Abstract
Partly due to poor blood–brain barrier drug penetration the treatment options for many brain diseases are limited. To safely enhance drug delivery to the brain, glutathione PEGylated liposomes (G-Technology®) were developed. In this study, in rats, we compared the pharmacokinetics and organ distribution of GSH-PEG liposomes using an autoquenched fluorescent tracer after intraperitoneal administration and intravenous administration. Although the appearance of liposomes in the circulation was much slower after intraperitoneal administration, comparable maximum levels of long circulating liposomes were found between 4 and 24 h after injection. Furthermore, 24 h after injection a similar tissue distribution was found. To investigate the effect of GSH coating on brain delivery in vitro uptake studies in rat brain endothelial cells (RBE4) and an in vivo brain microdialysis study in rats were used. Significantly more fluorescent tracer was found in RBE4 cell homogenates incubated with GSH-PEG liposomes compared to non-targeted PEG liposomes (1.8-fold, p < 0.001). In the microdialysis study 4-fold higher (p < 0.001) brain levels of fluorescent tracer were found after intravenous injection of GSH-PEG liposomes compared with PEG control liposomes. The results support further investigation into the versatility of GSH-PEG liposomes for enhanced drug delivery to the brain within a tolerable therapeutic window.
Introduction
The blood–brain barrier (BBB) maintains homeostasis in the brain by selective transport of metabolic compounds, while excluding harmful compounds from the blood circulation. These barrier properties prevent adequate BBB crossing of many high potential “would-be” central nervous system (CNS) drugs. Most hydrophilic molecules are not able to cross the BBB due to the presence of tight junctions between the brain endothelial cells preventing paracellular transport. Transport of other more lipophilic small molecules that could potentially cross the barrier is often limited because they are substrate for efflux transporters like the p-glycoprotein (Pgp), multidrug related protein (MRP) or breast cancer resistance protein (BCRP) transporters [Citation1–3]. Many strategies to enhance the BBB transport of drugs have therefore been investigated. These strategies are either focused on locally circumventing the BBB by direct injections in the brain or through the blood stream for widespread delivery [Citation4–7].
For the blood-to-brain delivery of drugs via the blood stream, advanced drug delivery systems like nanocarriers can best be used. Some examples of commonly used nanocarriers are liposomes, solid lipid nanoparticles, albumin nanoparticles and polymeric nanoparticles [Citation8]. Drug delivery using nanocarriers may increase the bioavailability and stability of drugs and decrease the (peripheral) toxicity. Furthermore, drugs delivered by liposomes are described to be able to bypass the efflux transporters at the BBB [Citation9,Citation10]. Moreover, liposomal formulation allows a wide range of compounds, like small molecules, peptides, proteins and RNA to be encapsulated without changing their function and protecting them against degradation and potential immune responses. Coating of liposomes with polyethylene glycol (PEG) further ensures a prolonged circulation time in plasma allowing prolonged dosing intervals. As a result, liposomes have been used in clinical practice for several years and are shown to be effective and safe for human use (e.g. Doxil®, Ambisome®). Liposomes can furthermore be functionalized with ligands to target specific cell types or tissues, including the brain. Recently, several excellent reviews presented an overview of the development status of carrier-based drug delivery systems for CNS indications [Citation11,Citation12].
Nutrient transporters at the BBB can also be used to facilitate drug delivery into the brain. For example, the sodium-dependent (active) glutathione transporter has a preferential expression in CNS and BBB and is present in all mammalian species [Citation13–15]. Glutathione (GSH) is an endogenous tripeptide that plays a central role in the detoxification of intracellular metabolites. Exogenously administered GSH has a well-established and good safety profile, and, due to its antioxidant-like properties, it is used as a functional food ingredient and as supportive intravenous therapy in cancer, Parkinson Disease and HIV.
to-BBB has developed the G-Technology® that is based on the new use of GSH as a drug-targeting ligand combined with liposomal drug delivery. to-BBB’s lead product is glutathione PEGylated (GSH-PEG) liposomal doxorubicin (2B3-101), which is based on the already marketed PEGylated liposomal doxorubicin (Doxil®/Caelyx®). Using 2B3-101, the brain delivery of doxorubicin was safely enhanced by approximately 5-fold (unpublished data), resulting in a reduced brain tumor growth and improved survival [Citation16]. Currently, 2B3-101 is investigated in a clinical phase I/IIa study in patients with recurrent glioma or brain metastases of solid tumors (clinicaltrials.gov NCT01386580, NCT01818713). GSH-PEG liposomes have also been used to enhance the sustained delivery and efficacy of methylprednisolone [Citation17]. More recently, a mechanistic brain microdialysis study using DAMGO, an antinociceptive peptide, showed a 2-fold increase in brain extracellular fluid (ECF) concentrations of the free drug at steady-state plasma concentrations [Citation18]. Two recent publications by independent groups confirmed delivery of GSH-coated nanoparticles to the brain [Citation19,Citation20].
