Abstract
Objective: Determine the independent association between time-specific placental growth factor (PIGF)—a marker of placental vasculature—and infant birth weight in offspring of mothers with preexisting type 1 and 2 diabetes. Methods: A total of 150 women were recruited from Joslin Diabetes Center’s/Beth Israel Deaconess Medical Center’s Diabetes in Pregnancy Program. PlGF was measured up to four times during pregnancy. Infant birth weight and covariate data were collected from medical records. Hemoglobin A1c was assessed from drawn blood samples. We used generalized linear and log-binomial models to calculate the change in continuous birth weight, as well as macrosomia for every unit change in time-specific ln-transformed PlGF, respectively. Models were adjusted for potential confounders. Results: Approximately 75% of women had type 1 diabetes. Third trimester PlGF levels were significantly associated with infant birth weight (r = 0.24, p = 0.02 at 27–34 weeks; r = 0.26, p < 0.009 for 36–40 weeks). After full adjustment, there was a 6.1% and 6.6% increase in birth weight for gestational age percentile for each unit increase in ln-transformed PlGF level at 27–34 weeks and 35–40 weeks, respectively (95% CI for 27–34 weeks gestation: 1.1, 11.0, and 95% CI for 35–40 weeks gestation: 0.7%, 12.5%). We found a statistically significant increased risk of macrosomia among women with higher ln-transformed PlGF levels (RR: 1.72; 95% CI: 1.09, 2.70). Associations were not mediated by hemoglobin A1c. Conclusions: Third trimester PlGF levels were associated with higher birth weight in women with preexisting diabetes. These findings may provide insight to the pathophysiology of fetal overgrowth in women with diabetes.
Introduction
Large for gestational age (LGA) and macrosomia occur in approximately 50% of pregnancies complicated by preexisting type 1 diabetes (Citation1,Citation2). Women with preexisting type 2 diabetes have a similar risk of macrosomia as those with type 1 diabetes (Citation3). The underlying mechanisms of excess fetal growth in women with preexisting diabetes are not completely understood. The Pedersen Hypothesis suggests that maternal hyperglycemia results in fetal hyperglycemia, which causes fetal hyperinsulinemia and excessive accretion of fetal fat (Citation4). While blood glucose during pregnancy is assuredly one of the pathways involved in fetal overgrowth, the inconsistency of studies associating blood glucose and fetal growth indicates that there are other factors at play (Citation3, Citation5, Citation6–Citation8, Citation9,Citation10). Despite good blood glucose control, women with preexisting diabetes appear to have large babies. (Citation1) As such, new factors need to be identified to better understand fetal overgrowth in the offspring of women with preexisting diabetes.
Among other factors that may affect fetal growth is placental growth factor (PlGF), an angiogenic growth factor produced by the placenta (Citation11). Higher PlGF levels may signal better vasculature, which could result in increased glucose transport in women with preexisting diabetes during hyperglycemic excursions, leading to excess glucose exposure to the offspring in utero. While several studies have shown that lower PlGF levels are associated with intrauterine growth restriction (Citation12–Citation15), few have evaluated higher PlGF levels and their association with higher birth weight, particularly among those with preexisting diabetes (Citation16–Citation17). In one study by Kuc et al., first trimester PlGF was associated with LGA in women with preexisting diabetes (Citation17). However, this study did not adjust for potential confounding factors such as maternal first trimester BMI, nor did it evaluate potential mediation by HbA1c or multiple time points across pregnancy. Assessment of multiple time points would allow for better determination of sensitive windows in which PlGF concentrations may best predict high infant birth weight in offspring of women with diabetes. Of interest are two studies that suggest that third trimester PlGF levels may be associated with important adverse pregnancy outcomes in women with and without diabetes, such as preeclampsia (Citation18,Citation19). Given the association between preeclampsia and birth weight (Citation19), it is important to extend these findings to look at trimester-specific PlGF levels and their association with birth weight among women with diabetes, a group at high risk of delivering a high birth weight offspring.
Therefore, we evaluated the association between time-specific maternal PlGF levels and infant birth weight in a population of women with preexisting diabetes. We assessed potential confounders, including maternal age and pre-pregnancy BMI. We also evaluated mediation by HbA1c across these time periods. We posited that higher PlGF levels in the third trimester, during the period of rapid fetal growth and increased hyperglycemic excursions due to increasing insulin resistance, would be associated with higher infant birth weight, as an indicator of increased fetal growth. We also hypothesized that PlGF would have a direct and indirect effect on birth weight that would be most apparent in the third trimester, with partial mediation through HbA1c either in first or in the third trimester.
