Abstract
Objective: The Elecsys® immunoassay sFlt-1/PlGF ratio and the Triage® PlGF assay were compared (in a prospective, multicenter, case-control study) for diagnosis of preeclampsia/hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome. Methods: Women in European perinatal care centers with singleton pregnancies were enrolled: 178 cases had confirmed preeclampsia and 391 controls had normal outcome. Patients in the preeclampsia/HELLP syndrome group were matched pairwise by gestational week to healthy controls (1:2). Maternal blood samples were analyzed using (a) fully automated Elecsys PlGF and Elecsys sFlt-1 immunoassays with two cutoffs (early-onset [<34 weeks] ≤33, ≥85; late-onset [≥34 weeks] ≤33, ≥110), and (b) Triage PlGF immunoassay (single cutoff). Diagnostic performance and utility were assessed. Results: Respectively, 83 and 95 women had early-onset or late-onset preeclampsia/HELLP syndrome. The overall diagnostic performance of the Elecsys immunoassay sFlt-1/PlGF ratio (area under the curve [AUC] 0.941) was higher than for Triage PlGF (AUC 0.917). The Elecsys immunoassay sFlt-1/PlGF ratio sensitivity and specificity was: 94.0% (95% confidence interval [CI] 86.5–98.0) and 99.4% (95% CI: 96.8–99.9) for early-onset preeclampsia; and 89.5% (95% CI: 81.5–94.8) and 95.4% (95% CI: 91.7–97.8) for late-onset preeclampsia. The Triage assay sensitivity and specificity was: 96.4% (95% CI: 89.8–99.3) and 88.5% (95% CI: 82.8–92.8) (early-onset); and 90.5% (95% CI: 83–96) and 64.5% (95% CI: 57.8–70.9) (late onset). Conclusions: The fully automated Elecsys immunoassay sFlt-1/PlGF ratio provides improved diagnostic utility over the Triage PlGF assay with improved specificity for the clinical management of pregnant women with suspected preeclampsia/HELLP syndrome.
Introduction
Preeclampsia continues to be a leading cause of maternal and fetal morbidity and mortality worldwide with an incidence of 3%–5% (Citation1–Citation4). At least 42% of all maternal deaths annually, and 15% of all preterm deliveries, are due to preeclampsia (Citation5,Citation6). The heterogeneity of preeclampsia clinical presentation often complicates timely and accurate diagnosis and atypical cases continue to remain a daily challenge for the practicing obstetrician (Citation7).
The diagnosis of preeclampsia typically relies on the measurement of maternal blood pressure and proteinuria (Citation8). However, these two parameters are imprecise and have a low predictive value for disease progression and associated adverse outcomes. Furthermore, standardization of these methods can be difficult (Citation9,Citation10).
An imbalance of circulating angiogenic factors plays a central role in preeclampsia (Citation11,Citation12). Pregnant women who develop preeclampsia have an increased ratio of soluble fms-like tyrosine kinase-1 (sFlt-1) to placental growth factor (PlGF), resulting from increased sFlt-1 and decreased PlGF levels (Citation11,Citation13–Citation15). The marked increase in the circulating sFlt-1 concentration commences 5 weeks prior to the preeclampsia onset, accompanied by decreasing PlGF and vascular-endothelial growth factor levels (Citation14). Levels of circulating angiogenic factors may act as biomarkers for preeclampsia diagnosis.
Immunoassays to detect circulating angiogenic factors are available for clinical use in the diagnosis of preeclampsia. Two are compared in this study: the fully automated Elecsys® immunoassay sFlt/PlGF ratio on the cobas electrochemiluminescence immunoassay platform (Roche Diagnostics, Mannheim, Germany) and the Triage® PlGF assay (Alere, San Diego, CA).