In this study, we have used a fluorescent tracer, carboxyfluorescein (CF) that is autoquenching within the core of the liposomes [Citation21]. This approach allows for straightforward and fast quantification of intact liposomes in blood and tissue homogenates. The aim of this study was to characterize the specific cell uptake, pharmacokinetics and the organ distribution of GSH-PEG liposomes, with a focus on the brain. Specifically, we first investigated the stability of the liposomes in culture medium and performed in vitro uptake studies of PEGylated liposomes in brain endothelial cells to show the beneficial effect of GSH coating on the specificity of cell uptake. Second, to investigate possible effects of route of administration on systemic exposure and distribution of GSH-PEG liposomes we compared intraperitoneal (IP) or intravenous (IV) injection in rats. Finally, to accurately quantify the amount of free fluorescent tracer in the brain and to show the benefit of GSH coating a microdialysis study was performed in rats after IV administration of GSH-PEG liposomes, non-targeted PEG liposomes and free fluorescent tracer.
Methods
Preparation and characterization of the liposomes
Liposomes with an encapsulated fluorescent tracer (CF) were prepared using an ethanol injection method and a post-insertion of GSH-PEG micelles or PEG micelles. In short, liposomes consisting of HSPC (Lipoid, Cham, Switzerland), cholesterol (Sigma-Aldrich, Zwijndrecht, the Netherlands) and 1% mPEG2000-DSPE (Lipoid, Cham, Switzerland) were made by dissolving the lipids in 96% ethanol and mixing this with a 36 mg/mL 5(6)-carboxyfluorescein (CF, Sigma-Aldrich, Zwijndrecht, the Netherlands) solution at 60 °C. After extrusion through 200/200, 200/100 and 100/100 nm filters (Whatman, Piscataway, NJ), liposomes were purified using Zebaspin desalting columns (Thermo Scientific, Etten-Leur, the Netherlands) equilibrated with PBS to remove CF from the outside of the liposomes. Reduced GSH (Sigma-Aldrich, Zwijndrecht, the Netherlands) and DSPE-PEG2000-maleimide (NOF, Grobbendonk, Belgium) were incubated at a 1.5:1 molar ratio for 2 h at room temperature to form GSH-PEG-DSPE micelles. GSH-PEG-DSPE or mPEG2000-DSPE micelles (for the control liposomes without GSH) were added to the liposomes. The resulting molar total percentage of PEG in the liposomes was 5%, for the GSH-coated liposomes 4% GSH-PEG-DSPE and 1% mPEG-DSPE (already included in the liposomes) and 5% mPEG-DSPE for the control liposomes. After post-insertion of the micelles the liposomes were purified using Zebaspin desalting columns equilibrated with PBS to remove the excess of GSH. The liposomes were sterile filtered using 0.2 μm filters and stored at 4 °C.
For the studies in which we compared liposomes with and without GSH, the in vitro studies and the in vivo microdialysis study, we produced one batch liposomes of which a part was post-inserted with GSH-PEG-DSPE micelles and the other part with mPEG-DSPE micelles. For the pharmacokinetics and distribution study in rats a third batch of liposomes post-inserted with GSH-PEG-DSPE was used. The encapsulated CF in the three batches was measured after purification of the liposomes. At the concentration CF we used to produce the liposomes (36 mg/mL) the fluorescent signal is autoquenched. The resulting concentration of CF inside the liposomes after purification is also autoquenching. This means that the total fluorescent signal can only be measured after release of the autoquenched CF from the liposomes. The autoquenched CF was effectively released from the liposomes with 50% v/v isopropanol. We used free CF for a standard curve to quantify total CF in the liposomes. After measurement of the CF concentration the batches were diluted or concentrated to reach 2 mg/mL (quenched) CF in the formulations.
The amount of lipids in the liposome samples was analyzed using an HPLC method. In short, a Perkin Elmer Series 200 pump with an autoinjector, autosampler and an ELSD detector (Alltech, the Netherlands) were used. A C18 (Kinetex, 15 cm × 4.6 mm i.d.; 2.6 μm, Phenomenex, cat# 00F-4496-E0) column equipped with a guard column was used for analysis of HSPC, cholesterol, GSH-PEG-DSPE and mPEG-DSPE. Column temperature was set to 45 °C. The chromatographic conditions are a gradient elution of 0–10% eluent A (0.1 M ammonium acetate, pH 6.0) and eluent B (methanol) were pumped with a flow rate of 1.5 mL/min. The total run time was 20 min. The nitrogen gas flow of the ELSD was set to 1.3 mL/min (5 bar) and the used temperature was 38 °C. The size of the liposomes was measured using dynamic light scattering (Malvern Zetasizer, Worcestershire, UK).