Methods
Study population
Study subjects were women with type 1 or type 2 diabetes recruited from Joslin Diabetes Center’s and Beth Israel Deaconess Medical Center’s Diabetes in Pregnancy program (Boston, MA) between August 2004 and May 2008. The parent study and recruitment practices have been described elsewhere (Citation18). Briefly, during the first trimester of pregnancy, women with preexisting type 1 or 2 diabetes, who had a singleton pregnancy, were recruited into the study (n = 159). Blood samples were collected from study participants at routine pregnancy time points, with a possibility of up to four blood samples (i.e. blood samples were collected at 11–18, 19–26, 27–34, and 35–40 weeks gestation). At 11–18 weeks, 74.7% of the population had blood samples available, 61.3% at 19–26 weeks, 66.7% at 27–34 weeks, and 65.3% at 35–40 weeks. Samples were stored at –80°C. We excluded n = 9 women from this study due to no available data on PlGF or infant birth weight information, for an analytic data set of n = 150 women with preexisting type 1 or 2 diabetes.
PIGF assays
For assay performance, blood samples were frozen at –80°C and thawed once. We analyzed ethylenediaminetetraacetic acid plasma or serum samples for free PlGF using Access Immunoassay system’s automated prototype assays (Beckman Coulter, Chaska, MN). These assays involved a one-step immunoenzymatic “sandwich” assay and have been described elsewhere (Citation20,Citation21). The PlGF upper limit was 10,000 pg/mL and the CV for this assay is 3% for within- and between-run precision. The PlGF assays were performed in one batch by a single technician, who was not aware of the study participant’s medical history or the birth weight of the offspring. Due to the skewedness of this variable, we natural log-transformed PlGF. Study participants could have up to four measurements of PlGF based on the above-mentioned time points in pregnancy. We evaluated PlGF levels at each specific pregnancy time point (i.e. 11–18, 19–26, 27–34, and 35–40 weeks gestation) to determine whether there was a window of time that was more relevant with regard to higher PlGF and infant birth weight.
Hemoglobin A1c (HbA1c)
Starting in the first trimester, HbA1c was measured during study participant’s prenatal visits, as a part of their standard clinical care during pregnancy for all women with preexisting type 1 or 2 diabetes. We used standard commercial assays—Roche Hitachi Tina-quant immunoassay (Basel, Switzerland) and Integra Hemoglobin A1c Generation 2 immunoassay (Basel, Switzerland). For the present analysis, we evaluated information on the first prenatal HbA1c (baseline) and the HbA1c closest to delivery to determine whether blood glucose control affected birth weight. This measure was evaluated continuously, as well as dichotomized at HbA1c ≥48 mmol/mol v. <48 mmol/mol (≥6.5% vs. HbA1c <6.5%).
Infant birth weight
At the time of delivery, infants born to study participants were weighed in grams. Birth weight for gestational age was calculated as a percentile based on birth weight and the length of gestation. The latter was calculated from the estimated date of confinement (confirmed by ultrasound) and the date of delivery (Citation22). For conciseness, we use birth weight percentile to represent birth weight for gestational age percentile.
Covariates
Maternal age (continuous), race/ethnicity (i.e. Black, Asian, Hispanic vs. White), history of diabetes complications (yes vs. no for personal history of retinopathy, hypertension, or nephropathy), and parity (1 or more vs. 0 not including the index pregnancy) were collected from medical record data. We also collected data from medical records on preeclampsia in the index pregnancy, type of diabetes (1 vs. 2), and years with diabetes (continuous). Information on pre-pregnancy or first trimester body mass index (BMI, continuous) was collected from weight and height measurements obtained at the specified time points (weight in kg/height in m2).
Statistical analysis
We calculated means and standard deviations for normally distributed continuous variables. We calculated medians and the 25th and 75th percentiles for skewed continuous variables and the frequency and percentages for categorical variables.
Unadjusted associations between ln-transformed PIGF at each gestational age group and birth weight for gestational age percentile were examined with Pearson Product-Moment correlations. We also calculated the correlation between HbA1c at baseline and at delivery with birth weight percentile.
