Abstract
Reactive oxygen species (ROS) may attack several types of tissues and chronic exposure to ROS may attenuate various biological functions and increase the risk of several types of serious disorders. It is known that treatments with ROS attack neurons and induce cell death. However, the mechanisms of neuronal change by ROS prior to induction of cell death are not yet understood. Here, it was found that treatment of neurons with low concentrations of hydrogen peroxide induced neurite injury, but not cell death. Unusual bands located above the original collapsin response mediator protein (CRMP)-2 protein were detected by western blotting. Treatment with tocopherol or tocotrienols significantly inhibited these changes in neuro2a cells and cerebellar granule neurons (CGCs). Furthermore, prevention by tocotrienols of hydrogen peroxide-induced neurite degeneration was stronger than that by tocopherol. These findings indicate that neurite beading is one of the early events of neuronal degeneration prior to induction of death of hydrogen peroxide-treated neurons. Treatment with tocotrienols may protect neurite function through its neuroprotective function.
Keywords::
Introduction
Oxidative stress is known to result in the production of various types of reactive oxygen species (ROS), including superoxide, hydrogen peroxide and hydroxyl radicals [Citation1]. ROS may attack several types of tissue. Chronic exposure to ROS may gradually increase the risk of development of several types of serious disorders, such as cancer, arteriosclerosis and neurodegenerative disorders, due to the enhancement of oxidative injury, e.g. lipid peroxidation, attenuation of antioxidant enzyme activity and other effects. We previously found apoptosis 7 days after exposure to ROS in rodent models [Citation2]. These changes were not noted after stress alone. It has been reported that ROS are generated from glycation reaction [Citation3,Citation4] and amyloid plaque [Citation5–7]. These findings indicate that one of the reasons for the development of these diseases is related to ROS generation and the accumulation of ROS-derived oxidative injury. ROS are related to ageing. In 1956, Harman [Citation8] had already reported the relationship between ROS and ageing, i.e. the free-radical theory of ageing. Thus, if ROS-derived oxidation in living tissues accelerates ageing via attenuation of various biological functions, treatment with antioxidant substances may be beneficial in opposing ageing. It is well known that ROS attack polyunsaturated fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [Citation9,Citation10]. The brain has high concentrations of these polyunsaturated fatty acids and uses a large amount of oxygen, accounting for one-fifth of total oxygen consumption [Citation11]. The brain is therefore at great risk of ROS damage at all times. However, the relationship between ROS and degeneration of neuronal functions during ageing remains to be elucidated in detail.
Vitamin E is one of the most common natural lipophilic vitamins. Its most important function is as an antioxidant. Recently, other possible functions of vitamin E have been considered, including prevention of neurodegenerative disorders and enhancement of immune response [Citation12–14]. In our previous studies we found that treatment with α-tocopherol significantly prevented cognitive dysfunction, such as that involving learning and memory, in rodent models of exposure to ROS in several maze tasks [Citation2,Citation15]. Moreover, chronic deficiency of α-tocopherol in young rats results in deterioration of cognitive function. Rates of learning and memory retention in these trials were similar to those in old animals. These results indicated that ROS is one of the causes of cognitive dysfunction during ageing. Using immunohistochemistry, we also identified neuronal apoptosis [Citation2] and β-amyloid-like proteins in the CA1 region of the rat hippocampus [Citation16]. However, at this point, it is difficult to maintain a neuronal network, because neurons have already died. It is important to find early events of neuronal change before the induction of death in ROS-exposed neurons.
To clarify early events of ROS-induced neuronal degeneration, we focused on neurite formation, which plays an important role in neurotransmission. Furthermore, synapses exist on the distal ends of neurites. Neurites exhibit expansion and contraction and a large number of substances including proteins and lipids are carried from the cell body by the axonal transport system. It is well known that neurite degeneration is classified as anterograde and retrograde. In Wallerian degeneration, neurite induces anterograde degeneration. On the other hands, with a decrease in neuritic ATP or neuro-growth factors (NGF)-free medium, neurite degeneration begins distally and proceeds in a retrograde fashion, in a process of dying back [Citation17,Citation18]. These phenomena indicate that neurites are more sensitive than the cell body to types of outer stimulation such as deprivation of NGF, deficiency of zinc ion and other factors [Citation18,Citation19].
Collapsin response mediator proteins (CRMPs) belong to a family of cytoplasmic proteins in the brain. As one member of this protein family, CRMP-2 plays a crucial role in neurite polarity and axon guidance [Citation20–22]. Changes in CRMP-2 level have been implicated in various neurodegenerative disorders, including Alzheimer's disease, ischemia and Wallerian degeneration [Citation23–26]. Induction of the cleaved form of CRMP-2 protein by calpain 1 in the presence of calcium ions is involved in the common process of neurite degeneration [Citation18]. However, the relationship between ROS-induced neurite degeneration and changes in CRMP-2 protein remains to be elucidated in detail.
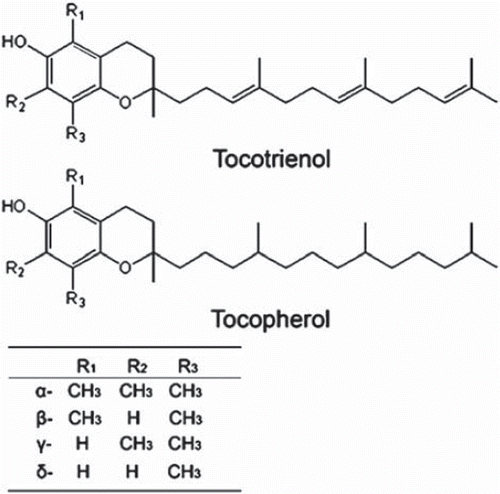
Tocotrienols, which include four different isoforms (α-, β-, γ- and δ-tocotrienol), comprise a vitamin E family (). One structural feature of tocotrienols is their possession of an unsaturated phytyl chain. It has been reported that rice oat bran and olive oils contain relatively large amounts of α-tocotrienol, while rice oat bran and palm oils contain relatively large amounts of γ- and δ-tocotrienol [Citation27]. Reported biological functions of tocotrienols include induction of apoptosis in cancer cells [Citation28,Citation29], antioxidant activity and suppression of 3-hydroxy-3-methyglutaryl coenzyme A (HMG CoA) reductase [Citation30,Citation31]. Khanna et al. [Citation32] reported that α-tocotrienol prevented glutamate-induced neuronal degeneration via modulation of 12-lipoxygenase. These researchers suggested the possibility of neuroprotective effects of this agent. Several types of evidence indicate that the biological activity of tocotrienols is stronger than that of tocopherols.