Liposome stability and uptake in rat brain endothelial cells
Rat brain endothelial cells (RBE4 cells, obtained from Prof. P.O. Couraud, Inserm, Paris) [Citation22] were cultured in Dulbecco’s Modified Eagle Media with 10% Fetal Calf Serum, 1% Penicillin–Streptomycin, 1% Glutamine and 1% Non-Essential Amino Acid (Lonza Verviers). The liposome uptake in cells and the liposome stability in culture medium were determined in the same experiment. For the experiments, the cells were seeded in a 6-wells plate and grown until confluency. Liposomes were diluted in culture medium (450 μg/mL based on HSPC concentration) and incubated with the cells for 4 and 24 h. During this incubation culture medium samples were collected at 0, 1, 2, 4, 6, 8 and 24 h to quantify the amount of CF in the liposomes and possible released CF in culture medium. The culture medium samples were diluted in PBS and fluorescence was measured at 485/538 nm. After the first measurement, the same samples were mixed with equal amounts of isopropanol to release the encapsulated quenched CF and were measured again at the same wavelength. The total amount of CF and the amount of released CF in culture medium was expressed as percentage of the start value at T = 0.
After incubation with the liposomes the cells were washed with PBS and cell lysates were made using 500 μL 0.1 M sodium hydroxide (NaOH). Fluorescence of CF in the lysates was measured in 0.1 M NaOH and again after release of quenched CF from the liposomes with isopropanol 1:1 v/v. The amount of protein in the cell lysates was quantified using a BCA protein assay (Thermo Scientific, Etten-Leur, the Netherlands). The concentration CF in the cell lysates was expressed as ng CF per mg of protein in the lysates. A t-test (unpaired, two-tailed) was used for statistical analysis of the difference in CF uptake after treatment with GSH-PEG or PEG liposomes.
The uptake experiments were also done on slide chambers (ibidi, Martinsried, Germany) for microscopy. GSH-PEG and PEG liposomes were diluted in culture medium and incubated with the cells at 4 and 37 °C for 4 h. After 4 h incubation, cells were washed with cold PBS followed by fixation with formalin solution, neutral buffered, 10% (Sigma-Aldrich, Zwijndrecht, the Netherlands). For microscopic analysis, cells were mounted with Prolong®Gold Antifade reagent with DAPI (Invitrogen, Bleiswijk, the Netherlands).
Pharmacokinetics and distribution in rats
The pharmacokinetics and distribution study was performed in male Wistar rats (Charles River Deutschland, Sulzfeld, Germany) at WIL Research Europe BV (‘s-Hertogenbosch, the Netherlands). The study protocol was approved by the Animal Ethics Committee of WIL Research (DEC 00-34 and 12-117) and all animal procedures were performed in accordance with Dutch laws on animal experimentation. The animals were housed in groups of 4 per cage under a 12-h light/12-h dark cycle with free access to water and food. Four 8-week-old animals per group were injected with the GSH-PEG liposomes. The different routes of administration were IV tail vein injection or IP injection. The liposomes were dosed based on CF concentration and the animals received 2.5 mg/kg CF. Different dilutions of liposomes were used for the different administration routes to ensure a good distribution of the solution after IP injections. The injection volumes for IV were 1.25 mL/kg (stock 2.0 mg CF/mL) and for IP 8 mL/kg (stock 0.3 mg CF/mL).
Blood samples were collected from the tail vein of all rats. In case of IV dosing, however, the samples during the first 2 h after administration were collected from the saphenous vein to avoid potential contamination from the injection site. Approximately 300 μL (tail) or 100 μL (saphenous) blood samples were collected into tubes containing K3-EDTA (Greiner Bio-One GmbH, Alphen a/d Rijn, the Netherlands) at the following time points: pre-dose, 0.25, 0.5, 1, 2, 4, 8 and 24 h after dosing. The samples were centrifuged and plasma was stored in the fridge (2–8 °C) until further analysis. Ten μL of the samples and a standard curve of free CF in rat plasma were transferred in duplicate to a 96-wells black plate and 100 μL 0.1 M NaOH per well was added. Fluorescence was measured at 485/538 nm. After addition of 100 μL isopropanol to each well, to release all CF from the liposomes, the plates were measured again.
