Abstract
The aim of this study was to evaluate the localization of collagen modifying enzymes (CMEs) on fibroblastic reticular cells (FRCs) and follicular dendritic cells (FDCs) in non-neoplastic lymphoid tissues and various malignant lymphomas. The expression of prolyl 4-hydroxylase 1 (P4H1), lysyl hydroxylase 3 (LH3), and protein disulfide isomerase (PDI) was frequently observed on FRCs and FDCs in the germinal center (GC), except for the mantle zone. The expression of CMEs was lower in most lymphomas than in their respective postulated normal counterparts. The ratio of transglutaminase II+ FRCs/CD35+ FDCs was also lower in follicular lymphomas (FL) than in other lymphomas. The mRNAs of some CMEs (P4H1, prolyl 4-hydroxylase 3, LH3, and heat shock protein 47) were confirmed in almost all lymphomas. These results indicate that lymphoma cell proliferation suppresses/decreases the number of CMEs expressing FRCs and FDCs in most lymphomas.
Introduction
Two types of lymphoid stromal cells, fibroblastic reticular cells (FRCs) and follicular dendritic cells (FDCs), support the three-dimensional framework of paracortex/T-cell area and lymphoid follicles, respectively. Human FRCs support cell migration including CCR7+ T-cells, and produce/secrete and ensheath collagen-rich reticular fibers composed of collagens I, III and IV covered by the extracellular matrix (ECM), elastin and tenascin.[Citation1–3] In mice, FRCs expressed transcripts for collagens I, III, IV, V, VI, XIV and XVI.[Citation4] Tenascin-C (TN-C) plays the morphoregulatory role during development and tissue remodeling.[Citation5] Transglutaminase II (TGII) corresponding to FRCs is associated with stabilization of the ECM and cell adhesion.[Citation6,Citation7] FDCs trap and retain immune complexes, present antigens to germinal center (GC) B-cells, select activated B-cells with higher affinity to antigens, and support the differentiation of memory B cells and plasma cells.[Citation8] FDCs can be identified by antibodies such as CD21, CD23, CD35 and S-100 protein,[Citation9] and express collagens I and IV, but not collagen III.[Citation10] These findings suggest FRCs/FDCs is associated with collagen production.
Some collagen modifying enzymes (CMEs) such as prolyl 4-hydroxylase (P4H), prolyl 3-hydroxylase (P3H), lysyl hydroxylase (LH), protein disulfide isomerase (PDI), and heat shock protein 47 (HSP47) were identified in the intracellular endoplasmic reticulum.[Citation11] P4H is an α2β2 tetramer; two catalytic sites are involved in the α-subunit, while the β-subunit is identical to PDI.[Citation12] PDI functions as a disulfide isomerase and chaperone to facilitate the proper folding of nascent proteins.[Citation13] P3H1 was reported to preferentially localize in tissues expressing fibril-forming collagens such as collagens I, II and III.[Citation12] The human LH family consists of three isoenzymes, but does not associate with specific collagen types.[Citation14,Citation15] A previous study showed that HSP47 stabilized the triple helical folding intermediates of procollagen.[Citation16]
P4H is expressed by some FDCs, fibroblasts of the interfollicular area and the capsule of the human tonsils,[Citation17] lysyl hydroxylase 3 (LH3) by reticular cells in the red pulp of the adult mouse spleen,[Citation18] and HSP47 by FRCs in the chicken spleen.[Citation19] However, the types of CMEs that localize in human FRCs/FDCs not only in non-neoplastic lymphoid tissues, but also in malignant lymphomas remain unknown. The aim of this study was to analyze the localization of CMEs in malignant lymphomas and their postulated normal counterparts in non-neoplastic lymphoid tissues, and to estimate the role of FRCs/FDCs in both tissues.