The Triage PlGF assay (with a cutoff value of <36 pg/mL to confirm a preeclampsia diagnosis) has previously demonstrated comparable sensitivity and specificity in the diagnosis of early-onset preeclampsia to the Elecsys immunoassay sFlt-1/PlGF ratio assay (using fixed cutoff values) (Citation16). Recent evidence suggested that the sFlt-1/PlGF ratio cutoff of 85, although optimized for high specificity in preeclampsia diagnosis, was not optimal in terms of sensitivity to rule out preeclampsia (Citation17–Citation19). With accumulating evidence for the importance of the sFlt-1/PlGF ratio as a diagnostic and prognostic marker, different cutoffs for the Elecsys immunoassay sFlt-1/PlGF ratio have been defined to allow an assessment of early-onset (≤33, ≥85) and late-onset (≤33, ≥110) disease, and better reflect the putatively different pathophysiologies of early-onset and late-onset preeclampsia (Citation13). The sFlt-1/PlGF ratio can also predict the occurrence of preeclampsia-related adverse maternal and fetal outcomes, irrespective of the presence of a diagnosis of preeclampsia according to current standard diagnostic criteria (Citation20). Rana et al. demonstrated a significantly improved prediction rate of adverse outcomes among preeclampsia patients presenting at <34 weeks’ gestation (Citation20).
The aim of the current study was to compare the Elecsys immunoassay sFlt-1/PlGF ratio and the Triage PlGF assay with a focus on their diagnostic utility with regard to preeclampsia and HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, using a modified cutoff model in early and late phases of gestation.
Material and methods
Study population
Samples (n = 569) from singleton pregnancies in a previously reported multicenter case-control study from nine European perinatal care centers were used (the local ethics committees and institutional review boards approved the study, and all subjects gave their written informed consent before participation) (Citation13). The original study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The present study comprised 178 cases with confirmed preeclampsia and 391 controls with a normal pregnancy outcome: the only selection criteria for the present study was that cases/controls had both plasma and serum samples available, to enable comparison of the diagnostic performance of the Elecsys sFlt-1/PlGF immunoassay (which requires serum) and the Triage PlGF assay (which requires plasma samples). For the preeclampsia cases, blood samples were only taken at the visit when preeclampsia was diagnosed (Citation21). Preeclampsia was defined according to the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy (Citation22). HELLP syndrome was defined as previously published (Citation21). The pregnancy outcome was defined as normal if the mother was not diagnosed with any form of preeclampsia or HELLP syndrome and if the infant was not diagnosed with intrauterine growth restriction (as per local guidelines). Early and late gestational phases were defined as <34 weeks and ≥34 weeks, respectively.
Patients with any form of preeclampsia or HELLP syndrome were combined into one group for overall analysis. Patients in the preeclampsia/HELLP syndrome group with a negative or eventful outcome were matched pairwise by gestational week to a healthy control in a 1:2 manner, resulting in a double sample size of the control group. For each case subject, two control subjects were matched by gestational age: the only requirements for the matching were similar gestational age (<2 weeks’ difference), similar gestational phase (early or late), and the requirement that each subject must not contribute more than one visit. Therefore, a control patient only contributed samples either to the early-onset or the late-onset preeclampsia group, and no repeated samples were used in the case-control cohort, as per the methodology previously described (Citation13).
Samples and immunoassays
Ethylenediaminetetraacetic acid (EDTA) plasma samples from maternal blood were analyzed on Alere Triage MetroPro point-of-care analyzers at Bioscientia Labor Ingelheim, Germany, using the Triage PlGF immunoassay (Alere, San Diego, CA) according to the manufacturers’ instructions. The published cutoff value of <36 pg/mL was used (Citation16). The Elecsys immunoassay sFlt/PlGF ratio assay (Roche Diagnostics, Mannheim, Germany) was used to quantify the sFlt/PlGF ratio, as previously described (Citation13). Single measurements in serum were performed for sFlt-1 and PlGF using the fully automated Elecsys PlGF and Elecsys sFlt-1 immunoassays. Both assays are sandwich immunoassays based on electrochemiluminescence technology (Citation23). In the present study, an sFlt-1/PlGF ratio with a cutoff of ≤33 (“rule out”) and ≥85 (“rule in”) was used for the early gestational phase (before gestational week 34), and ≤33 (“rule out”) and ≥110 (“rule in”) for the late gestational phase (at or after gestational week 34), as previously described (Citation13). Triage PlGF values and Elecsys immunoassay sFlt-1/PlGF ratios were measured in the same subjects in different sample aliquots.
Statistics
In a receiver operator characteristic (ROC) analysis (Citation24), the overall area under the curve (AUC) of the Elecsys immunoassay sFlt-1/PlGF ratio curve and the Triage PlGF assay curve were assessed, as previously described (Citation13). In addition, the AUCs for the two gestational phases were also assessed. Sensitivity and specificity, plus 95% confidence interval (CI), for the two assays were assessed for all cutoffs mentioned above. 95% CIs were calculated according to DeLong et al. (Citation25).