We used general linear models (GLMs) to calculate beta coefficients and 95% confidence intervals to assess the association between ln-transformed time-specific PlGF and continuous birth weight percentile, as well as ln-transformed time-specific PlGF and continuous HbA1c at baseline and delivery. BMI, parity, years of DM, history of retinopathy, and history of other diabetes-related complications (i.e. hypertension, nephropathy, etc.) were tested as potential confounders. Of these, only maternal age altered the beta for PlGF by >10%. Therefore, our final model only included maternal age.
We posited that HbA1c was a potential mediator of the association between PlGF and birth weight percentile. For assessing mediation, we used the Baron and Kenney method, where we evaluated whether the inclusion of these potential mediators in the adjusted model attenuated the association between our exposure and outcome of interest (Citation23). To start, we evaluated the association between baseline and delivery HbA1c and birth weight percentile. HbA1c at baseline and delivery were evaluated in separate models. For this, confounders (as mentioned earlier) of baseline or delivery HBA1C ≥ 48 mmol/mol (≥ 6.5%) were tested in univariate GLM predicting birth weight for gestational age percentile. We constructed the following models using GLM: (Citation1) unadjusted, (Citation2) maternal age adjusted, (Citation3) maternal age, race, maternal BMI, years with type 1 diabetes, history of retinopathy, and history of nephropathy adjusted. For the association between HbA1c at delivery and birth weight percentile, we also included preeclampsia in the model. Where mediation was not present, direct effect reporting was determined to be appropriate. All statistical analyses were performed with SAS version 9.4. Two-tailed p-values <0.05 were considered to indicate statistical significance.
Results
In this population of women with preexisting type 1 or type 2 diabetes, the majority of women had type 1 diabetes (74.5%). Most women in the population were overweight or obese, with 70% having a BMI ≥ 25. The majority of this population was white (87.3%). A fair number had other preexisting complications, such as retinopathy (32.4%) or a history of hypertension (16.1%). Preeclampsia affected 12.7% of the pregnancies. The mean baseline HbA1c was 52.0 mmol/mol (SD: 14.4) (6.9% (SD: 1.3)). The time-specific PlGF levels are shown in . Approximately, 25% of infants born to mothers with predelivery HbA1c < 42.1 mmol/mol (<6.0%) were LGA. The mean birth weight for infants was 3523 g (SD: 745), with a mean birth weight for gestational age of almost 71% (SD: 26.7) (). A total of 0.7% of infants were small-for-gestational age (SGA), 55.8% were average-for-gestational age (AGA), and 43.5% were large-for-gestational age (LGA). Macrosomia was present in 20.7% of the offspring (n = 31).
Table 1. Characteristics of women with preexisting type 1 and type 2 diabetes in pregnancy (n = 150).
PlGF and birth weight for gestational age
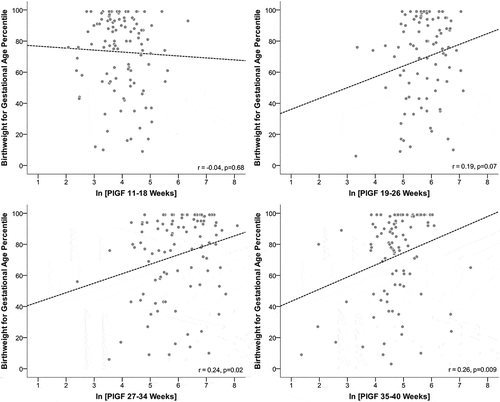
shows the association between ln-transformed PlGF at discrete time points (Panels A–D). The strongest correlations between PlGF and birth weight percentile occurred at 27–34 weeks (r = 0.24, p = 0.02) and 34–40 weeks (r = 0.26, p = 0.009). When evaluating the unadjusted and adjusted associations between ln-transformed time-specific PlGF levels, we found associations to be most significant later in pregnancy, with the strongest association at 35–40 weeks (adj. PlGF β: 6.6%; 95% CI: 0.7%, 12.5%) (). When restricting to women without preeclampsia, associations were similar (adj. β: 7.0%; 95% CI: 1.5%, 12.4%; data not shown). Adjustment for maternal BMI, race/ethnicity, and diabetes-related complications did not alter associations.
Figure 1. Unadjusted association between ln-transformed PlGF by gestational age and birth weight for gestational age percentile among women with preexisting diabetes (n = 150).