Materials and methods
Tissue specimens
A total of 69 patients diagnosed at Yamagata University Hospital, Yamagata Prefectural Shinjo Hospital, and Nihonkai General Hospital between 1996 and 2013 participated in this study. Biopsied and resected tissue specimens including non-neoplastic lymphoid tissues (n = 10) including lymph nodes and tonsils were obtained from patients with reactive lymphadenopathy and chronic tonsillitis, respectively. Neoplastic tissue specimens were obtained from patients with mature B-cell neoplasms including small lymphocytic lymphoma (SLL) (n = 2), extranodal marginal zone lymphoma of mucosa-associated lymphoid tissues (MALT) (n = 2), nodal marginal zone lymphomas (NMZL) (n = 3), follicular lymphomas (FL) [n = 16; Grade 1 (n = 5), Grade 2 (n = 5) and Grade 3 (n = 6)] except in situ FL,[Citation20] mantle cell lymphomas (MCL) (n = 5), diffuse large B-cell lymphomas not otherwise specified [n = 10; GC B-cell-like subgroup (GCB type DLBCL) (n = 5), non-GC B-cell-like subgroup (non-GCB type DLBCL) (n = 5)], mature T-cell neoplasms including enteropathy-associated T-cell lymphoma (EATL) (n = 1), peripheral T-cell lymphomas (PTCL) not otherwise specified (n = 4), angioimmunoblastic T-cell lymphomas (AITL) (n = 6) and classical Hodgkin lymphomas included nodular sclerosis classical Hodgkin lymphoma (NS-cHL) (n = 5) and mixed cellularity classical Hodgkin lymphoma (MC-cHL) (n = 5). The Research Ethics Committee (H25-102) of Yamagata University Faculty of Medicine, Yamagata, Japan, approved this study. Tissues were fixed in buffered 10% formalin for 6–12 h at room temperature, embedded in paraffin. Tissue specimens of the normal liver (n = 1) and pancreatic cancers (n = 2) were used as positive controls for reverse transcription polymerase chain reaction (RT-PCR) of CMEs.[Citation21–24]
Antibodies, immunohistochemistry and immunofluorescence double stain
Immunohistochemistry was performed as described previously,[Citation25] using antibodies against P4H1 (rabbit IgG, polyclonal, ProteinTech, Chicago, IL), P4H3 (rabbit polyclonal, Sigma-Aldrich, Tokyo, Japan), P3H1 (3C7; mouse IgG2a,κ, Abnova, Taipei, Taiwan), PLOD3 (LH3) (rabbit IgG, polyclonal, ProteinTech, Chicago, IL), P4H, β-subunit (P4HB = PDI) (3-2B12; mouse IgG1,κ, LifeSpan Biosciences, Seattle, WA), HSP47 (rabbit polyclonal, Santa Cruz, Santa Cruz, CA), CD35 (RLB25; mouse IgG2b, Novocastra, Leica Biosystems, Nussloch, Germany), CD35 (EPR6602; rabbit IgG, Epitomics, Burlingame, CA), S-100 protein (rabbit polyclonal, Nichirei, Tokyo, Japan), TGII antibody (CUB7402; mouse IgG1, Acris, Herford, Germany) and TN-C (EPR4219; rabbit IgG, Epitomics, Burlingame, CA). Immunoreactive positive cells in non-neoplastic lymph nodes and tonsils were estimated in four areas including the marginal zone, mantle zone, GC and paracortex/interfollicular area in at least 50 areas of each follicle, identified by CD23 immunostaining (1B12; mouse IgG1κ, Novocastra, Leica Biosystems, Nussloch, Germany).[Citation26] The staining index (SI) was calculated by multiplying the staining intensity and cellularity/proportion scores, described by King et al.[Citation27] (Supplemental Table I). These SIs that obtained in the malignant lymphoma were compared with in their postulated normal counterpart areas respectively, in accordance with WHO Classification fourth edition.[Citation28]
Immunofluorescence double staining using paraffin-embedded tissue sections was performed as described previously.[Citation25,Citation29] After observations, hematoxylin-eosin staining was used as counterstaining. The SIs of the double positive cells of CMEs and FRCs/FDCs markers were calculated as with immunohistochemistry. The staining intensity of double positive cells was scored as 0 (negative) or 1 (positive).
RT-PCR
The paraffin-embedded tissue sections of positive controls, lymph node (n = 1), tonsil (n = 1) and malignant lymphoma (n = 14) were used. RT-PCR was performed as previously described.[Citation30] WaxFree RNA (TrimGen, Sparks, MD) was used to extract mRNA. Primer sequences were 5′-CATGACCCTGAGACTGGAAA-3′ and 5′-GCCAGGCACTCTTAGATACT-3′ for human P4H1,[Citation31] 5′-CCAAACGGCTCTTTTCTCTCA-3′ and 5′-GCCACCTTGCCAACTTGGA-3′ for human P4H3, 5′-CTGGGCCTGGGAGAGGAGTG-3′ and 5′-TCACGTCGTAGCTATCCACAAACAT-3′ for human LH3,[Citation32] 5′-CGCCATGTTCTTCAAGCCA-3′ and 5′-CATGAAGCCACGGTTGTCC-3′ for human HSP47,[Citation33] and 5′-CAGAGCAAGAGAGGCATCCT-3′ and 5′-ACGTACATGGCTGGGGTG-3′ for human β-actin as the internal control. We used primers for human P4H3 designed by PrimerBank (http://pga.mgh.harvard.edu/primerbank/).