The purpose of this study was to determine whether treatment of neurons with hydrogen peroxide induces neurite degeneration in one of the early events before induction of cell death and to determine the prevention by α- and γ-tocotrienols of such effects in hydrogen peroxide-treated neurons. We therefore examined neurite formation and CRMP-2 protein levels in hydrogen peroxide-treated neuronal cells. We collected basic data in this research and in the near future hope to examine the relationship between ROS-derived neurite degeneration and cognitive dysfunction during ageing.
Materials and methods
Animals and reagents
The wild-type C57BL/6 mouse strains used were obtained from Japan SLC, Inc. (Hamamatsu, Japan). α-tocopherol was purchased from Tama Biochemical Co., Ltd (Tokyo, Japan). α- and γ-tocotrienols were kindly provided by Eisai Food & Chemical Co., Ltd (Tokyo, Japan). All other chemical agents were obtained from either Wako Pure Chemicals Industries, Ltd. (Osaka, Japan) or Sigma-Aldrich Corporation (St. Louis, MO). All animal experiments were performed with the permission of the Animal Protection and Ethics Committee of the Shibaura Institute of Technology. All tissue culture plates and dishes were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ). All other reagents were purchased from Sigma-Aldrich.
Cell cultures
Neuro2a cells from mouse neuroblastoma C1300 tumour were originally obtained from the Human Science Research Resources Bank (HSRRB) (#IFO50081) (Osaka, Japan). The cells were grown in minimum essential medium (MEM) containing 5% heat-inactivated foetal calf serum (FCS) (Biological Industries, Beit Haemek, Israel), 50 U/mL penicillin and 50 μg/mL streptomycin and were plated on polyethyleneimine-coated well plates. In order to elicit neurite extension, FCS concentration was decreased from 5 to 1%. After 72 h, the cells were used in experiments.
Primary cultures of cerebellar granule cells (CGCs) were prepared from 7-day-old post-natal mice as described previously, with some modifications [Citation33]. Briefly, the cerebellum was dissected after decapitation, cleaned free of meninges and then treated with 0.125% (w/v) trypsin in MEM for 10 min at 37°C. The reaction was terminated by adding feed medium consisting of MEM, 25 μg/mL streptomycin, 25 U/mL penicillin and 5% FCS supplemented with K+ to a final concentration of 25 mM. After trituration, the cells were suspended and plated onto 6- or 24-well dishes coated with 0.1% polyethyleneimine; this was designated Day 0 (DIV0). At Day 1, cytosine-D- arabinofuranoside was added to a final concentration of 1μM to prevent non-neuronal cell proliferation. CGCs were used in experiments on Day 3.
Judgement of cell death
Cell survival was assessed by trypan blue dye exclusion assay. Cells at a density of 5.0–7.0 × 104 cells/well were treated with each concentration of hydrogen peroxide in the presence or absence of 5 μM of each isoform of vitamin E. After 24 h of treatment with each concentration of hydrogen peroxide, the same volume of 0.8% (w/v) trypan blue in phosphate-buffered saline (PBS) was added and incubated for 20 min in a CO2 incubator. The cells were then washed in PBS at least three times. The number of cells that had died was counted per area and presented as the percentage of the total number of cells (100–150 s). At least three wells were analysed for all experimental cultures. Experiments were repeated three times. Counting was independently confirmed by a student not involved in this study. Data were analysed using Student's t-test, with findings of p < 0.01 considered significant.
Neurite degeneration
Axon or dendrite degeneration was evaluated by monitoring morphological hallmarks of neurite degeneration such as beading and fragmentation, as described previously with some modifications [Citation20,Citation35]. Twenty-four hours after treatment with hydrogen peroxide, neuro2a cells were fixed with 4% (w/v) paraformaldehyde (PFA) in PBS for 15 min at 4°C. Photomicrographs of the cells were taken on an Olympus IX81 phase-contrast microscope (Olympus Corp., Tokyo, Japan) equipped with a DP71 digital camera (Olympus), stored and then processed on a personal computer. At least 50 neurites were evaluated for beading formation per exposure. In the case of CGCs, after 18 h of transfection, the cells were treated with 5 μM hydrogen peroxide in the presence or absence of 10 μM of α-tocopherol. Eight hours after treatment with hydrogen peroxide, the cells were fixed with 4% PFA solution and observed under a confocal laser scanning microscope (Fluoview; Olympus). The number of beadings per 100 μm length of neurites was determined. At least 30 neurites were evaluated per exposure. Data were plotted as the mean ± SD of results of three independent experiments. Counting was independently confirmed by a student not involved in this study. Data were analysed using Student's t-test, with findings of p < 0.01 considered significant.