Results
Cases and control patient populations
In total, 83 women presented with early-onset preeclampsia/HELLP (<34 weeks) and 95 with late-onset preeclampsia/HELLP syndrome (≥34 weeks) (). There were no significant differences in age, height, and weight between patients with early-onset or late-onset preeclampsia and their gestational age-matched controls. Also, the overall characteristics of the selected cases (based on availability of both a plasma and serum sample for analysis) were similar to the overall cohort of 569 cases from the initial study (Citation13).
Table 1. Demographics of the control subjects and patients.
Diagnostic performance
The diagnostic performances of the Elecsys immunoassay sFlt-1/PlGF ratio and Triage PlGF assay were similar. In the ROC analysis, the overall AUC of the Elecsys immunoassay sFlt-1/PlGF ratio curve (0.941; 95% CI: 0.9188–0.9632) was higher than that obtained by the Triage PlGF assay (0.9172; 95% CI: 0.8918–0.9426) (). The AUCs for the two gestational phases were comparable between the two assays, however, with discriminatory power appearing to be lower for both assays in the diagnosis of late-onset preeclampsia (). The AUCs for the early gestational phase were 0.9822 (95% CI: 0.9651–0.9993) with Elecsys immunoassay and 0.9744 (95% CI: 0.9551–0.9937) with Triage, while for the late gestational phase these values were 0.8884 (95% CI: 0.8461–0.9307; Elecsys immunoassay) and 0.8552 (95% CI: 0.8091–0.9013; Triage).
Figure 1. ROC curve for Elecsys immunoassay sFlt-1/PlGF ratio versus Triage PlGF assay. The AUC is 0.941 (95% CI: 0.9188–0.9632) for the Elecsys immunoassay sFlt-1/PlGF ratio and 0.9172 (95% CI: 0.8918–0.9426) for the Triage PlGF assay.
AUC = area under the curve; CI = confidence interval; PlGF = placental growth factor; ROC = receiver operating characteristics; sFlt-1 = soluble fms-like tyrosine kinase-1.
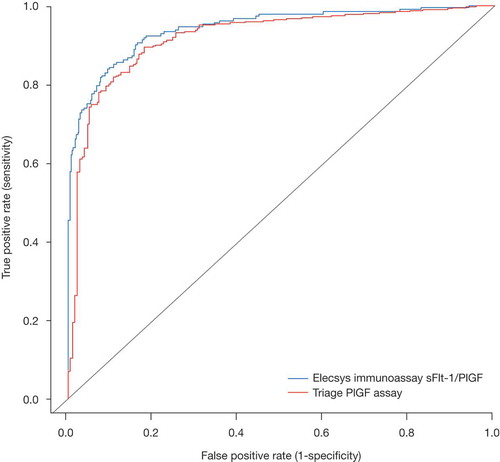
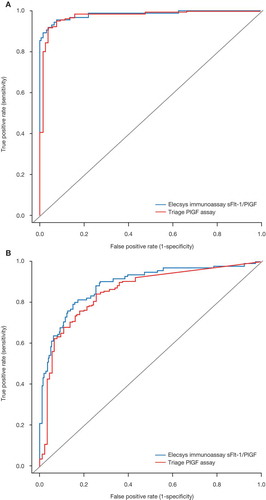
Figure 2. ROC curves for Elecsys immunoassay sFlt-1/PlGF ratio versus Triage PlGF assay in (A) early and (B) late gestational phases. The AUCs for early gestational phase were 0.9822 (95% CI: 0.9651–0.9993) (Elecsys immunoassay ratio) and 0.9744 (95% CI: 0.9551–0.9937) (Triage assay), and for late gestational phase were 0.8884 (95% CI: 0.8461–0.9307) (Elecsys immunoassay ratio) and 0.8552 (95% CI: 0.8091–0.9013) (Triage assay).
AUC = area under the curve; CI = confidence interval; PlGF = placental growth factor; ROC = receiver operator characteristic; sFlt-1 = soluble fms-like tyrosine kinase-1.