Statistical analysis
As the number of malignant lymphoma cases is low, 10 SIs were scored in a case. The Kruskal–Wallis test was used for the SIs. If cases were considered to be significant, Bonferroni’s multiple comparison test was further applied. The Mann–Whitney test was carried out for the SIs of malignant lymphomas and their normal counterpart areas of these lymphomas. Differences with p < 0.05 were considered to be significant in each case.
Results
Expression of TGII and TN-C in non-neoplastic lymphoid tissues
TGII and TN-C were mainly expressed on reticular forming cells in marginal zone and T-cell area in non-neoplastic lymphoid tissues, but not on lymphocytes []. TGII was observed in the cell cytoplasm and extracellular space, and TN-C was expressed extracellularly. TGII was expressed on endothelial cells and TN-C was expressed on perivascular space too []. We could recognize them as vessels and exclude them form SIs, used by hematoxylin-eosin stain as counterstain. The SIs of TGII and TN-C in the marginal zone and T-cell area were higher than those in the mantle zone and GC in both the lymph nodes and tonsils (p < 0.01). TGII and TN-C showed similar staining patterns [], which indicated that the staining pattern was coadunate to the FRC distribution in marginal zone and T-cell area unlike the FDCs meshwork in GC and mantle zone.
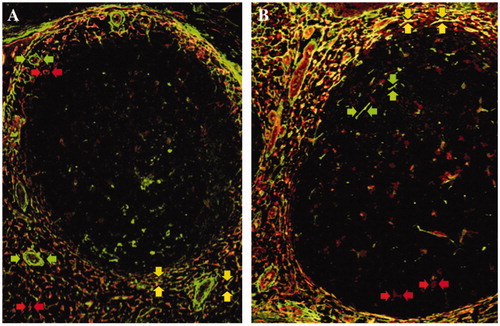
Figure 1. Immunofluorescence double staining of transglutaminase II and tenascin-C in non-neoplastic lymphoid tissues. Transglutaminase II (rhodamine, red arrows) and tenascin-C (FITC, green arrows) were mainly expressed in the marginal zone and T-cell area in a reactive lymph node (A) and tonsil (B). Double positive cells of transglutaminase II and tenascin-C were also detected in the same areas (merged, yellow arrows).
Expression of CMEs on FRCs and FDCs in non-neoplastic lymphoid tissues
Expression of CMEs in non-neoplastic lymphoid tissues was evaluated in immunohistochemical single stain ( and ). In accordance with FRCs’ reticular network and FDCs’ meshwork, all CMEs were expressed in non-neoplastic lymphoid tissues, even if there is a difference in SIs. The SIs of P4H1, LH3 and PDI in the marginal zone, GC and T-cell area were higher than those in the mantle zone (p < 0.01). P4H3, P3H1 and HSP47 SIs were low in any area. Furthermore, we confirmed which cells expressed any CMEs in immunofluorescence double stain of CMEs and FRCs/FDCs markers ( and ). The SIs of the double positive cells of CMEs and FRCs markers in the marginal zone and T-cell area were higher than those in the mantle zone or GC (p < 0.05). The SIs of the double positive cells of CMEs, except for P3H1 and PDI, and the FDCs markers were higher in GC than the mantle zone (p < 0.01). The SIs of P3H1 were very low. P3H1 and PDI on FDCs could not be detected completely because of the absence of S-100 protein+ FDCs of the mantle zone. According to these results, CMEs in non-neoplastic lymphoid tissues expressed on FRCs/FDCs, mainly. In particular, P4H1, LH3 and PDI were high-expressed. Some or many PDI+ lymphocytes were observed in each area, except for the mantle zone.
Figure 2. The expression of collagen modifying enzymes in non-neoplastic lymphoid tissues. The expression of prolyl 4-hydroxylase 1 (A), lysyl hydroxylase 3 (B), and protein disulfide isomerase (C) was more frequently observed in the marginal zone (MaZ) and germinal center (GC) than in the mantle zone (MnZ) or interfollicular area (IF). A, B, and C are serial sections of the tonsil. Transglutaminase II (D and F; rhodamine, red arrows) was predominantly expressed in the marginal zone and T-cell area, as was lysyl hydroxylase 3 (E and F; FITC, green arrows) as well as in the germinal center in a lymph node. Double positive cells of transglutaminase II and lysyl hydroxylase 3 were localized in the marginal zone and T-cell area (F; merged, yellow arrows).