Western blotting
After 24 h of treatment with hydrogen peroxide, the samples were collected and used in western blotting as described previously, with some modifications [Citation35]. The lysates were centrifuged and protein content was determined using a Bio-Rad protein assay according to the manufacturer's procedure. Protein extracts (15 μg) were separated on 10% SDS-polyacrylamide gels and transferred to immobilon transfer membranes (PVDF; Millipore, Billerica, MA). The membranes were washed and then incubated in blocking solution (Tris-HCl-buffered saline, pH 7.6 (TBS), containing 0.1% Tween20 and 3% bovine serum albumin (BSA)) for 1 h at room temperature (R/T). The membranes were washed in TBS containing 0.1% Tween20 and then treated with anti-human CRMP-2 (C4G) mouse IgG monoclonal antibody (#11096, Immuno-Biological Laboratories Co., Ltd, Gunma, Japan) at 1:400 dilution overnight at 4°C. Anti-mouse IgG HRP antibody (Promega Corp., Madison, WI) was used as a secondary antibody at 1:4000 dilution for 1 h at R/T. Chemiluminescent signals were generated by incubation with the detection reagents (ECL Western Blotting Analysis Reagent; GE Healthcare UK Ltd., Buckinghamshire, UK) according to the manufacturer's procedure. For normalization of each band of CRMP-2, the membranes were re-probed with anti-β actin antibodies (Abcam Plc., Cambridge, UK). The relative intensity of CRMP-2 protein was determined using ImageQuant TL (GE Healthcare). Western blotting experiments were performed at least three times.
Immunocytochemical analysis
Neuro2a cells were washed and fixed with 4% PFA in PBS for 15 min at 4°C. The cells were then blocked with 10% goat serum in PBS for 1 h at R/T and probed with antibodies to CRMP-2 at 1:400 dilution in PBS containing 1% goat serum, 1% BSA and 0.05% Triton X-100 for 1 h at R/T. The cells were then incubated with Alexa Fluor 488-coupled anti-mouse IgG antibody (Molecular Probes Inc., Eugene, OR) at 1:500 dilution as a secondary antibody diluted in PBS for 1 h at R/T. Photomicrographs of neuro2a cells were taken on a IX81 phase-contrast microscope equipped with a digital camera, stored and then processed on a personal computer.
Transfection
CGCs were transfected according to the calcium phosphate method [Citation36]. An equal volume of solution containing 250 mM CaCl2 and a pEGFP-C1 mammalian expression vector (Clontech Takara Bio Inc., Tokyo, Japan) was added to 2 × HEPES-buffered saline (140 mM NaCl, 1.5 mM Na2HPO4 and 50 mM HEPES: pH 7.05). After 1 min, the mixture was added drop-wise to the cells at 1 DIV. After 18 h, the cells were washed with KRB containing 5 mM EGTA for 30 s with MEM containing 2% dimethyl sulphoxide for 1 min. Transfection efficiency was within 10–20%. The transfected cells were observed with a confocal laser scanning microscope.
Results
Optimization of hydrogen peroxide concentration in neuro2a cells
Neuro2a cells, a neuroblastoma cell line, are usually roughly round and do not have long neurites. In order to induce neurite extension in neuro2a cells, we changed FCS concentration from 5 to 1% and maintained it for 72 h. Neuro2a cells with neurite extension were treated with hydrogen peroxide as oxidative stress. Twenty-four hours of treatment with hydrogen peroxide induced significant death of neuro2a cells (). Moreover, treatment with hydrogen peroxide enhanced the death of neuro2a cells in a concentration-dependent fashion (0.1, 0.5, 1, 2 and 5 μM). Treatment with 1 μM of hydrogen peroxide induced ∼ 40% death of neuro2a cells. In this case, large numbers of neurites exhibited fragmentation (). This result showed that less than 0.5 μM of hydrogen peroxide did not induce the death of neuro2a cells. Since the purpose of this study was to detect early events of cell degeneration before induction of cell death, we chose less than 0.5 μM hydrogen peroxide and used it to produce hydrogen peroxide-treated samples in subsequent experiments.
Figure 2. Vitamin E prevents cell death after treatment of neuro2a cells with hydrogen peroxide. Neuro2a cells were treated with α-tocopherol (5 μM), α-tocotrienol (5 μM) or γ-tocotrienol (5 μM) in the presence or absence of various concentrations of hydrogen peroxide. After 24 h, the number of dead neuro2a cells was counted by trypan blue dye exclusion assay. Each column represents the mean of results of three independent experiments. At least three wells were used per experiment. Data were analysed by Student's t-test, with findings of p < 0.01 considered significant.
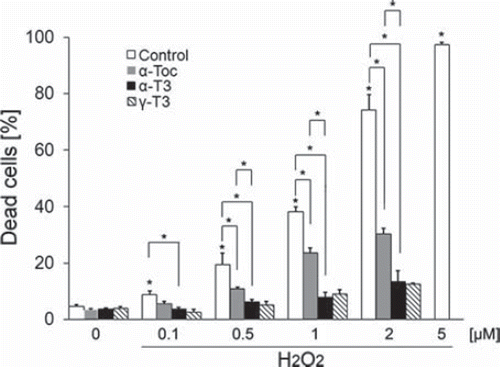
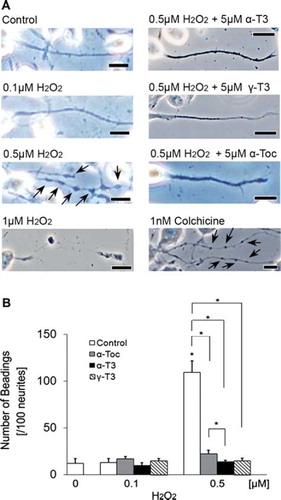
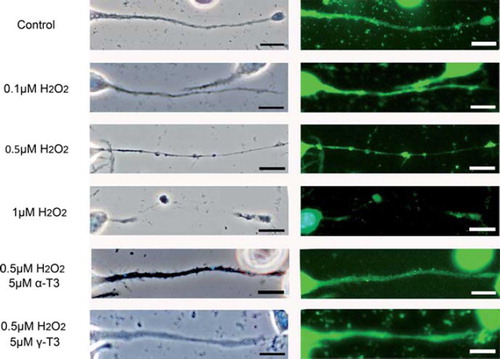
Figure 3. Treatment of neuro2a cells with low concentrations of hydrogen peroxide induces neurite beading, which is prevented by vitamin E. Neuro2a cells were treated with α-tocopherol (5 μM), α-tocotrienol (5 μM) or γ-tocotrienol (5 μM) in the presence or absence of various concentrations of hydrogen peroxide. After 24 h, the cells were fixed with 4% PFA in PBS. Photomicrographs of the cells were taken and analysed on a personal computer. The scale bar is 10 μm. Arrows indicate beading on the degenerating neurites of neuro2a cells (A). The results of quantitative analysis of neurite beading are shown (B). Each column represents the mean of results of three independent experiments. At least three wells were used per experiment. Data were analysed by Student's t-test, with findings of p < 0.01 considered significant.