Diagnostic utility
Sensitivity and specificity data for preeclampsia detection (defined as single cutoff with Triage and two cutoffs with Elecsys immunoassay) are shown in . The specificity was higher using the Elecsys immunoassay sFlt-1/PlGF ratio for both early-stage and late-stage gestation, in particular for the late gestational phase (≥34 weeks of gestation; 95.4% specificity using the Elecsys immunoassay ratio at cutoff 110 vs. 64.5% using the Triage PlGF assay). Sensitivity was comparable between the Triage PlGF assay and the Elecsys immunoassay sFlt-1/PlGF ratio for both early-stage and late-stage gestation. sFlt-1/PlGF ratio and PlGF results for cases and controls by preeclampsia/HELLP status, along with the cutoffs applied for both systems, are shown in . Of note, the equivocal zone between the lower and upper sFlt-1/PlGF cutoffs contains a mixture of cases and controls that could not be accurately separated from each other using either system. The results for the sFlt-1/PlGF ratio and PlGF results for cases and controls by gestational age are shown in .
Table 2. Sensitivity and specificity of Elecsys immunoassay sFlt-1/PlGF ratio and Triage PlGF assays in the positive diagnosis of preeclampsia.
Figure 3. Scatterplot showing Elecsys immunoassay sFlt-1/PlGF ratio and Triage PlGF results for cases and controls. The horizontal dashed lines illustrate the Elecsys immunoassay ratio cutoffs, the vertical dashed line represents the Triage assay cutoff. Both the vertical and the horizontal scale are shown in logarithmic scale.
HELLP = hemolysis, elevated liver enzymes, low platelets; PlGF = placental growth factor; sFlt-1 = soluble fms-like tyrosine kinase-1.
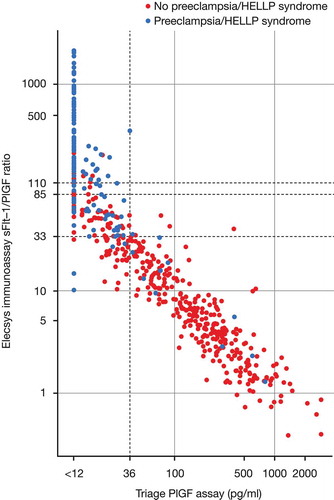
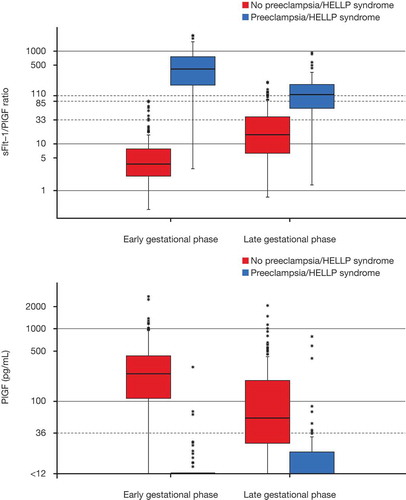
Figure 4. Boxplots showing (A) sFlt-1/PlGF ratio and (B) PlGF result for cases and controls by gestational age with cutoffs for each system.The box represents the median and first and third quartiles (the 25th and 75th percentiles). The upper whisker extends from the box to the highest value that is within 1.5 × interquartile range (IQR). The lower whisker extends from the box to the lowest value within 1.5 × IQR. Data beyond the end of the whiskers are outliers and plotted as points. Early and late gestational phases were defined as <34 and ≥34 weeks, respectively. The vertical scales in (A) and (B) are shown in logarithmic scale. The cutoffs for each assay system are illustrated by dashed horizontal lines.
HELLP = hemolysis, elevated liver enzymes, low platelets; PlGF = placental growth factor; sFlt-1 = soluble fms-like tyrosine kinase-1.
Conclusion
The aim of the present study was to compare the Elecsys immunoassay sFlt-1/PlGF ratio and Triage PlGF assay with a focus on their diagnostic utility in preeclampsia and HELLP syndrome. Previous studies have consistently demonstrated that the automated measurement of sFlt-1, PlGF, and the calculation of the sFlt-1/PlGF ratio are reliable tools for the diagnosis of preeclampsia (Citation19,Citation21,Citation26,Citation27). This study suggests that the fully automated Elecsys immunoassay sFlt-1/PlGF ratio, utilizing different cutoffs for early and late gestation, provides improved diagnostic utility for the clinical management of pregnant women with suspected preeclampsia/HELLP syndrome over the Triage PlGF assay. The Elecsys immunoassay sFlt1/PlGF ratio demonstrated considerably greater specificity compared with the Triage PlGF assay while having comparable sensitivity, underlining its usefulness in ruling in and ruling out preeclampsia for at-risk pregnant women. Nevertheless, ROC analyses suggested that both assays appeared to have comparable diagnostic accuracy. In addition, diagnostic performance of both the Elecsys sFlt-1/PlGF ratio immunoassay and the Triage PlGF assay in diagnosing preeclampsia/HELLP syndrome appeared better in early gestation compared with late gestation.