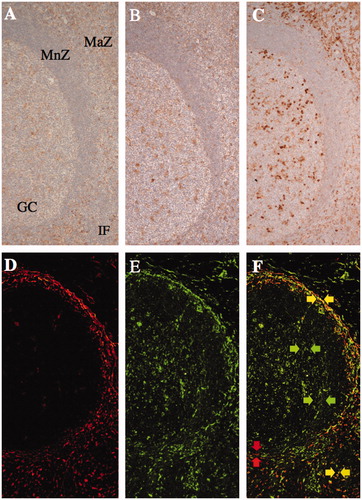
Table I. The staining index of transglutaminase II, tenascin-C, and collagen modifying enzymes on fibroblastic reticular cells and follicular dendritic cells in non-neoplastic lymph nodes.
Table II. Comparison of the staining index of collagen modifying enzymes and the frequency of transglutaminase II+ fibroblastic reticular cells and CD35+ follicular dendritic cells between malignant lymphomas and their respective postulated normal counterparts.
Comparison of the expression of CMEs, TGII and CD35 between malignant lymphomas and postulated normal counterparts
Expression of CMEs in malignant lymphomas was evaluated in immunohistochemical single stain (). In the same as non-neoplastic lymphoid tissues, all CMEs+ cell was distributed looks like the FRCs’ reticular network and the FDCs’ meshwork in malignant lymphomas too, even if there is a difference in degree. And these SIs in malignant lymphomas were compared with the SIs in the areas of their normal counterparts in non-neoplastic lymphoid tissues. The SIs of P4H1 and PDI were higher in MCL, NS-cHL and MC-cHL than in their normal counterparts (p < 0.05) (). The SIs of both P3H1 and HSP47 were high in NS-cHL. Any SI of CMEs was low in SLL, FL (any grade), non-GCB type DLBCL, PTCL/EATL and AITL. The SIs of P4H1, P4H3, LH3 and HSP47 were low in GCB-type DLBCL. According to these results, CMEs expression in most of lymphomas, except MCL and cHL, was less than their normal counterparts. There is no significant difference of CMEs SIs among FLs, any grade. About PDI, it was not expressed on lymphoma cells in all malignant lymphomas. And we confirmed some CMEs were expressed on FRCs/FDCs in some malignant lymphomas compared with their normal counterparts [].
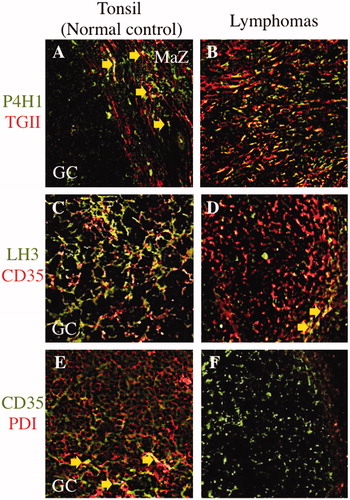
Figure 3. Comparison of three selected collagen modifying enzymes on transglutaminase II+ fibroblastic reticular cells or CD35+ follicular dendritic cells in between malignant lymphomas and postulated normal counterparts. Prolyl 4-hydroxylase 1 (P4H1) was expressed in the germinal center (GC) and marginal zone (MaZ) (A; FITC, green colored) and transglutaminase II+ fibroblastic reticular cells (TGII+ FRCs) were localized in the MaZ (A; rhodamine, red colored) in the tonsil. There were a few of P4H1+TGII+ FRCs in MaZ (A; merged, yellow colored and arrows). In nodular sclerosis classical Hodgkin lymphoma, P4H1+TGII+ FRCs were proliferating (B; merged, yellow colored) at the background of Hodgkin–Reed–Sternberg cells (data not shown). Lysyl hydroxylase 3 (LH3) was expressed in the GC (C; FITC, green colored) and CD35+ follicular dendritic cells (CD35+ FDCs) were localized in the GC (C; rhodamine, red colored) in the tonsil. There were a lot of LH3+CD35+ FDCs in GC (C; merged, yellow colored). In follicular lymphoma, there were a few of LH3+CD35+ FDCs in the neoplastic follicle (D; merged, yellow colored and arrows). Protein disulfide isomerase (PDI) was expressed in the GC (E; rhodamine, red colored) and CD35+ FDCs were localized in the GC (E; FITC, green colored) in the tonsil. PDI+CD35+ FDCs were scattered in GC (E; merged, yellow colored and arrows). In follicular lymphoma, no PDI+CD35+ FDCs was existed in the neoplastic follicle (E; FITC, green colored).