Tocotrienols prevent hydrogen peroxide-induced neurite degeneration in neuro2a cells
Treatment with each isoforms of vitamin E significantly prevented the death of hydrogen peroxide-treated neuro2a cells (). The preventive effects of α- and γ-tocotrienols were significantly stronger than that of α-tocopherol in hydrogen peroxide-treated neuro2a cells. This result indicates that the neuroprotective effect of tocotrienols is stronger than that of α-tocopherol. To detect early events of cell degeneration, we checked neurite formation by neuro2a cells with phase-contrast microscopy. Treatment with hydrogen peroxide induced neurite degeneration in neuro2a cells in a dose-dependent fashion (0.1, 0.5 and 1 μM) ( and ). Treatment with 0.1 μM of hydrogen peroxide did not affect the neurite morphology of neuro2a cells (). Almost no beading was observed. However, treatment with 0.5 μM hydrogen peroxide induced abnormal morphology and significantly induced neurite beadings. Fragmented nuclei were not found on Hoechst 33258 staining. This finding indicates that treatment with 0.5 μM hydrogen peroxide induced neurite degeneration of neuro2a cells but not induction of a large number of cell death. More than 1 μM hydrogen peroxide completely eliminated neurite formation in neuro2a cells. Our results indicate that neurite degeneration occurred as one of the early events in hydrogen peroxide-treated neuro2a cells. Treatment with each isoform of vitamin E significantly inhibited neurite beading in hydrogen peroxide-treated neuro2a cells. The inhibitory effects of α- and γ-tocotrienol were in this respect significantly stronger than that of α-tocopherol. To examine the efficacy of tocotrienols in preventing this neurite degeneration, further experiments were performed with tocotrienols rather than tocopherols.
Tocotrienols prevented hydrogen peroxide-induced CRMP-2 protein denaturation on western blot analysis
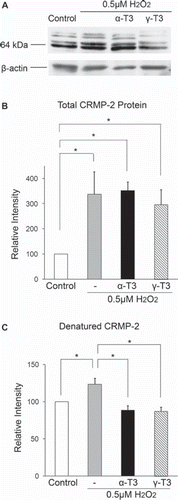
To clarify the mechanism of neurite degeneration after treatment with hydrogen peroxide in neuro2a cells, we measured CRMP-2 protein level using a C4G monoclonal antibody. CRMP-2 protein is localized in axons, growth cones and the cell body and plays a crucial role in neurite formation [Citation22]. On western blotting, two original bands of CRMP2 (64 and 72-kDa) were detected for a mouse brain homogenate sample on the data sheet of IBL Co., Ltd (http://www.ibl-japan.co.jp/eng/index.htm). Touma et al. [Citation18] reported that the 72-kDa band is a minor component of CRMP-2 protein. Treatment of neuro2a cells with hydrogen peroxide induced two unusual bands located above the two original bands of CRMP-2 protein (). Treatment with α- or γ-tocotrienol significantly inhibited the appearance of these unknown bands. The relative intensity of total CRMP-2 protein was significantly increased after treatment of neuro2a cells with hydrogen peroxide in the presence or absence of each tocotrienol (). Furthermore, treatment of neuro2a cells with hydrogen peroxide significantly increased the unusual bands of CRMP-2 protein (). Treatment of hydrogen peroxide-treated neuro2a cells with α- or γ-tocotrienol inhibited induction of unusual CRMP-2 protein, but not total CRMP2 protein induction. The ratio of band intensities for the unusual CRMP-2 protein was after treatment of hydrogen peroxide-treated neuro2a cells with each tocotrienol similar to that for the control. These results showed that tocotrienols increased CRMP-2 protein while decreasing unusual CRMP-2 protein.
Figure 4. Denatured CRMP-2 proteins appear after treatment of neuro2a cells with hydrogen peroxide. Neuro2a cells after treatment with hydrogen peroxide in the presence or absence of α-tocotrienol (5 μM) or γ-tocotrienol (5 μM) were lysed and used for western blotting analysis. The same membranes were re-probed and used for the detection of β-actin (A). CRMP-2 band intensities were calculated using ImageQuant TL. Relative band intensities of total CRMP-2 protein are shown (B). Relative band intensities of denatured CRMP-2 protein are plotted as a ratio of the intensity of bands for total CRMP-2 protein. Control values were set to 100%. All scores are normalized to the band intensity of β-actin (C). Each column represents the mean of three independent experiments. Data were analysed by Student's t-test, with findings of p < 0.05 considered significant.
Localization of CRMP-2 protein by immunocytochemical analysis
CRMP-2 protein plays crucial roles in neurite function, such as neurite outgrowth and transport function [Citation36]. We examined the localization of CRMP-2 protein by immunocytochemical analysis using C4G antibody (see Materials and methods). shows that CRMP-2 protein was found not only throughout the neurites but also in the region of beadings in hydrogen peroxide-induced neurite degeneration in neuro2a cells.