The overall performance of the Triage PlGF assay in confirming a diagnosis of preeclampsia has been previously published (Citation16). For the Elecsys immunoassay sFlt-1/PlGF ratio different cutoffs have been proposed for aid in diagnosis of preeclampsia/HELLP syndrome (Citation19,Citation21,Citation26). The different cutoff values for early-onset (≤33, ≥85) and late-onset preeclampsia disease (≤33, ≥110) used in the Elecsys sFlt-1/PlGF immunoassay in this study increase the diagnostic accuracy of the test. The range of values between the lower and upper cutoff levels also identifies patients whom preeclampsia cannot be confidently ruled in or ruled out, and for whom repeated monitoring would be clinically appropriate. At the current time, there are no formal guidelines regarding the use of PlGF or the sFlt-1/PlGF ratio; however, consensus statements have been developed by international experts on the clinical use of the Elecsys immunoassay sFlt-1/PlGF ratio (and consequent patient management based on the result) in cases of suspected preeclampsia or in women at high risk of the syndrome (Citation28).
The increased diagnostic specificity obtained by the use of Elecsys immunoassay sFlt-1/PlGF ratio led to fewer false-positive results than the Triage PlGF assay alone. This is a clinically relevant finding, which should support more appropriate clinical decisions and reduce the number of patients undergoing inappropriate lengthy hospitalization for suspected preeclampsia or to rule out other secondary diagnoses (Citation27). This hypothesis is supported by , which shows the higher diagnostic specificity of the sFlt-1/PlGF ratio >85 for early-onset or >110 for late-onset preeclampsia/HELLP syndrome compared with the PlGF assay (with the cutoff of <36 pg/mL, many control samples were flagged as being at risk). Given that preeclampsia is associated with a shorter time to delivery, patients identified by the Elecsys immunoassay sFlt-1/PlGF ratio as being particularly at risk would be immediately (and appropriately) hospitalized. In addition, the high sensitivity and specificity when using the two gestational-age cutoffs defined for the sFlt-1/PlGF ratio offer physicians a greater degree of confidence to “rule in” or “rule out” preeclampsia. Therefore, while the PlGF and sFlt-1/PlGF ratio assays have comparable technical performance, the application of the Elecsys immunoassay sFlt-1/PlGF ratio appears to offer a greater degree of diagnostic utility in the clinic.
In women with suspected preeclampsia presenting at <34 weeks, the circulating sFlt-1/PlGF ratio predicted adverse outcomes (including iatrogenic delivery, small for gestational age birth weight, fetal/neonatal death) occurring within 2 weeks (Citation20). Lai et al. also demonstrated that testing by PlGF and sFlt-1 at 30–33 weeks, they could identify all pregnancies developing preeclampsia and requiring delivery within the subsequent 4 weeks (Citation29). Serial measurements of angiogenic factors allow dynamics in serum values to be assessed and permit a risk-adapted prenatal care of these patients.
Limitations of the present study include the retrospective case-control study design. In addition, the Elecsys immunoassay sFlt-1/PlGF ratio cutoffs and the Triage PlGF cutoff have only been validated for these immunoassays. Optimal cutoffs for sFlt-1/PlGF and PlGF alone, respectively, may be different using assays from other manufacturers. A randomized controlled study using the dual cutoff Elecsys sFlt-1/PlGF ratio immunoassay is warranted in a study population at high risk of preeclampsia to support the findings from the present analysis.
In summary, data from the present study suggest that the Elecsys immunoassay sFlt-1/PlGF ratio, combined with appropriate gestational age-related cutoff values, could better help identify individuals with high risk of developing preeclampsia, compared with the PlGF measurement alone, thus allowing the optimization of their care.