Distribution of FRCs/FDCs in malignant lymphomas
In the same way as CMEs, expression of TGII+ FRC/CD35+ FRC in malignant lymphomas was evaluated (). The SIs of TGII was higher in GCB type DLBCL, AITL, NS-cHL, MC-cHL, MCL and MALT/NMZL (p < 0.01) and lower in FL (any grade), SLL and PTCL/EATL (p < 0.05) than in their normal counterparts (). The SIs of CD35 were higher in MALT/NMZL and non-GCB type DLBCL (p < 0.05) and lower in FL (any grade), GCB type DLBCL, AITL, NS-cHL, MC-cHL and MCL (p < 0.01) than in their normal counterparts.
By the use of SIs of TGII and CD35, FRCs/FDCs distributions among malignant lymphomas were comparatively investigated. The TGII SI was higher than the CD35 SI in SLL, MALT/NMZL, MCL, GCB type DLBCL, non-GCB type DLBCL, PTCL/EATL, NS-cHL and MC-cHL (p < 0.01). The TGII SI was lower than the CD35 SI in FL, except for Grade 2 (p < 0.01). The SI ratios of TGII/CD35 were low (1:1.5) in FL (any grade), but slightly higher in almost lymphomas (PTCL/EATL 27.8:1, SLL 20:1, GCB and non-GCB type DLBCL 7.7:1, MALT/NMZL 6:1, NS and MC-cHL 3.4:1, MCL 1.6:1, and AITL 1.3:1) (). Among FLs, Grade 1, 2 and 3, the SI ratios of TGII/CD35 were not significant different (data not shown). In a few cases of FLs (any grade), TGII+ cells were scattered in neoplastic follicles []. TGII+ cells were diffusely observed in SLL, MALT/NMZL and DLBCL (GCB and non-GCB type). TGII+ cells intermingled with the CD35+ cells meshwork MCL and AITL []. Hodgkin–Reed–Sternberg (HRS) cells were observed in or outside of the TGII+ cell network with independent of CD35+ cell meshwork in cHLs.
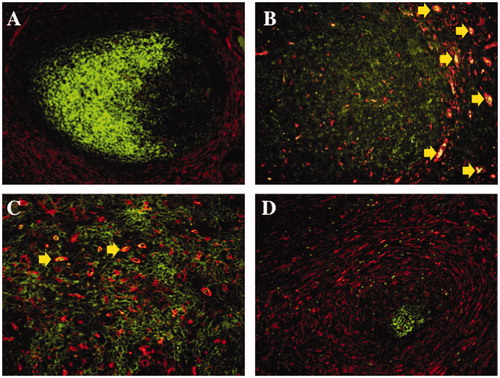
Figure 4. Immunofluorescence double staining of transglutaminase II and CD35 in malignant lymphomas and a non-neoplastic lymphoid follicle. In normal tonsil, transglutaminase II+ fibroblastic reticular cells (TGII+ FRCs) were mainly localized in the marginal zone and T-cell area (A; rhodamine, red colored), and CD35+ follicular dendritic cells (CD35+ FDCs) were localized in the germinal center (A; FITC, green colored). In follicular lymphoma, TGII+ FRCs were scattered in a neoplastic follicle (B; rhodamine, red colored) with the background of densely packed CD35+ FDCs (B; FITC, green colored). In angioimmunoblastic T-cell lymphoma, TGII+ FRCs (C; rhodamine, red colored) were intermingled with CD35+ FDCs meshwork (C; FITC, green colored). In nodular sclerosis classical Hodgkin lymphoma, TGII + FRCs were proliferating (D; rhodamine, red colored) around an atrophic follicle of CD35 + FDCs (D; FITC, green colored). Erythrocytes existed intravascular areas (B and C; merged, yellow arrows).
Therefore, TGII+ FRCs were observed in all types of lymphomas, and CD35+ FDCs were observed in GC-forming lymphomas including MCL, FL, AITL and some cHL, respectively [].
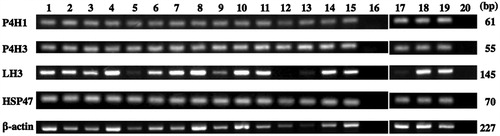
Expression of CME mRNAs by RT-PCR
β-Actin mRNA as the housekeeping gene was expressed in all cases. The mRNAs of P4H1, P4H3, LH3 and HSP47 were expressed in positive controls. The mRNAs of P4H1, P4H3 and HSP47 were expressed in all cases of non-neoplastic tissues and lymphomas [], while LH3 mRNA was expressed in almost all cases except EATL.