Figure 5. CRMP-2 protein is localized in regions of neurite beading in neuro2a cells. Immunohistochemical analysis of neuro2a cells using C4G antibody. Neuro2a cells were treated with each concentration of hydrogen peroxide (0.1, 0.5, 1 μM) in the presence or absence of α-tocotrienol (5 μM) or γ-tocotrienol (5 μM). After 24 h, the cells were fixed with 4% PFA solution. Phase-contrast (left) or fluorescence photomicrographs were taken (right). The scale bar is 10 μm. The method of immunohistochemical analysis is described in Materials and methods.
Hydrogen peroxide induces neurite degeneration in EGFP-transfected CGCs
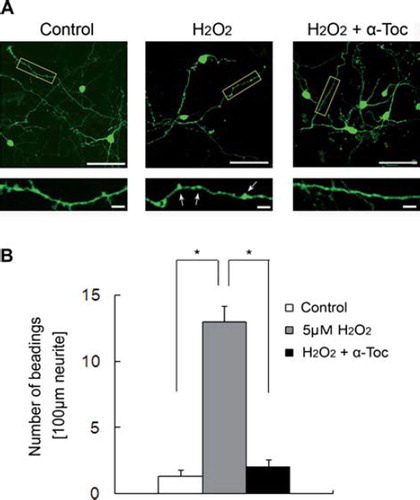
To obtain more findings about hydrogen peroxide-induced neurite degeneration, we examined neurite morphology using primary culture of mouse CGCs. For judgement of neurites in CGCs, we prepared a pEGFP1-C1 mammalian expression vector and transfected it into CGCs using calcium-phosphate methods [Citation35]. After 18 h of transfection, neurite degeneration had been induced in hydrogen peroxide-treated CGCs. Treatment of CGCs with hydrogen peroxide induced significant neurite beading. In contrast, treatment of hydrogen peroxide-treated CGCs with α-tocopherol significantly inhibited neurite degeneration ( and ). The promotion of neurite degeneration in CGCs was similar to that in neuro2a cells.
Figure 6. Neurite beading was induced by treatment of EGFP-transfected CGCs with hydrogen peroxide. (A) Each image was taken by confocal microscopy. After 18 h of transfection with EGFP, the CGCs were treated with 5 μM hydrogen peroxide in the presence or absence of 10 μM α-tocopherol. Magnified images represent the regions defined in the yellow box of each fluorescence photomicrograph. Arrows show beading along the neurites. The scale bar is 50 μm (upper panels) or 10 μm (lower panels). (B) The results of quantitative analysis of neurite beading in hydrogen peroxide-treated CGCs. Each column represents the mean of three independent experiments. Data were analysed by Student's t-test, with findings of p < 0.01 considered significant. The method of transfection is described in Materials and methods.
Discussion
Hydrogen peroxide induces neurite degeneration before induction of death of neuro2a cells
We used hydrogen peroxide in this study to detect early events of ROS-induced cell degeneration before induction of cell death. Hydrogen peroxide is ROS and a by-product of the super-oxide dismutase reaction in living tissues. Before starting this study, we optimized hydrogen peroxide concentration. Treatment of neuro2a cells with hydrogen peroxide induced cell death in a dose-dependent fashion (). Treatment with 1 μM of hydrogen peroxide induced ∼ 40% death of neuro2a cells. Less than 0.5 μM hydrogen peroxide did not induce the death of a large number of cells. Rates of cell death were ∼ 8.9% (0.1 μM) and 20% (0.5 μM). We chose less than 0.5 μM of hydrogen peroxide and used it to produce hydrogen peroxide-treated samples in subsequent experiments.
We thus attempted to detect early events of hydrogen peroxide-induced neuronal degeneration before induction of cell death and focused on neurite formation. Neurite formation plays important roles in neurotransmission. As noted above, neuro2a cells are usually roughly round. In order to induce neurite extension in neuro2a cells, we changed FCS concentration from 5 to 1% and maintained it for 72 h. In this condition, the neuro2a cells were starved and the neurites of neuro2a cells grew to more than 10-times normal length. After optimization of hydrogen peroxide concentration (), we treated neuro2a cells with 0.5 μM hydrogen peroxide for 24 h and found neurite degeneration, but not cell death on phase-contrast microscopy (). We found no nuclear condensation or fragmentation on trypan blue dye exclusion assay and Hoechst33258 staining (data not shown). Large numbers of beadings appeared in the neurites (). Beading formation is well known as a major index of neurite degeneration [Citation35]. Colchicine, which acts as an inhibitor of microtubule polymerization by binding to tubulin, was used as a positive control and administered to neuro2a cells. Microtubules, a component of cytoskeletal proteins, are ubiquitously present in cells. It is well known that microtubules play an important role in axonal extension [Citation17,Citation37]. Furthermore, many kinds of protein are presented in neurites and these proteins are necessary for control of microtubule dynamics, including polymerization, stabilization and destabilization [Citation38]. After 24 h treatment with 1 nM colchicine, the cells exhibited neurite beading. These findings suggest that treatment with a low concentration of hydrogen peroxide induces neurite degeneration via effects on microtubule polymerization. The cell body was not or was only weakly affected by a low concentration of hydrogen peroxide. However, it is unclear whether treatment with hydrogen peroxide affects microtubule directly or indirectly.