When assessing HbA1c as a mediator of the association between PlGF and birth weight percentile, neither baseline HbA1c nor delivery HbA1c appeared to mediate the association between PlGF and birth weight percentile (). Specifically, we did not find an association between late third trimester ln-transformed PlGF and HbA1c at baseline or delivery (HbA1c at baseline: β: –0.13; 95% CI: –0.36, 0.11 and HbA1c at delivery: β: 0.03; 95% CI: –0.10, 0.17).
Table 2. Associations between ln-transformed time-specific PlGF and birth weight for gestational age percentile among women with preexisting diabetes (n = 150).
PlGF, LGA, and macrosomia
In addition to evaluating birth weight percentile as a continuous outcome, we also evaluated the association between ln-transformed PlGF and LGA (>90th percentile in birth weight) and ln-transformed PlGF and macrosomia (birth weight >4000 g). The association between ln-transformed PlGF and LGA appeared to be trending in the expected positive direction, but did not reach statistical significance (OR for PlGF levels at 12–18 weeks gestation: 1.02 (95% CI: 0.63, 1.64) versus OR for PlGF levels at 36–40 weeks gestation: 1.58; 0.96, 2.58). However, we found a statistically significant association for the more extreme birth weight outcome of macrosomia, with women with diabetes who had higher late third trimester PlGF levels having a 1.72 increased risk of delivering an infant with macrosomia (95% CI: 1.09, 2.07).
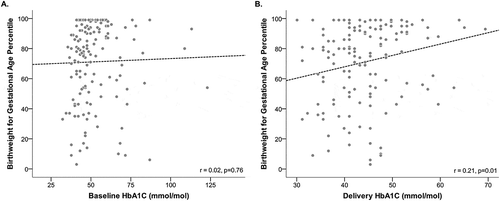
Hemoglobin A1c and birth weight for gestational age
In (Panels A and B), we show the crude association between continuous HbA1c at baseline and delivery with birth weight for gestational age. While baseline continuous HbA1c is not associated with birth weight percentile, delivery continuous HbA1c is associated with birth weight (r = 0.21, p = 0.01).
In , we present the crude and adjusted associations between HbA1c at baseline and delivery and birth weight percentile for gestational age. When evaluating dichotomized baseline A1c measures, we found a significant increase in birth weight percentile for gestational age if maternal baseline hemoglobin HbA1c ≥48 mmol/mol (≥6.5%), after adjustment for maternal age, race, and history of retinopathy and nephropathy. Specifically, if a mother had a baseline HbA1c ≥48 mmol/mol (≥6.5%), then her infant had a birth weigh percentile for gestational age that was 8.8% higher than an infant born to a mother that had a baseline HbA1c <48 mmol/mol (<6.5%) (95% CI: 0.4%,17.2%). When assessing dichotomized HbA1c at delivery, we found that mothers with a HbA1c ≥48 mmol/mol (≥6.5%) delivered an infant whose birth weight percentile for gestational age was 9.9% higher than a mother who had a delivery HbA1c <48 mmol/mol (<6.5%) (95% CI: 0.8%, 19.1%). Adjustments for maternal BMI, race/ethnicity, and diabetes-related complications did not alter these associations.
Table 3. Associations between HbA1C and birth weight for gestational age percentile among women with preexisting diabetes (n = 150).
Discussion
In this single-center study, we found a modest but significant association between later pregnancy PlGF levels and infant birth weight percentile among women with preexisting diabetes. In fact, we also found a significant association of late third trimester PlGF and macrosomia for mothers with diabetes. A significant relationship of third trimester HbA1c was also seen with birth weight percentile, but the degree to which third trimester HbA1c explains birth weight was similar to third trimester PlGF levels. Interestingly, the associations between PlGF and birth weight were not mediated by pregnancy HbA1c levels. These findings suggest that both PlGF in later pregnancy and HbA1c could be independent predictors of infant birth weight in women with preexisting diabetes and that women with preexisting diabetes who have higher PlGF levels may be at increased risk of delivering an infant of higher birth weight.