Figure 5. Reverse transcription polymerase chain reaction of collagen modifying enzymes. Positive controls for mRNAs of prolyl 4-hydroxylase 1 (P4H1), prolyl 4-hydroxylase 3 (P4H3), lysyl hydroxylase 3 (LH3), and heat shock protein 47 (HSP47) were shown in lanes 1 and 17. The mRNAs of P4H1, P4H3 and HSP47 were expressed in non-neoplastic tonsils and lymph nodes (lanes 2 and 3, respectively) and lymphomas (lane 4, small lymphocytic lymphoma; lane 5, nodal marginal zone lymphoma; lane 6, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue; lane 7, mantle cell lymphoma; lanes 8 to 10, follicular lymphoma grades 1, 2 and 3, respectively; lane 11, peripheral T-cell lymphoma; lane 12, enteropathy-associated T-cell lymphoma; lane 13, angioimmunoblastic T-cell lymphoma; lane 14, nodular sclerosis classical Hodgkin lymphoma; lane 15, mixed cellularity classical Hodgkin lymphoma; lane 18, diffuse large B-cell lymphoma not other specified, germinal center B-cell-like subgroup; lane 19, diffuse large B-cell lymphoma not other specified, non-germinal center B-cell-like subgroup), while LH3 mRNA was expressed in most cases, except for enteropathy-associated T-cell lymphoma. Lanes 16 and 20, negative control.
Discussion
Firstly, immunohistochemical and immunofluorescence double staining indicated that both TGII and TN-C antibodies may be used as FRC markers, as previously described.[Citation5,Citation11]
Secondly, FRCs in the marginal zone and T-cell area and GC FDCs highly expressed P4H1, LH3 and PDI. The expressions of P4H3, P3H1 and HSP47 were lower in any area than in other CMEs, nevertheless P3H1 and HSP47 among CMEs were specific and restricted to collagen biosynthesis in the intracellular endoplasmic reticulum,[Citation12,Citation16] as demonstrated in the HSP47-KO mouse.[Citation34] This result suggested incomplete collagen biosynthesis in FRCs/FDCs. Unexpectedly, FRCs/FDCs expressed P4H3 at low levels in this study. P4H3 has been associated with the biosynthesis of collagen III,[Citation12] which is one of the main components of FRCs’ reticular fibers. This result suggested the low frequency of collagen III biosynthesis in mature lymphoid tissues, though it was essential for maturing.
Localization of CMEs in hematopoietic tumors is still not fully understood. In this study, PDI was not expressed on lymphoma cells themselves in all malignant lymphomas, but expressed on B-cell chronic lymphocytic leukemia cells, previously.[Citation35,Citation36] There were a few reports investigating FRCs/FDCs distribution in malignant lymphomas. GC-derived lymphomas including FL, AITL and nodular lymphocyte predominant HL had FDCs, and cHL has FDCs and fibroblasts.[Citation8,Citation9,Citation37] The recognization of FDCs’ pattern was useful in identifying early lymphomas of follicular origin such as FL, MCL and AITL.[Citation9] It could not obtain definitive diagnosis of these lymphomas, but be helpful as a factor of differential diagnosis. The recognizing FRCs’ patterns in malignant lymphomas were rare. Some malignant lymphomas contain TGII+ FRCs.[Citation7]
Thirdly, this is first study that demonstrated the localization of CMEs on FRCs/FDCs in malignant lymphomas (Supplemental Fig. 1 and Table II). An interesting finding was the lower expression of CMEs such as P4H1, LH3 and PDI in the majority of malignant lymphomas, except for MCL and cHL, than that of their normal counterparts. A decrease in CMEs may be closely related to the decrease in FRCs, FDCs or both populations (). This result suggested that lymphoma cell proliferation disturbs the lymphoid tissue construction with the reduction of FRCs/FDCs in most of malignant lymphomas. And this may contribute to induction of lymphoma cell involvement to another lymph node or other parenchymas, because movement of lymphoma cells was mediated by fibroblasts, probably FRCs.[Citation20] In mice study, FRCs had the role of the lymphoma-permissive niche formation.[Citation38] On the other hand, in cases of in situ FL, the secondary follicle immunoarchitecture consisting vimentin+ FRCs and CD21+CD23+ FDCs remained recognizable.[Citation20] Because of no selection in situ FLs in this study, we will challenge investigating FRCs/FDCs populations in their lymphomas in the future.