After the last blood sampling, the animals were first anesthetized using isoflurane/nitrous oxide vapor (Abbott B.V., Hoofddorp, the Netherlands) and then given Buprecare® (0.3 mg buprenorphin/mL, AST Farma/Animal care Ltd, Oudewater, the Netherlands) 0.06 mg/kg, i.p. as a premedication analgesic and were deeply anaesthetized by ip dosing of Ketalin® (100 mg ketamine/mL, Ceva Sante Animale BV, Naaldwijk, the Netherlands) and Dexdomitor® (0.5 mg dexmedetomidin/mL, Pfizer Animal Health, Capelle a/d IJssel, the Netherlands) diluted together in saline at respectively 25 and 0.38 mg/kg body weight. The animals were subsequently sacrificed by whole body (in situ) perfusion using heparinized saline (0.9% NaCl). After perfusion, the following organs were collected: liver, spleen, kidney, lung, brain and spinal cord. The organs were stored in saline on ice and analyzed on the same day. Small pieces (around 50 mg) of the tissues were taken and the weight was measured. The tissues were homogenized in 500 μL 0.1 M NaOH using a homogenizer (Polytron PT-1300D). Tissue homogenates of untreated animals were used to prepare the standard curves with free CF. After centrifugation at 15 000 × g for 10 min 100 μL of the supernatants was transferred to a 96-wells black plate and fluorescence was measured at 485/538 nm. After measurement 100 μL isopropanol was added to the samples in the 96-wells black plate to release CF from the liposomes and the samples were measured again at the same wavelength. The amount of CF measured after release of CF from the liposomes with isopropanol was corrected for the background levels measured before the addition of isopropanol to quantify only the encapsulated liposomal CF in the tissue homogenates.
Microdialysis study in rats
The study protocol was approved by the Animal Ethics Committee of Leiden University and all animal procedures were performed in accordance with Dutch laws on animal experimentation. After arrival, all animals (male Wistar WU rats (Charles River Deutschland, Sulzfeld, Germany)) were housed at the Leiden University animal facilities under standard environmental conditions with ad libitum access to food and water. Between surgery and experiments, the animals were kept individually in Makrolon type three cages for 7 days to recover from the surgical procedures.
Cannulas were implanted in nine animals in the left femoral artery and vein for blood sampling and drug administration, respectively. Subsequently, the animals were chronically instrumented with a CMA/12 microdialysis guide (CMA/Microdialysis AB, Stockholm, Sweden) for sampling in brain ECF. The position of the microdialysis guide is: 1.0 mm posterior, 3.0 mm lateral, 3.4 mm ventral, relative to the bregma. The microdialysis dummies were replaced by the microdialysis probe one day prior to the experiment (CMA/12 Elite, Polyarylethersulfone, molecular weight cut-off 20 kDa, CMA/Microdialysis AB, Stockholm, Sweden, with a semi-permeable membrane length of 4 mm). The recovery of CF in the microdialysis probe was determined in vitro in microdialysis buffer at a flow rate of 2 μL/min and a CF concentration of 10 ng/mL and was found to be 40 ± 3.7%.
All animals received an IV infusion of a CF formulation during 10 min with an automated pump (Pump 22 Multiple Syringe Pump, Harvard Apparatus, Holliston, MA) at the start of the dialysis experiment. The different groups received GSH-PEG liposomal CF (7.5 mg/kg CF) and controls: PEG-liposomal CF (7.5 mg/kg) and free CF (5 mg/kg). Microdialysis samples were collected during 30 min intervals starting 1 h prior to the infusion until 12 h after the infusion. After weighing the microdialysis vials, they were stored at −80 °C until analysis. For CF analysis in the microdialysis, 25 μL of sample was transferred to a 96-wells black plate and 100 μL of 0.1 M NaOH was added. Standard curves of free CF were made in the same buffer as used for the microdialysis. Fluorescence was measured at 485/538 nm.
For the determination of CF plasma concentrations, blood samples of 100 μL were taken from the arterial cannula at t = −15′ (blank), 10′, 1 h, 2 h, 4 h, 6 h, 8 h, 10 h and 12 h. All blood samples were temporarily stored in heparin (10 IU)-coated Eppendorf tubes before being centrifuged for 15 min at 5000 rpm. The plasma was then pipetted into clean Eppendorf tubes and stored at −20 °C before analysis. For CF analysis, the samples and standard curve of free CF in rat plasma were transferred to a 96-wells black plate 10 μL in duplicate and 100 μL 0.1 M NaOH was added. Fluorescence was measured at 485/538 nm. After the addition of 100 μL isopropanol to each well, the plates were measured again to quantify total CF in plasma.
Results
Characteristics of the liposomes
The lipid levels and size of the three batches of liposomes were measured and are shown in . Liposome batches 1 and 2 were used for the studies where we compared GSH-PEG liposomes with control PEG liposomes. They are used for the in vitro uptake study in rat brain endothelial cells and for the in vivo microdialysis study in rats. Batch 3 was used for the pharmacokinetics and distribution study in rats. All liposomes had similar compositions and allowed for direct comparisons of the different studies.
Table 1. Characteristics of CF containing liposomes.