Neuroprotective effect of tocotrienols is stronger than that of tocopherol in neuro2a cells
Treatment with vitamin E (α-tocopherol, α-tocotrienol or γ-tocotrienol) significantly prevented neurite beadings in hydrogen peroxide-treated neuro2a cells ( and ). Treatment with each type of vitamin E prevented the death of hydrogen peroxide-treated neruo2a cells in a dose-dependent fashion (data not shown). These findings indicated that one of the reasons for neurite degeneration was oxidative injury. However, the inhibitory effects of α- and γ-tocotrienol were in this respect significantly stronger than that of α-tocopherol. The number of beadings on hydrogen peroxide-treated neuro2a cells did not differ between treatment with α- or γ-tocotrienol. Tocopherols and tocotrienols are well known antioxidant substances [Citation11,Citation31]. We have been examining the relationship between the antioxidant functions of α-tocopherol and oxidative injury using in vivo and in vitro models [Citation2,Citation15,Citation16]. Recently, several lines of evidence have shown that the antioxidant activity of tocotrienols is stronger than those of tocopherols. Furthermore, tocotrienols are now believed to have other beneficial effects (e.g. induction of apoptosis in cancer cells [Citation28,Citation29], enhancement of cholesterol-lowering activity [Citation39] and anti-angiogenic effects [Citation31]). One such function is neuroprotective [Citation32]. In this study, treatment with α-tocopherol, α- or γ-tocotrienol significantly inhibited neurite degeneration in hydrogen peroxide-treated neuro2a cells. These findings indicated that tocotrienols exhibited not only antioxidant but neuroprotective effects in our model. Furthermore, the neuroprotective effects of α- and γ-tocotrienol from ROS were significantly stronger than that of α-tocopherol [Citation30,Citation31]. In this study, the same findings were observed. The preventive effects of tocotrienols from hydrogen peroxide were significantly stronger than that of α-tocopherol. For these reasons, we used tocotrienols for subsequent experiments in our study.
Treatment with hydrogen peroxide increased induction of total CRMP-2 protein in neuro2a cells
To determine the mechanism of hydrogen peroxide-induced neurite beading in neuro2a cells, we examined CRMP-2 protein levels using western blotting and immunohistochemical analysis. CRMPs belong to a family of cytoplasmic proteins in the brain. Several lines of evidence have shown that CRMP-2 protein plays crucial roles in neurite polarity and axon guidance [Citation37]. Gu and Ihara [Citation40] showed expression of CRMP-2 protein in neuro2a cells. Over-expression of CRMP-2 protein induces multiple axons in cultured hippocampal neurons [Citation41]. Changes in CRMP-2 protein level have been implicated in various neurodegenerative disorders, including Alzheimer's disease and ischemia [Citation23–26]. CRMP-2 protein is cleaved by calpain 1 and induces neurite degeneration in mice [Citation18]. In the present study, total CRMP-2 protein level in hydrogen peroxide-treated neuro2a cells increased to three times the normal level (). This finding showed that treatment with low concentrations of hydrogen peroxide enhances induction of CRMP-2 protein in neuro2a cells. Total CRMP-2 protein levels in hydrogen peroxide-treated neuro2a cells were unchanged in the presence or absence of tocotrienols. This finding showed that treatment with tocotrienols did not affect CRMP-2 production in neuro2a cells. Neurons may protect neurite function against ROS through enhancement of CRMP-2 protein production. It is very important for neurons to maintain neurite function. Synapses, which play crucial roles in neurotransmission, exist at the distal ends of neurites. Several types of substances such as trophic factors, neurotransmitters and other proteins are carried by axonal transport. On the other hand, waste substances are carried from the neurites to the cell body by the ubiquitin-proteasome or autophagy pathway [Citation19]. The neurites of 0.5 μM hydrogen peroxide-treated neuro2a cells were quite fine, while the neurite of 1 μM hydrogen peroxide-treated neuro2a cells were completely fragmented (). CRMP-2 protein is involved in microtubule organization [Citation23]. In this study, treatment with colchicine significantly induced neurite beadings in neuro2a cells. Enhancement of the production of CRMP-2 protein may protect neurite function against hydrogen peroxide through protection of microtubule components. The transport of many types of substances between the cell body and the distal end of neurites may be stopped, with gradual accumulation of such substances, since microtubule composition is altered by some changes in ROS-derived CRMP-2 protein.
Treatment with tocotrienol prevents induction of unusual CRMP-2 protein in hydrogen peroxide-treated neuro2a cells
On western blotting, we detected two unknown bands which reacted with anti-human CRMP-2 (C4G) mouse monoclonal antibody in hydrogen peroxide-treated neuro2a cells. These bands appeared above the two original bands of CRMP-2 protein (). We detected multiple bands after treatment of neuro2a cells with hydrogen peroxide. Unfortunately, we could not identify the two unusual bands in hydrogen peroxide-treated neuro2a cells. Several lines of evidence have characterized CRMP-2 protein. The possibility of existence of sub-types and various phosphorylation forms of CRMP-2 protein have been suggested [Citation21–23,Citation25,Citation40,Citation42]. Yuasa-Kawada et al. [Citation22] showed that CRMP-2 protein was found to include two sub-types they termed CRMP-2A and -2B proteins. These two proteins have opposite effects. CRMP-2B protein exists in axons and dendrites and induces axon branching and a reduction of axon length, while CRMP-2A protein exists only in axons and blocks the effects of CRMP-2B [Citation21]. Both proteins are involved in maintenance of neurite morphology via changes in microtubule organization. Anti-human CRMP-2 (C4G) mouse monoclonal antibody detected both CRMP-2A and -2B protein [Citation40]. In this study, we used C4G-antibody with western blotting and immunohistochemical assay and detected multiple bands after treatment of neuro2a cells with hydrogen peroxide. It is one of the possibilities that these unknown bands are related to these sub-types of CRMP-2 protein. Slight appearance of the unknown bands was noted with normal neuro2a cells. Very small numbers of control cells died and/or exhibited neurite beadings ( and ). It is possible that the unknown two bands of CRMP-2 were made from a weakened neurite. Another possibility is that small amounts of the unusual CRMP-2 protein may appear anytime in neuro2a cells in the presence or absence of ROS. The cells may maintain a balance between induction of unusual CRMP-2 protein and elimination of it. We are presently continuing to examine the mechanism of induction of the two unknown bands of CRMP-2 protein in hydrogen peroxide-treated neurons.