Conversely, the expression of CMEs was higher in MCL and cHL than in their normal counterparts, together with an increasing FRCs population and decreasing FDCs population. This result suggested that CMEs+ FRCs were associated with some lymphoma cell proliferation, because patient-derived xenograft (PDX) of lymphoma cells could not survive without FRCs, previously.[Citation38] Therefore, if stromal cell targeting therapy in lymphomas will be developed, FRCs would be a main target.
Fourthly, RT-PCR, as qualitative test, confirmed the expression of P4H1, P4H3, LH3 and HSP47 mRNAs in normal lymphoid tissues and malignant lymphomas (), although low level expression of P4H3 and HSP47 by immunohistochemistry. This may have been due to the low levels of translation and protein-biosynthesis of P4H3 and HSP47. However this result was affected by CMEs+ other stromal cells except for FRCs/FDCs.
In conclusion, the present study demonstrated that P4H1, LH3 and PDI were frequently expressed, whereas other CMEs were not, on FRCs/FDCs populations in lymphoid tissues. Therefore, FRCs/FDCs may have a function of collagen production. And, the majority of malignant lymphomas infrequently expressed CMEs, which may have been due to the suppression of FRCs/FDCs population by active lymphoma cell proliferation. Conversely, MCL and cHL frequently had CMEs together with an increasing number of the FRCs population. Collectively, these results indicate that the balance between CMEs-expressing FRCs/FDCs populations may change the architectural conformation of respective malignant lymphoma subtypes.
Supplemental_Table_1.doc
Download MS Word (50.5 KB)Supplemental_Figure_1.doc
Download MS Word (164 KB)ICMJE_Forms_for_Disclosure_of_Potential_Conflicts_of_Interest.zip
Download Zip (10 MB)Acknowledgements
The authors are grateful to Hitoshi Suzuki, Hiromi Murata and Junko Takeda for their valuable assistance during this study. This work was supported by a Grant-in-Aid for Scientific Research (c) (25460451) in Japan.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.3109/10428194.2015.1107907
All authors have read the journal’s policy on conflicts of interest and have none to declare.
References
- Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Rev Immunol. 2009; 9: 618–629.
- Kaldjian EP, Gretz JE, Anderson AO, et al. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Internat Immunol. 2001; 13: 1243–1253.
- Brown FD, Turley SJ. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J Immunol. 2015; 194: 1389–1394.
- Malhotra D, Fletcher AL, Astarita J, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nature Immunol. 2012; 13: 499–510.
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dynam. 2000; 218: 235–259.
- Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 2009; 36: 659–670.
- Thomazy VA, Vega F, Medeiros LJ, et al. Phenotypic modulation of the stromal reticular network in normal and neoplastic lymph nodes: tissue transglutaminase reveals coordinate regulation of multiple cell types. Am J Pathol. 2003; 163: 165–174.
- Rezk SA, Nathwani BN, Zhao X, et al. Follicular dendritic cells: origin, function, and different disease-associated patterns. Hum Pathol. 2013; 44: 937–950.
- Carbone A, Gloghini A. Follicular dendritic cell pattern in early lymphomas involving follicles. Adv Anat Pathol 2014; 21: 260–269.
- Bofill M, Akbar AN, Amlot PL. Follicular dendritic cells share a membrane-bound protein with fibroblasts. J Pathol 2000; 191: 217–226.
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004; 20: 33–43.
- Myllyharju J. Intracellular post-translational modifications of collagens. In: Brinckmann J, Notbohm H, Muller PK, editors. Collagen: primer in structure, processing and assembly. Berlin: Springer-Verlag; 2005. p 115–147.
- Fonseca C, Soiffer R, Ho V, et al. Protein disulfide isomerases are antibody targets during immune-mediated tumor destruction. Blood 2009; 113: 1681–1688.
- Takaluoma K, Lantto J, Myllyharju J. Lysyl hydroxylase 2 is a specific telopeptide hydroxylase, while all three isoenzymes hydroxylate collagenous sequences. Matrix Biol. 2007; 26: 396–403.
- Myllyla R, Wang C, Heikkinen J, et al. Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3). J Cell Physiol. 2007; 212: 323–329.
- Koide T, Nagata K. Collagen biosynthesis. In: Brinckmann J, Notbohm H, Muller PK, editors. Collagen: primer in structure, processing and assembly. Berlin: Springer-Verlag; 2005. p 85–114.
- Bosseloir A, Heinen E, Defrance T, et al. Moabs MAS516 and 5B5, two fibroblast markers, recognize human follicular dendritic cells. Immunol Lett. 1994; 42: 49–54.