GSH-PEG liposomes are specifically taken up by rat brain endothelial cells
Experiments in rat brain endothelial cells were performed to investigate the effects of glutathione coating on cell uptake of PEGylated liposomes. In the same experiments, the stability of CF liposomes was determined in culture medium. After 24 h incubation, no significant differences in liposomal or released CF from PEG and GSH-PEG liposomes were found at 37 °C. Some release of CF in culture medium was found for both liposomal formulations reaching 0.5 ± 0.1% (GSH-PEG) and 0.4 ± 0.1% (PEG) of the total liposomal CF concentration after 24 h (). This demonstrated the stability of both liposomal formulations at physiological conditions.
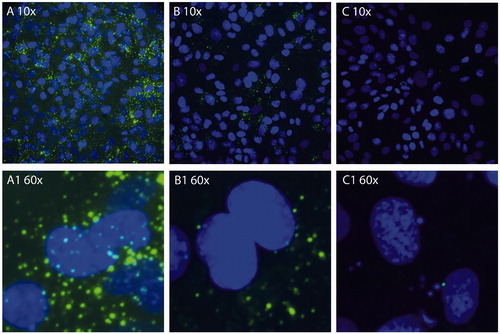
Figure 1. (A) Stability of liposomal CF formulations in culture medium at 37 °C. No difference in release was observed between GSH-PEG and PEG liposomes. Release reached a maximum of 0.5 ± 0.1% (GSH-PEG) and 0.4 ± 0.1% (PEG) of the total liposomal CF concentration after 24 h. (B) CF in cell lysates of RBE4 cells treated with liposomes. Liposomal CF levels were measured after release of CF from the liposomes in the lysates using isopropanol.
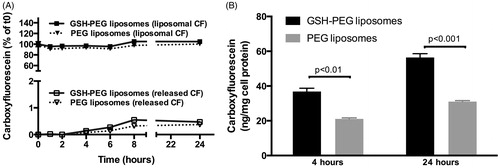
Uptake of CF-containing liposomes with and without GSH coating was quantified by measuring fluorescence in cell lysates. The total fluorescent signal was measured after release of CF from intact liposomes in the cell lysates with isopropanol and corrected for the CF levels before addition of isopropanol to quantify the amount of CF from intact liposomes in the cell lysates. The fluorescent signal increased greater than 60-fold after the release of quenched CF with isopropanol indicating that CF in the cell lysates was mainly present in intact liposomes. The uptake of intact liposomes was significantly higher for the GSH-PEG liposomes compared to PEG liposomes after 4 (p < 0.01) and 24 h (p < 0.001; ). Furthermore, uptake of CF liposomes in RBE4 cells was shown using fluorescent microscopy. A stronger fluorescent signal was seen in cells incubated with GSH-PEG liposomes indicating more uptake of these liposomes compared to PEG liposomes at 37 °C. This uptake was not visible after incubation of RBE4 cells with GSH-PEG liposomes at 4 °C. These data demonstrate the advantage of using GSH as targeting agent, and the reduced uptake at 4 °C indicates an involvement of an active uptake mechanism for the GSH formulation ().
GSH-PEG liposomes have sustained levels in the circulation after IV and IP injection
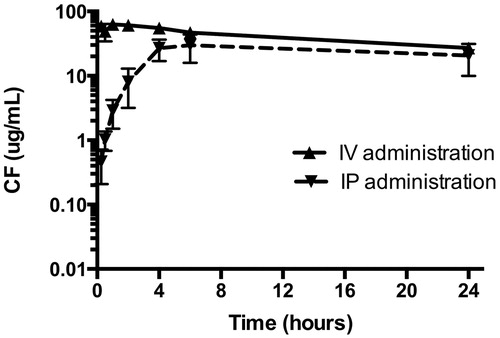
GSH-PEG liposomes were administered as an IV bolus injection or IP injected. The collected plasma samples were analyzed for CF levels. The total CF levels measured after release of CF from the liposomes with isopropanol was corrected for the background levels of fluorescence measured before isopropanol treatment resulting in the liposomal CF level in the samples. After IP administration, the liposomes are slowly entering the blood stream and remain significantly below the levels of IV administration during the first 4 h (). Blood levels of liposomal CF remained high for at least 24 h after IV and IP injection. During this sampling period, area under the curve for liposomal CF was 983 ± 97 μg h/mL and 665 ± 118 μg h/mL after IV and IP administration, respectively. At the end of the blood sampling (after 24 h), tissue distribution was also determined.