CRMP-2 protein was on immunocytochemical analysis found not only throughout the neurites but also in the region of beading in hydrogen peroxide-induced neurite degeneration in neuro2a cells. These findings showed that hydrogen peroxide may induce CRMP-2 protein alteration. This unusual CRMP-2 protein may gradually accumulate in the region of neurites of neuro2a cells and may, thus, induce neurite beadings. However, the results of characterization of the unknown CRMP-2 protein were unclear.
Administration of α- or γ-tocotrienol significantly attenuated the induction of unusual CRMP-2 protein in hydrogen peroxide-treated neuro2a cells (). Tocotrienols displayed not only antioxidant but also neuroprotective effects. Treatment with tocotorienols did not affect induction of normal CRMP-2 protein and prevented induction of the unusual CRMP-2 protein in hydrogen peroxide-treated neuro2a cells. We are currently continuing to study the relationship between hydrogen peroxide-induced neurite degeneration and CRMP-2 protein and the possibility of prevention by vitamin E of such dysfunction in neuronal cells.
Induction of neurite beadings in hydrogen peroxide-treated CGCs and prevention of them by α-tocopherol
To obtain more findings regarding the relationship between hydrogen peroxide and neurite degeneration, we finally examined neurite morphology using EGFP-transfected primary culture of mouse CGCs (). Treatment of CGCs with hydrogen peroxide induced significant neurite beadings (). In contrast, treatment of hydrogen peroxide-treated CGCs with α-tocopherol significantly inhibited neurite degeneration. These findings strongly suggest that treatment with low concentrations of hydrogen peroxide induces neurite beadings in ex vivo models. Neurite degeneration may be induced as one of the early events in neuronal dysfunction. We are continuing to study the neuroprotective effects of tocotrienols in CGC models.
Conclusions
In summary, this study raises the possibility that treatment with low concentrations of hydrogen peroxide induces neurite degeneration, but not cell death, via some changes in CRMP-2 protein. After treatment with low concentration of hydrogen peroxide, neurites are gradually thin and induces large numbers of beadings. Further investigation is needed to determine the mechanism of hydrogen peroxide-induced neurite degeneration, such as induction of abnormal CRMP-2 protein, impairment of microtubule assembly, or there factors. Chronic exposure to ROS may accelerate ageing via dysfunction of various biological activities. As noted above, in the 1950s, Harman [Citation8] had already reported the relationship between ROS and ageing, i.e. the free-radical theory of ageing. Furthermore, it has been reported that ROS may be related to a large number of neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease [Citation9,Citation12,Citation42]. Several lines of evidence have suggested a relationship between phosphorylation of CRMP-2 protein and Alzheimer's disease [Citation22,Citation23,Citation25,Citation43]. In the near future, we plan to examine the relationship between ROS-derived neurite degeneration and age-related neurodegenerative disorders.
Acknowledgement
We would like to thank Kouichi Abe and Hiroyuki Yoshimura, Eisai Food & Chemical Co., Ltd., for providing us with the tocotrienols. We would also like to thank Satoko Kato, Hokkaido University, for their initial contribution to this project. Finally, the authors thank Dr Kazuhiko Suzuki for his valuable advice on establishment of the culture samples.
Declaration of interest
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan and Eisai Food & Chemical Co., Ltd. (Tokyo, Japan). This study was also supported by a grant-in-aid for Project Research from the Shibaura Institute of Technology (Tokyo, Japan) and the Sasagawa Scientific Research Grant from The Japan Science Society (#22-401) (Tokyo, Japan). The authors confirm that we have no conflict of interest.
This paper was first published online on Early Online on 24 March 2011.
References
- Sies H . Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82:291–295.
- Fukui K , Omoi N , Hayasaka T , Shinkai T , Suzuki S , Abe K , Urano S . Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci 2002;959:275–284.
- Brouwers O , Niessen PM , Ferreira I , Miyata T , Scheffer PG , Teerlink T , Schrauwen P , Brownlee M , Stehouwer CD , Schalkwijk CG . Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;288: 1374–1380.
- Baydas G . Effects of vitamin E on the brain in diabetes. Preedy VR , Watson RR . The Encyclopedia of vitamin E. CAB International; 2007. 487–493.
- Cecchi C , Fiorillo C , Baglioni S , Pensalfini A , Bagnoli S , Nacmias B , Sorbi S , Nosi D , Relini A , Liguri G . Increased susceptibility to amyloid toxity in familial Alzheimer's Disease. Neurobiol Aging 2007;28:863–876.
- Hadid LK , Lee MT , Yang J . Inhibitors of catalase-amyloid interactions protect cells from beta-amyloid-induced oxidative stress and toxicity. J Biol Chem 2010;285: 38933–38943.
- Ko SY , Lin YP , Lin YS , Chang SS . Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic Biol Med 2010;49:474–480.
- Harman D . Aging; a theory based on free radical and radiation chemistry. J Gerontrol 1956;11:298–300.
- Urano S , Sato Y , Otonari T , Makabe S , Suzuki S , Ogata M , Endo T . Aging and oxidative stress in neurodegeneration. Biofactors 1998;7:103–112.
- Omoi NO , Arai M , Saito M , Takatsu H , Shibata A , Fukuzawa K , Sato K , Abe K , Fukui K , Urano S . Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidyl choline liposomes, and protective effect of vitamin E. J Nutr Sci Vitaminol 2006;52:248–255.
- Onodera K , Omoi NO , Fukui K , Hayasaka T , Shinkai T , Suzuki S , Abe K , Urano S . Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Radic Res 2003;37:367–372.
- Sano M , Ernesto C , Thomas RG , Klauber MR , Schafer K , Grundman M , Woodbury P , Growdon J , Cotman CW , Pfeiffer E , Schneider LS , Thal LJ . A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's disease cooperative study. N Engl J Med 1997;336:1216–1222.
- Adolfsson O , Huber BT , Meydani SN . Vitamin Eenhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing Capacity. J Immunol 2001;167:3809–3817.