The majority of studies evaluating the association between PlGF and birth weight have focused on populations without preexisting diabetes. Poon et al. found an association between lower PlGF levels and SGA among women without preexisting diabetes (Citation15). In the few studies that have evaluated PlGF and birth weight in women with preexisting diabetes, findings are inconclusive, likely due to differences in timing and measurement of PlGF across studies. For example, no association was found between PlGF and birth weight by Loukovaara and others (Citation14). However, PlGF was based on cord blood samples at the time of delivery, which may not represent maternal levels of PlGF during pregnancy. A study by Gutaj et al. found an association between low PlGF and SGA. Only one study to our knowledge evaluated the upper end of birth weight by assessing macrosomia among women with preexisting diabetes (Citation17). However, they only evaluated first trimester measures of PlGF (Citation17). In the present study, we evaluated maternal PlGF levels at multiple time points in women with preexisting diabetes and found third trimester levels to be the strongest predictor of birth weight in this population.
Third trimester PlGF may be involved in higher birth weight among women with preexisting diabetes through having a better vascularized (“healthier”) placenta. As such, higher PlGF could be a marker of more effective transport of glucose and other nutrients to the fetal circulation. In women with preexisting diabetes, this more effective transport of glucose could lead to fetal overgrowth due to higher glucose transport to the fetus during times of hyperglycemic excursions, leading to greater likelihood of fetal overgrowth. In fact, larger placentas have been found in women with LGA infants (Citation24).
While it is well-accepted that fetal overgrowth is at least in part due to maternal hyperglycemia leading to increased fetal fat accretion through fetal hyperinsulinemia (Citation4, Citation25), in the present study, there was an association between third trimester PlGF and birth weight that was independent of HbA1c. Specifically, PlGF was not associated with HbA1c in this population of women with preexisting diabetes. This finding suggests that with regard to birth weight, PlGF may operate as a part of a separate pathway for high birth weight. The lack of an association between PlGF and HbA1c may suggest that PlGF is permissive of chronic and acute fetal glucose exposure. On the other hand, HbA1c, as an average of glucose over an extended time period, may not be able to capture acute hyperglycemic excursions that could lead to fetal overgrowth (Citation26) in the context of good placental vasculature.
This study has several limitations. First, this is a small study of 150 women with type 1 or type 2 diabetes. Mechanisms involving birth weight may differ by type of diabetes, particularly due to other lifestyle and comorbid chronic disease factors that are involved that may differ between these two groups. Second, we used complete case assessment methods to evaluate the association between time-specific PlGF levels and infant birth weight percentile. While the majority of women had PlGF measurements at multiple time points (84%), with 70% having measures in the third trimester, it is possible that those women who made it to the third trimester have higher PlGF levels with the chance of having a stronger effect in this subgroup compared to all women in the overall population. That said, women who make it this far into pregnancy with preexisting diabetes may be among the healthiest and have better functioning placenta that allow for the greater transfer of glucose and other fuels that may lead to increased fetal growth. Future studies will need to further investigate these findings.
Despite these limitations, this study has several strengths. First, this is among the first studies to evaluate PlGF at multiple time points in a population of women with preexisting type 1 and type 2 diabetes. Second, we assessed the direct and indirect effects of PlGF on birth weight percentile, positing that A1c might partially mediate the association. Third, we adjusted for a variety of factors and stratified by pre-pregnancy BMI to determine the association between time-specific PlGF and birth weight percentile. Finally, our outcome was assessed both as continuous birth weight percentile and as clinically relevant measures of LGA and macrosomia.
In conclusion, third trimester PlGF levels independently predicted higher birth weight percentile and were associated with macrosomia in pregnancies complicated by preexisting diabetes. HbA1c also predicted infant birth weight percentile in this population. However, PlGF and HbA1c were independent of one another. With birth weight being the result of multiple pathways, the finding of a positive association between PlGF and birth weight may warrant further investigation into the physiology of excess fetal growth in women with preexisting diabetes. Given that LGA and macrosomia are major adverse pregnancy outcomes among women with preexisting diabetes, future studies are needed to determine the underlying mechanism between PlGF and higher infant birth weight in this high-risk group of women.
Acknowledgments
The authors would like to thank Dr. S. Ananth Karamanchi, who assisted with the original data collection, with partial support from in Pfizer Inc. and Merck & Co. However, the authors of the present study were not funded by either Pfizer or Merck & Co. We would also like to thank the study subjects as well as Suzanne Ghiloni for nursing expertise and Breda Curran for administrative assistance in the Joslin/Beth Israel Diabetes and Pregnancy Program.