Distribution of intact GSH-PEG liposomes to tissues
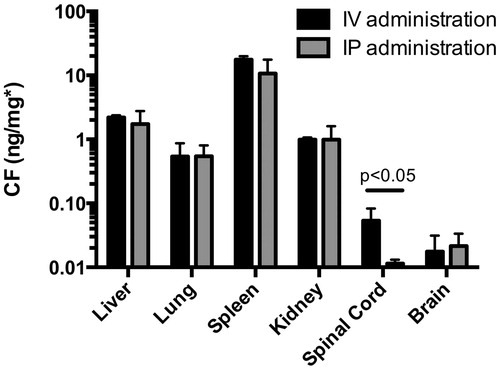
Tissue homogenates were prepared and, using isopropanol, liposomal encapsulated CF was released. The levels of released CF were corrected for the background levels measured in the homogenates without isopropanol resulting in measurement of liposomal CF only. Tissue levels of liposomal CF after IV or IP administration were not statistically different except for the levels in spinal cord (p < 0.05), where IV administration resulted in higher tissue uptake. The highest CF concentrations were found in the spleen, followed by liver, lung and kidney. CF levels from intact liposomes were detectable in the brain homogenates ().
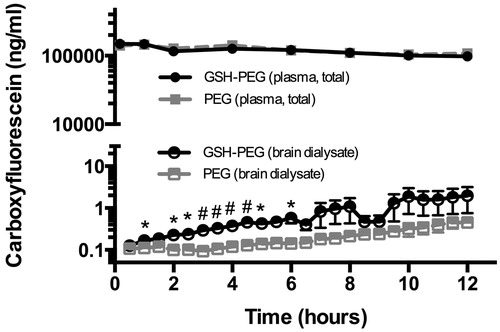
GSH coating of liposomes increased concentration of CF in brain microdialysates
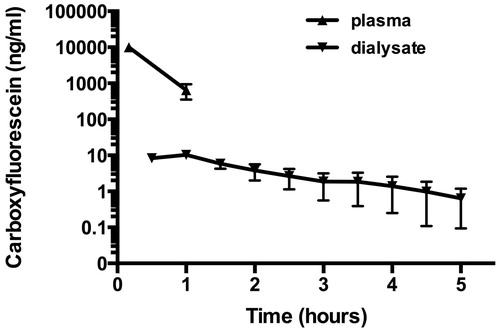
In order to obtain a more precise quantification of CF levels passing the blood–brain barrier and reaching the parenchyma, a microdialysis study was performed. The CF concentrations after IV injections of free CF, CF containing GSH-PEG and PEG liposomes were measured in blood and in brain microdialysates and are shown in and , respectively. Free CF has a relatively fast clearance from the circulation, as the CF concentrations in blood were below detection limit after 2 h. However, retention in the brain was found since the levels of free CF in the brain after administration of free CF remained detectable for at least 4.5 h (). No differences in plasma levels of CF were found for the GSH-PEG liposomes compared with the PEG liposomes. Microdialysis was used to measure free CF in the brain extracellular fluid and significantly higher levels were found for the GSH-PEG liposomes compared to the PEG liposomes in the first 6 h of the experiments ().
Discussion
The presence of the blood–brain barrier is one of the challenges for drug development for brain diseases. GSH-PEG liposomes are used to safely enhance drug delivery across the BBB. A phase I/IIA study with GSH-PEG liposomal doxorubicin (2B3-101) for treatment of brain tumors is ongoing (see www.clinicaltrials.gov; NCT01386580). Studies using a similar approach in several other disease models like pain, viral encephalitis and neuroinflammation showed beneficial effects of GSH-PEG liposomes when compared to non-targeted liposomes and free drugs [Citation17,Citation18,Citation23]. A mechanistic validation assay using microdialysis has shown that the delivery of free drug to the extracellular fluid of the brain was specifically enhanced by using GSH-PEG liposomes; increasing the amount of glutathione on the outside of the liposomes resulted up to a five times higher free drug concentration compared to non-targeted liposomes [Citation24]. However, these additional mechanistic studies may lead to a better understanding of drug delivery using GSH-PEG liposomes.
To gain further insight and to further optimize drug delivery to the brain using the G-Technology we investigated the pharmacokinetics and brain delivery using CF-loaded liposomes. These liposomes contain autoquenched CF what allows for straightforward and fast analysis of intact liposomes in complex biological samples like culture medium, cell lysates, blood and tissue homogenates. The stability of the liposomes was shown in culture medium and in blood. In culture medium, <0.5% of the total encapsulated CF was released after 24 h. This stability of the liposomes was confirmed in the in vivo studies were only a small amount of background fluorescence (<2% for all measurements) was detectable in blood. The stable encapsulation in the long circulating liposomes enables quantification of the CF distribution in intact liposomes.
In vitro uptake experiments in brain endothelial cells showed mainly uptake of intact liposomes since the difference between background signal and the signal from CF that could be released from the liposomes was more than 60-fold. Intact liposomes were present in the cells with only very limited release of CF from the liposomes during the 24-h experiments. This uptake of intact liposomes was seen for both the GSH-PEG as well as the control liposomes; however, uptake of the GSH-PEG liposomes was significantly 1.8-fold higher in brain endothelial cells after 24 h incubation. The lower uptake of GSH-PEG liposomes at 4 °C compared to 37 °C suggest that an energy-dependent uptake mechanism is involved. Further research is necessary to elucidate what endocytosis routes and what the molecular nature of the GSH transporter/receptor is.