- Salo AM, Sipila L, Sormunen R, et al. The lysyl hydroxylase isoforms are widely expressed during mouse embryogenesis, but obtain tissue- and cell-specific patterns in the adult. Matrix Biol 2006; 25: 475–483.
- Kasai K, Nakayama A, Ohbayashi M, et al. Immunohistochemical characteristics of chicken spleen ellipsoids using newly established monoclonal antibodies. Cell Tissue Res. 1995; 281: 135–141.
- Carbone A, Gloghini A. Emerging issues after the recognition of in situ follicular lymphoma. Leukemia Lymphoma 2014; 55: 482–490.
- Annunen P, Helaakoski T, Myllyharju J, et al. Cloning of the human prolyl 4-hydroxylase alpha subunit isoform alpha(II) and characterization of the type II enzyme tetramer. The alpha(I) and alpha(II) subunits do not form a mixed alpha(I)alpha(II)beta2 tetramer. J Biol Chem 1997; 272: 17342–17348.
- Kukkola L, Hieta R, Kivirikko KI, et al. Identification and characterization of a third human, rat, and mouse collagen prolyl 4-hydroxylase isoenzyme. J Biol Chem. 2003; 278: 47685–47693.
- Wang C, Valtavaara M, Myllyla R. Lack of collagen type specificity for lysyl hydroxylase isoforms. DNA Cell Biology 2000; 19: 71–77.
- Cao D, Maitra A, Saavedra JA, et al. Expression of novel markers of pancreatic ductal adenocarcinoma in pancreatic nonductal neoplasms: additional evidence of different genetic pathways. Modern Pathol. 2005; 18: 752–761.
- Meng HX, Li HN, Geng JS, et al. Decreased expression of follicular dendritic cell-secreted protein correlates with increased immunoglobulin A production in the tonsils of individuals with immunoglobulin A nephropathy. Transl Res. 2015; 166: 281–291.
- Orui H, Yamakawa M, Imai Y. Proliferation and apoptosis of follicular lymphocytes: relationship to follicular dendritic cell-associated clusters. Immunology 1997; 90: 489–495.
- King RJ, Coffer AI, Gilbert J, et al. Histochemical studies with a monoclonal antibody raised against a partially purified soluble estradiol receptor preparation from human myometrium. Cancer Res. 1985; 45: 5728–5733.
- Swerdlow SH, Campo E, HN L, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008.
- Robertson D, Savage K, Reis-Filho JS, et al. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008; 9: 13.
- Mitsui H, Ohtake H, Ohe R, et al. Frequent infiltration of S-100 protein + CCR5 + immature dendritic cells in damaged bile ducts of primary biliary cirrhosis compared to cholangiocellular carcinoma. Pathology Lab Med Int. 2013; 5: 9–19.
- Zhang C, Zhang MX, Shen YH, et al. TNF-alpha suppresses prolyl-4-hydroxylase alpha1 expression via the ASK1-JNK-NonO pathway. Arterioscler, Thromb Vasc Biol. 2007; 27: 1760–1767.
- van der Slot AJ, Zuurmond AM, Bardoel AF, et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003; 278: 40967–40972.
- Razzaque MS, Foster CS, Ahmed AR. Role of collagen-binding heat shock protein 47 and transforming growth factor-beta1 in conjunctival scarring in ocular cicatricial pemphigoid. Investigat Ophthalmol Visual Sci. 2003; 44: 1616–1621.
- Ishida Y, Kubota H, Yamamoto A, et al. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Molec Biol Cell. 2006; 17: 2346–2355.
- Kroning H, Kahne T, Ittenson A, et al. Thiol-proteindisulfide-oxidoreductase (proteindisulfide isomerase): a new plasma membrane constituent of mature human B lymphocytes. Scand J Immunol. 1994; 39: 346–350.
- Tager M, Kroning H, Thiel U, et al. Membrane-bound proteindisulfide isomerase (PDI) is involved in regulation of surface expression of thiols and drug sensitivity of B-CLL cells. Exp Hematol. 1997; 25: 601–607.
- Carbone A, Gloghini A, Cabras A, et al. The germinal centre-derived lymphomas seen through their cellular microenvironment. Br J Haematol. 2009; 145: 468–480.
- Sugimoto K, Hayakawa F, Shimada S, et al. Discovery of a drug targeting microenvironmental support for lymphoma cells by screening using patient-derived xenograft cells. Sci Rep. 2015;5:13054. DOI: 10.1038/srep13054.