Declaration of interest
MH and MG are employees of Roche Diagnostics International, Rotkreuz, Switzerland. BD and CD are employees of Roche Diagnostics GmbH, Penzberg, Germany. HS has worked as an advisor for Roche Diagnostics and has received fees for lecturing. SV has received grants from and acted as a consultant for Roche Diagnostics, and acted as a consultant for ThermoFisher. WK, PW, BS, and TM are employees of Bioscientia GmbH, contracted by Roche Diagnostics. LD has no conflicts of interest.
Contribution to authorship
All authors were involved in the writing of the manuscript and all authors made the decision to submit for publication.
Acknowledgments
The authors would like to thank Christoph Ehret for his statistical input and for his work on the statistical analysis plan and Michael Pfeffer for useful discussions on the manuscript content, both from Roche Diagnostics GmbH, Penzberg, Germany. An author, Carina Dinkel, has carried out the statistical analyses described in this article. ELECSYS and COBAS are trademarks of Roche.
Funding
The study was sponsored by Roche Diagnostics, who were involved in the study design, data collection, statistical analysis, and interpretation of data.Third-party writing assistance under the direction of the authors was provided by Martin Quinn, PhD, and Emma McConnell, PhD, both from Gardiner-Caldwell Communications (Macclesfield, UK), and was funded by Roche Diagnostics.
Additional information
Funding
References
- Hogberg U. The World Health Report 2005: ‘‘make every mother and child count’’ – including Africans. Scand J Public Health. 2005;33:409–411.
- Ray JG, Vermeulen MJ, Schull MJ, et al. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803.
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137.
- Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376:631–644.
- Noris M, Perico N, Remuzzi G. Mechanisms of disease: pre-eclampsia. Nat Clin Pract Nephrol. 2005;1:98–114.
- Roberts JM, Pearson GD, Cutler JA, et al. Summary of the NHBLI working group on research on hypertension during pregnancy. Hypertens Pregnancy. 2003;22:109–127.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200:481.e1–481.e7.
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131.
- Oliveira SM, Arcuri EA, Santos JL. Cuff width influence on blood pressure measurement during the pregnant-puerperal cycle. J Adv Nurs. 2002;38:180–189.
- Reinders LW, Mos CN, Thornton C, et al. Time poor: rushing decreases the accuracy and reliability of blood pressure measurement technique in pregnancy. Hypertens Pregnancy. 2006;25:81–91.
- Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658.
- Chaiworapongsa T, Chaemsaithong P, Yeo L, et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–480.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase–specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- Shibata E, Rajakumar A, Powers RW, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903.
- Benton SJ, Hu Y, Xie F, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol. 2011;205:469–471.
- Schiettecatte J, Russcher H, Anckaert E, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem. 2010;43:768–770.
- Gómez-Arriaga PI, Herraiz I, López-Jiménez EA, et al. Uterine artery Doppler and sFlt-1/PlGF ratio: usefulness in diagnosis of pre-eclampsia. Ultrasound Obstet Gynecol. 2013;41:530–537.
- Álvarez-Fernández I, Prieto B, Rodríguez V, et al. New biomarkers in diagnosis of early onset preeclampsia and imminent delivery prognosis. Clin Chem Lab Med 2014;52(8):1159–1168.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected pre-eclampsia. Circulation. 2012;125:911–919.
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PlGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161.e1–161.e11.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22.
- Roche Diagnostics GmbH: Elecsys PlGF Immunoassay, Package Insert. 2015-11, V 8.0.. Available at: https://pim-eservices.roche.com/(S(aou3rto4kqllwta02cqhham0))/pi/en/Documents/GetDocument?documentId=b4d1d21e-03cf-e411-19b6-00215a9b0ba8. Accessed February 17, 2016.
- Sing T, Sander O, Beerenwinkel N, et al. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845.
- Ohkuchi A, Hirashima C, Takahashi K, et al. Onset threshold of the plasma levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio for predicting the imminent onset of preeclampsia within 4 weeks after blood sampling at 19–31 weeks of gestation. Hypertens Res. 2013;36:1073–1080.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–58e8.
- Stepan H, Herraiz I, Schlembach D, et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol. 2015;45:241–246.
- Lai J, Garcia-Tizon Larroca S, Peeva G, et al. Competing risks model in screening for preeclampsia by serum placental growth factor and soluble fms-like tyrosine kinase-1 at 30-33 weeks’ gestation. Fetal Diagn Ther. 2014;35:240–248.