We investigated the pharmacokinetics and distribution of liposomal CF after IP and IV administration. High levels of long circulating liposomes were detectable in blood after both IP and IV injections. However, the maximum levels after IP administration, although comparable with the maximum levels after IV administration, were reached after 4 h. This result was in line with other studies by Allen et al. [Citation25], where slower appearance of liposomes in the circulation was shown after IP and SC administration compared with IV injections. For acute disease treatment, or where peak brain levels are required, IV injections seem to be more suitable since the peak blood levels of CF in liposomes were already reached in the first few minutes after injection. IV injections in preclinical research are furthermore preferred for a better translation to the clinical situation. We can conclude that IP administration can be used in some situations since it leads to high levels of liposomes in the circulation. The biodistribution of liposomes did not differ between tissues after IV or IP administration, except in the case of spinal cord, where IV administration resulted in a higher tissue uptake (p < 0.05 compared to IP administration). This unexpected finding requires further research to understand the significance of this result. In any case, the lower concentration of CF in the spinal cord did not correlate with a lower amount of CF in brain homogenates. It is important to mention that the liposomes used, for this study were very stable non-fusogenic PEGylated liposomes with a comparable composition as to-BBB’s lead product 2B3-101. The difference between IV and IP injection of more fusogenic liposomes may result in large differences in plasma pharmacokinetics and distribution, as shown before [Citation25]. In a published study with non-PEGylated amphothericin B containing liposomes (AmBisome), differences in distribution to several organs including kidney and lungs was shown after IV and IP administration [Citation26]. Other investigators, using IV- and IP-dosed doxorubicin-loaded liposomes, found a similar biodistribution [Citation27]. Beside the difference in PK and biodistribution, local effects of IV or IP administration of liposomal drug might have to be considered. For instance, liposomes containing alendronate were shown to affect endometriosis after IP injection due to the depletion of monocytes in the peritoneal cavity. This was not the case when the drug was administered IV [Citation28].
Fast clearance of free CF from the circulation was seen after bolus injection of free CF while intravenous bolus injections of liposomal CF result in high blood concentrations of CF. We used a brain microdialysis approach to investigate the influence of this increased plasma exposure on the brain uptake of CF. It is important to mention that the microdialysates only contain free CF because of the microdialysate membrane cut-off of 20 kDa. The actual CF concentrations in the brain are also higher due to the limitations related to the recovery of the microdialysis probe which was found to be around 40% for CF in an in vitro test. When measuring in the microdialysates from the animals treated with liposomal CF, we have to take into account that only released free CF can be detected. CF still present in the liposomes or intracellular released CF cannot be measured. The measurements in the perfused tissue homogenates () indeed show that there is CF in intact liposomes in the brain. However, brain microdialysis enables for the detection of free CF concentrations in the extracellular space as a function of time. We feel it is important to determine the free CF concentration as this can be seen as a model that provides information on the compound concentration that will be able to reach the target receptors. The microdialysis technique also excludes possible contamination of the samples caused by the high levels of liposomes that are still present in the circulation. Although this makes it difficult to draw quantitative conclusions about the levels of CF in the brain microdialysates some qualitative conclusions can be drawn about brain levels of CF after free CF versus liposomal CF administration: (1) In our study 30 min after free CF treatment peak levels of CF were found in brain microdialysates and detectable levels of CF were found up to 5 h after treatment. This indicates some retention of CF in the brain after free IV CF administration since the levels in blood were below detection limit within 2 h; (2) In the animals treated with liposomal CF, detectable levels of CF were found and increased up to 12 h after the IV administration. Since the liposomal CF levels in the circulation stay high for much longer (half-life >24 h) it is likely that CF is present in the brain for days. Unfortunately technical limitations of the brain microdialysis technique did not allow for sampling after 12 h to quantify this amount.
In line with the in vitro results, we detected more CF delivery to the brain when comparing GSH-PEG to non-targeted PEG liposomes. The difference was around 4-fold over the 12 h measurement period, already starting during the first 30 min after administration. We can exclude that this significant difference is driven by differences in stability of the liposomes or by the amount of free CF in the circulation since the pharmacokinetics of CF from both liposomal formulations is similar.
Conclusions
Liposomal formulations with autoquenched CF proved to be a valuable tool to investigate the benefits of glutathione targeting to the brain, and of liposomal encapsulation on pharmacokinetics and tissue distribution. The same liposomal formulation in combination with brain microdialysis showed significantly increased delivery of free CF to the brain with GSH-PEG coated liposomes compared with PEG control liposomes. The results support further investigation into the versatility of GSH-PEG liposomes for drug delivery to the brain within a tolerable therapeutic window.