Funding
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12HD051959) and the Department of Medicine Seed funds from the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center – Harvard/MIT 290 Health Sciences and Technology.
Disclosure statement
The authors of this paper declare no conflicts of interest.
Additional information
Funding
References
- Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia despite good glycaemic control in Type I diabetic pregnancy: results of a nationwide study in The Netherlands. Diabetologia 2002;45(11):1484–9.
- Persson M, Pasupathy D, Hanson U, Norman M. Birth size distribution in 3,705 infants born to mothers with type 1 diabetes: a population-based study. Diabetes Care 2011;34(5):1145–9.
- Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and meta-analysis. J Clin Endocrinol Metab 2009;94(11):4284–91.
- Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16(4):330–42.
- Landon MB, Gabbe SG, Piana R, Mennuti MT, Main EK. Neonatal morbidity in pregnancy complicated by diabetes mellitus: predictive value of maternal glycemic profiles. Am J Obstet Gynecol 1987;156(5):1089–95.
- Parfitt VJ, Clark JD, Turner GM, Hartog M. Maternal postprandial blood glucose levels influence infant birth weight in diabetic pregnancy. Diabetes Res 1992;19(3):133–5.
- Jovanovic-Peterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development: Diabetes in Early Pregnancy Study. Am J Obstet Gynecol 1991; 164 (1 Pt 1): 103–11.
- Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care 1992;15(10):1251–7.
- Kyne-Grzebalski D, Wood L, Marshall SM, Taylor R. Episodic hyperglycaemia in pregnant women with well-controlled Type 1 diabetes mellitus: a major potential factor underlying macrosomia. Diabet Med 1999;16(8):702–6.
- Roland JM, Murphy HR, Ball V, Northcote-Wright J, Temple RC. The pregnancies of women with Type 2 diabetes: poor outcomes but opportunities for improvement. Diabet Med 2005;22(12):1774–7.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111(5):649–58.
- Gutaj P, Wender-Ozegowska E, Iciek R, Zawiejska A, Pietryga M, Brazert J. Maternal serum placental growth factor and fetal SGA in pregnancy complicated by type 1 diabetes mellitus. J Perinat Med 2014;42(5):629–33.
- Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112(1):51–7.
- Loukovaara M, Leinonen P, Teramo K, Andersson S. Concentration of cord serum placenta growth factor in normal and diabetic pregnancies. BJOG 2005;112(1):75–9.
- Poon LC, Zaragoza E, Akolekar R, Anagnostopoulos E, Nicolaides KH. Maternal serum placental growth factor (PlGF) in small for gestational age pregnancy at 11(+0) to 13(+6) weeks of gestation. Prenat Diagn 2008;28(12):1110–5.
- Sundrani D, Khot V, Pisal H, Mehendale S, Wagh G, Joshi A, et al. Gestation dependant changes in angiogenic factors and their associations with fetal growth measures in normotensive pregnancy. PLoS One 2013;8(1):e54153.
- Kuc S, Wortelboer EJ, Koster MP, de Valk HW, Schielen PC, Visser GH. Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG 2011;118(6):748–54.
- Cohen AL, Wenger JB, James-Todd T, Lamparello BM, Halprin E, Serdy S, et al. The association of circulating angiogenic factors and HbA1c with the risk of preeclampsia in women with preexisting diabetes. Hypertens Pregnancy 2014;33(1):81–92.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83.
- Wothe D, Gaziano E, Sunderji S, Romero R, Kusanovic JP, Rogers L, et al. Measurement of sVEGF R1 and PlGF in serum: comparing prototype assays from Beckman Coulter, Inc. to R&D Systems microplate assays. Hypertens Pregnancy 2011;30(1):18–27.
- Christinger HW, Fuh G, de Vos AM, Wiesmann C. The crystal structure of placental growth factor in complex with domain 2 of vascular endothelial growth factor receptor-1. J Biol Chem 2004;279(11):10382–8.
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51(6):1173–82.
- Lepercq J, Taupin P, Dubois-Laforgue D, Duranteau L, Lahlou N, Boitard C, et al. Heterogeneity of fetal growth in type 1 diabetic pregnancy. Diabetes Metab 2001;27(3):339–44.
- Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008;31(5):1060–79.
- Hernandez TL, Barbour LA. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol Ther 2013;15(2):172–9.