- Azzi A , Ricciarelli R , Zingg JM . Non-antioxidant molecular functions of a-tocopherol (vitamin E). FEBS Lett 2002;519:8–10.
- Fukui K , Onodera K , Shinkai T , Suzuki S , Urano S . Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci 2001;928:168–175.
- Fukui K , Takatsu H , Shinkai T , Suzuki S , Abe K , Urano S . Appearance of amyloid beta-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. J Alzheimers Dis 2005;8:299–309.
- Kelly TA , Katagiri Y , Vartanian KB , Kumar P , Chen LL , Rosoff WJ , Urbach JS , Geller HM . Localized alteration of microtubule polymerization in response to guidance cues. J Neurosci Res 2010;88:3024–3033.
- Touma E , Kato S , Fukui K , Koike T . Calpain-mediated cleavage of collapsing response mediator protein (CRMP)-2 during neurite degeneration in mice. Eur J Neurosci 2007; 26:3368–3381.
- Yang Y , Kawataki T , Fukui K , Koike T . Cellular Zn2+chelators cause ‘dying-back’ neurite degeneration associated with energy impairment. J Neurosci Res 2007;85:2844–2855.
- Wang LH , Strimatter SM . A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci 1996;16:6197–6207.
- Fukata Y , Itoh T , Kimura T , Manager C , Nishimura T , Shiromizu T , Watanabe H , Inagaki N , Iwamatsu A , Hotani H , Kaibuchi K . CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 2002;4: 583–591.
- Yuasa-Kawada J , Suzuki R , Kano F , Ohkawara T , Murata M , Noda M . Axonal morphologenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur J Neurosci 2003;17:2329–2343.
- Yoshida H , Watanabe A , Ihara Y . Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer's disease. J Biol Chem 1998;273:9761–9768.
- Uchida Y , Ohshima T , Sasaki Y , Suzuki H , Yanai S , Yamashita N , Nakamura F , Takei K , Ihara Y , Mikoshiba K , Kolattukudy P , Honnorat J , Goshima Y . Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3β phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes cells 2005;10: 165–179.
- Cole AR , Noble W , van Aalten L , Plattner F , Meimaridou R , Hogan D , Taylor M , LaFrancois J , Gunn-Moore F , Verkhratsky A , Oddo S , LaFerla F , Giese KP , Dineley KT , Duff K , Richardson JC , Yan SD , Hanger DP , Allan SM , Sutherland C . Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer's disease progression. J Neurochem 2007;103:1132–1144.
- Petratos S , Li QX , George AJ , Hou X , Kerr ML , Unabia SE , Hatzinisiriou I , Maksel D , Aguilar MI , Small DH . The β-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain 2008;131:90–108.
- Wada S . Chemoprevention of tocotrienols: the mechanism of antiproliferative effects. Forum Nutr Basel Karger 2009;61: 204–216.
- Srivastava JK , Gupta S . Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun 2006; 346:447–453.
- Yap WN , Zaiden N , Tan YL , Ngoh CP , Zhang XW , Wong YC , Ling MT , Yap YL . Id1, inhibitor of differentiation, is a key protein mediating anti-tumor or responses of gamma-tocotrienol in breast cancer cells. Cancer Lett 2010;291:187–199.
- He L , Mo H , Hadisusilo S , Qureshi AA , Elson CE . Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo . J Nutr 1997;127:668–674.
- Miyazawa T , Shibata A , Sookwong P , Kawakami Y , Eitsuka T , Asai A , Oikawa S , Nakagawa K . Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol). J Nutri Biochem 2009;20:79–86.
- Khanna S , Roy S , Ryu H , Bahadduri P , Swaan PW , Ratan RR , Sen CK . Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem 2003;278:43508–43515.
- Suzuki K , Koike T . Brain-derived neurotrophic factor suppress programmed death of cerebellar granule cells through a posttranslational mechanism. Mol Chem Neuropathol 1997;30:101–124.
- Ikegami K , Koike T . Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience 2003;122:617–626.
- Jordan M , Schallhorn A , Wurm FM . Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 1996;24: 596–601.
- Kamata T , Subleski M , Hara Y , Yuhki N , Kung H , Copeland NG , Jenkins NA , Yoshimura T , Modi W , Copeland TD . Isolation and characterization of a bovine neuronal specific protein (CRMP-2) cDNA homologous to unc-33, a C elegans gene implicated in axonal outgrowth and guidance. Mol Brain Res 1998;54:219–236.
- Yamada KM , Spooner BS , Wessells NK . Axon growth: roles of microfilaments and microtubules. Proc Natl Acad Sci USA 1970;66:1206–1212.
- Poulain EE , Sobel A . The microtubule network and neuronal morphogenesis: dynamics and coordinated orchestration through multiple players. Mol Cell Neurosci 2010;43: 15–32.
- Parker RA , Pearce BC , Clark RW , Gordon DA , Wright JJ . Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem 1993;268: 11230–11238.
- Gu Y , Ihara Y . Evidence that collapsing response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem 2000;275:17917–17920.
- Inagaki N , Chihara K , Arimura N , Menager C , Kawano Y , Matsuo N , Nishimura T , Amano M , Kaibuchi K . CRMP-2 induces axons in cultured hippocampal neurons. Nature Neurosci 2001;4:781–782.
- Seet RC , Lee CY , Lim EC , Tan JJ , Quek AM , Chong WL , Looi WF , Huang SH , Wang H , Chan YH , Halliwell B . Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radic Biol Med 2010;48:560–566.
- Tahimic CG , Tomimatsu N , Nishigaki K , Fukuhara A , Toda T , Kaibuchi K , Shiota G , Oshimura M , Kurimasa A . Evidence for a role of collapsing response mediator protein-2 in signaling pathways that regulate the proliferation of non-neuronal cells. Biochem Biophys Res Commun 2006;340:1244–1250.