Declaration of interest
The work presented in this manuscript was sponsored by to-BBB technologies BV. The indicated authors are employees of to-BBB technologies BV. In addition, PJ Gaillard holds founder shares in to-BBB technologies BV. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
The authors thank Corine Visser (to-BBB technologies BV, Leiden, the Netherlands) for editorial assistance.
References
- Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 2004;104:29–45
- de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol 2007;47:323–55
- Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37:13–25
- Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics 2009;6:539–46
- Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother 2009;9:1519–27
- Dickson PI. Novel treatments and future perspectives: outcomes of intrathecal drug delivery. Int J Clin Pharmacol Ther 2009;47:S124–7
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron 2002;36:555–8
- Gaillard PJ, Visser CC, de Boer M, et al. Blood-to-brain drug delivery using nanocarriers. In: Hammarlund-Udenaes M, de Lange ECM, Thorne R, eds. Drug delivery to the brain – physiological concepts, methodologies and approaches. Chapter 15. New York: Springer; 2014:433–54
- Riganti C, Voena C, Kopecka J, et al. Liposome-encapsulated doxorubicin reverses drug resistance by inhibiting P-glycoprotein in human cancer cells. Mol Pharm 2011;8:683–700
- Huwyler J, Cerletti A, Fricker G, et al. By-passing of P-glycoprotein using immunoliposomes. J Drug Target 2002;10:73–9
- Pinzon-Daza ML, Campia I, Kopecka J, et al. Nanoparticle- and liposome-carried drugs: new strategies for active targeting and drug delivery across blood-brain barrier. Curr Drug Metab 2013;14:625–40
- Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today 2012;17:367–78
- Zlokovic BV, Mackic JB, McComb JG, et al. Evidence for transcapillary transport of reduced glutathione in vascular perfused guinea-pig brain. Biochem Biophys Res Commun 1994;201:402–8
- Kannan R, Kuhlenkamp JF, Jeandidier E, et al. Evidence for carrier-mediated transport of glutathione across the blood-brain barrier in the rat. J Clin Invest 1990;85:2009–13
- Kannan R, Chakrabarti R, Tang D, et al. GSH transport in human cerebrovascular endothelial cells and human astrocytes: evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res 2000;852:374–82
- Gaillard PJ, Appeldoorn CCM, Dorland R, et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One 2014;9:e82331
- Gaillard PJ, Appeldoorn CC, Rip J, et al. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J Control Release 2012;164:364–9
- Lindqvist A, Rip J, Gaillard PJ, et al. Enhanced brain delivery of the opioid peptide DAMGO in glutathione pegylated liposomes: a microdialysis study. Mol Pharm 2013;10:1533–41
- Mdzinarishvili A, Sutariya V, Talasila PK, et al. Engineering triiodothyronine (T3) nanoparticle for use in ischemic brain stroke. Drug Deliv Transl Res 2013;3:309–17
- Geldenhuys W, Mbimba T, Bui T, et al. Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. J Drug Target 2011;19:837–45
- Chen RF, Knutson JR. Mechanism of fluorescence concentration quenching of carboxyfluorescein in liposomes: energy transfer to nonfluorescent dimers. Anal Biochem 1988;172:61–77
- Roux F, Durieu-Trautmann O, Chaverot N, et al. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol 1994;159:101–13
- Gaillard PJ, Leyssen P, Appeldoorn CCM, et al. Development of brain-targeted liposomes with antiviral drugs for treating lethal viral encephalitis. [Abstract] Paper presented at the 8th International Conference on Cerebral Vascular Biology (CVB); 2009 Jun 28–July 2; Sendai, Japan
- Rip J, Appeldoorn CCM, Manca FM, et al. Receptor-mediated delivery of drugs across the blood-brain barrier. Pharmacology and Toxicology of the Blood-Brain Barrier: State of the Art, Needs for Future Research and Expected Benefits for the EU. Brussels, Belgium; 2010. doi: 10.3389/conf.fphar.2010.02.00025
- Allen TM, Hansen CB, Guo LS. Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection. Biochim Biophys Acta 1993;1150:9–16
- Chang T, Olson JA, Proffitt RT, Adler-Moore JP. Differences in tissue drug concentrations following intravenous versus intraperitoneal treatment with amphotericin B deoxycholate or liposomal amphotericin B. Med Mycol 2010;48:430–5
- Rosa P, Clementi F. Absorption and tissue distribution of doxorubicin entrapped in liposomes following intravenous or intraperitoneal administration. Pharmacology 1983;26:221–9
- Haber E, Afergan E, Epstein H, et al. Route of administration-dependent anti-inflammatory effect of liposomal alendronate. J Control Release 2010;148:226–33