sources of infection and increase awareness of signs and symptoms of bleeding during home administration of omacetaxine or between visits to the clinic.
In these trials, few patients required discontinuation of treatment due to hematologic toxicity, and persistent toxicity was rare, with most events resolved within 1–2 weeks during the early treatment cycles. Hematologic toxicity was not cumulative with continued treatment; rather, rates of grade ≥3 hematologic toxicities were reduced in later cycles, congruent with a switch to maintenance dosing. However, toxicities experienced in early cycles led to study discontinuation in some patients, thus possibly introducing a bias in patient selection. Future studies exploring alternative treatment schedules are warranted to examine whether myelosuppression (and all resulting sequelae) can be minimized.
Conclusions
Myelosuppression should be anticipated in patients with CML who are treated with omacetaxine. However, concerns regarding toxicity should not prevent omacetaxine administration as some patients could experience durable cytogenetic or hematologic responses with continued treatment. In heavily pretreated patients, frequent monitoring of laboratory parameters, use of adequate dose modifications, adequate patient training for signs and symptoms, and appropriate medical support are critical for the successful management of hematologic toxicity and continued treatment. For patients initiating omacetaxine, provision of informational pamphlets and maintaining close communication with oncology care providers (e.g. follow-up by phone) are important, particularly for patients who live far from the treatment center.
GLAL-2015-0230-File016.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File015.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File014.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File013.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File012.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File011.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File010.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File009.pdf
Download PDF (1 MB)GLAL-2015-0230-File008.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File007.pdf
Download PDF (1.2 MB)GLAL-2015-0230-File006.pdf
Download PDF (1.2 MB)Acknowledgements
The authors thank the investigators in the Omacetaxine-202 and -203 Study Groups, the patients and their families, as well as the clinical study staff for their dedication and support. The original research was sponsored by ChemGenex Pharmaceuticals Limited, Menlo Park, CA (now a wholly owned subsidiary of Teva Branded Pharmaceutical Products R&D, Inc. Frazer, PA, USA). Financial support for medical writing assistance from Jin Tomshine, PhD, of Powered 4 Significance LLC was provided by Teva Branded Pharmaceutical Products R&D.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Ahmed W, Van Etten RA. Signal transduction in the chronic leukemias: implications for targeted therapies. Curr Hematol Malig Rep 2013; 8: 71–80.
- Synribo® (omacetaxine mepesuccinate) [prescribing information]. North Wales, PA: Teva Pharmaceuticals USA, Inc.; 2012.
- Al Ustwani O, Griffiths EA, Wang ES, et al. Omacetaxine mepesuccinate in chronic myeloid leukemia. Expert Opin Pharmacother 2014; 15: 2397–2405.
- Quintas-Cardama A, Cortes JE, O’Brien S, et al. Dasatinib early intervention after cytogenetic or hematologic resistance to imatinib in patients with chronic myeloid leukemia. Cancer 2009; 115: 2912–2921.
- Tujebajeva RM, Graifer DM, Karpova GG, et al. Alkaloid homoharringtonine inhibits polypeptide chain elongation on human ribosomes on the step of peptide bond formation. FEBS Lett 1989; 257: 254–256.
- Wetzler M, Segal D. Omacetaxine as an anticancer therapeutic: what is old is new again. Curr Pharm Des 2011; 17: 59–64.
- Allan EK, Holyoake TL, Craig AR, et al. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia 2011; 25: 985–994.
- Chen R, Gandhi V, Plunkett W. A sequential blockade strategy for the design of combination therapies to overcome oncogene addiction in chronic myelogenous leukemia. Cancer Res 2006; 66: 10959–10966.
- Chen R, Guo L, Chen Y, et al. Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia. Blood 2011; 117: 156–164.
- Chen Y, Hu Y, Michaels S, et al. Inhibitory effects of omacetaxine on leukemic stem cells and BCR-ABL-induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice. Leukemia 2009; 23: 1446–1454.
- Cortes J, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood 2012; 120: 2573–2580.
- Cortes J, Digumarti R, Parikh PM, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate for chronic-phase chronic myeloid leukemia patients resistant to or intolerant of tyrosine kinase inhibitors. Am J Hematol 2013; 88: 350–354.
- Khoury HJ, Cortes J, Baccarani M, et al. Omacetaxine mepesuccinate in patients with advanced chronic myeloid leukemia with resistance or intolerance to tyrosine kinase inhibitors. Leuk Lymphoma 2015;56:120–127.
- Nicolini FE, Khoury HJ, Akard L, et al. Omacetaxine mepesuccinate for patients with accelerated phase chronic myeloid leukemia with resistance or intolerance to two or more tyrosine kinase inhibitors. Haematologica 2013; 98: e78–79.
- Cortes JE, Kantarjian HM, Rea D, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic- or accelerated-phase chronic myeloid leukemia: results with 24 months of follow-up. Cancer 2015; 121: 1637–1644.
- Cortes JE, Nicolini FE, Wetzler M, et al. Subcutaneous omacetaxine mepesuccinate in patients with chronic-phase chronic myeloid leukemia previously treated with 2 or more tyrosine kinase inhibitors including imatinib. Clin Lymphoma Myeloma Leuk 2013; 13: 584–591.
- Kantarjian HM, Cortes JE, Kim DW, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood 2014; 123: 1309–1318.
- Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369: 1783–1796.
- Rostad ME. Management of myelosuppression in the patient with cancer. Oncol Nurs Forum 1990; 17: 4–8.
- Damron B, et al. Platelet transfusions. In: Eaton L, Tipton J, eds. Putting evidence into practice: improving oncology patient outcomes: Oncol Nursing Soc 2009:257–265.
