Abstract
The pharmacokinetics and the local toxicity of commercial and liposome-encapsulated mepivacaine formulations injected intra-orally in rats were studied. Animals were divided in groups (n = 4–6) and treated with 0.1 mL of the formulations: 2% mepivacaine with 1:100,000 epinephrine (MVC2%EPI), 3% mepivacaine (MVC3%), and 2% liposome-encapsulated mepivacaine (MVCLUV). The results showed that the 2% liposome-encapsulated mepivacaine reduced Cmax, prolonged AUC0–∞ and t1/2 compared with 3% plain and 2% vasoconstritor-associated mepivacaine, after intraoral injection. In addition, it was also observed that liposomal mepivacaine might protect the tissue against local inflammation evoked by plain or vasoconstrictors-associated mepivacaine, giving supporting evidence for its safety and possible clinical use in dentistry.
Introduction
Local anesthesia is the most widely used method for controlling pain during the intra-operative period (CitationWhiteside & Wildsmith, 2001). Although it is an effective method, the conventional local anesthetic agents clinically used are characterized by short duration of analgesia and both systemic and local toxicity (CitationCox et al., 2003). For this reason, care should be taken regarding the right choice of local anesthetic with a preference for one that is most effective and has the lowest concentration of anesthetic salt and vasoconstrictor.
Liposomes have been extensively described in the literature for their use as drug carriers (CitationTorchilin, 2005; CitationSamad et al., 2007). The distinct advantage of liposomes is their structural versatility combined with their ability to encapsulate different compounds, including local anesthetics (CitationFelnerova et al., 2004).
The advantages of liposome encapsulated local anesthetics are slow drug release, prolonged anesthetic effect, and reduced toxicity for both the cardiovascular and central nervous system (CitationGesztes & Mezei, 1988; CitationLangerman et al., 1992; CitationBoogaerts et al., 1993a; Citationb; CitationMowat et al., 1996; CitationBucalo et al., 1998; Citationde Araujo et al., 2003). The vasoconstrictor is usually absent in liposome encapsulated anesthetics formulations. The pharmacological effectiveness of many liposomal-anesthetic preparations has been demonstrated by studies using animals (CitationMashimo et al., 1992; CitationBoogaerts et al., 1993a; Citationb; CitationMalinovsky et al., 1997; CitationGrant & Bansinath, 2001; CitationYu et al., 2002) and human beings (CitationLafont et al., 1994; Citation1996; CitationBoogaerts et al., 1994; CitationGrant et al., 2004).
Mepivacaine was the second amide to be introduced as a local anesthetic. It has a fast onset; similar to that of lidocaine, and it is a popular choice for a wide range of regional anesthetic procedures due to its safety (CitationMcLure & Rubin, 2005). Liposome encapsulated mepivacaine increased the analgesic effect in relation to amplitude and anesthetic duration (Citationde Araujo et al., 2004). Moreover, encapsulation of mepivacaine in liposomes greatly enhanced the infraorbital nerve block in rats when compared with the plain solution of this local anesthetic (CitationCereda et al., 2006).
In developing a new pharmaceutical form, such as liposomal mepivacaine, the International Conference on Harmonization requires pharmacokinetic and local toxicity assessments in animals. Thus, the aims of this study were to determine plasma concentrations and to evaluate the tissue reaction induced by liposomal mepivacaine compared with commercial formulations after intra-oral injections in rats, looking forward to its clinical use in dentistry.
Materials and methods
2% Mepivacaine with 1:100,000 epinephrine-MVC2%EPI (Mepiadre®-DFL Indústria e Comércio S.A., Rio de Janeiro, RJ, Brazil) and plain 3% mepivacaine-MVC3% (MepiSV®-DFL Ind. Com. S.A., Rio de Janeiro, RJ, Brazil) were purchased in local commerce. Liposomal suspension of 2% mepivacaine-MVCLUV was prepared with mepivacaine hydrochloride salt supplied by Cristália Ind. Quim. Farm. Ltda (Itapira, SP, Brazil). EDTA titriplex was purchased from Merck KGaA and sodium thiopental solution (Thiopentax®) from Cristália Ind. Quim. Farm. Ltda. Alpha-chloralose, urethane, alpha-tocopherol acetate, egg phosphatidylcholine, and cholesterol were obtained from Sigma Chem. Co (St Louis, MO). Acetonitrile used was of HPLC grade. Other reagents were of analytical grade.
Liposomal formulations: Preparation and serilization
A dry lipid film, containing egg phosphatidylcholine, cholesterol, and α-tocopherol at a 4:3:0.07 molar ratio was prepared by solvent evaporation under nitrogen flow (CitationBoogaerts et al., 1993a; Citationb; CitationFraceto et al., 2002). Multilamellar liposomes were obtained by adding 20 mM HEPES buffer, pH 7.4 (containing 154 mM NaCl) to the dry lipid film and vortexing the mixture during 5 min. Unilamellar-liposome vesicles (0.4 μm) were prepared by extrusion (12 cycles through 400 nm polycarbonate membrane, at 25°C) of the multilamellar vesicles. The total lipid concentration in the LUV was 5 mM (CitationCereda et al., 2008). Mepivacaine was added directly to the liposomes after extrusion, up to a concentration of 2% (corresponding to 70.7 mM of mepivacaine). Liposome formulations were incubated for 12 h and stored at 4°C until further use.
The preparations were sterilized by autoclaving (121°C, 1 atm, and 15 min). Afterward, the sterility was evaluated by microbiological test with Brain-Heart Infusion (BHI) and apyrogenicity was assessed by Endosafe® Limulus Amoebocyte Lysate Test.
Animals
Male Wistar rats, 250–350 g, were obtained from CEMIB–UNICAMP (Centro de Bioterismo, State University of Campinas, UNICAMP, SP, Brazil) and they received water and food ad libitum. Animals were randomly divided in groups (n = 4–6/group) and protocols were approved by the Institutional Committee for Ethics in Animal Research of the State University of Campinas (protocols #1067-1 and 871-1), which follows the recommendations of the Guide for the Care and Use of Laboratory Animals.
Pharmacokinetic study: Experimental design
The animals were divided into three groups and they received intra-orally 0.1 mL of one of the following treatments: Group 1, 3% plain mepivacaine (MVC3%); Group 2, 2% mepivacaine with 1:100.000 epinephrine (MVC2%EPI); and Group 3, liposomal 2% mepivacaine (MVCLUV). The selection of local anesthetic concentration in the liposomal formulation was determined by the efficacy of 2% liposomal mepivacaine shown in previous studies (Citationde Araujo et al., 2004; CitationCereda et al., 2006).
General anesthesia was performed with α-chloralose (50 mg/kg) and urethane (1 g/kg) before the injections. A prior pilot assay (data not shown) showed no influence of general anesthesia on the mepivacaine pharmacokinetics. An intravascular catheter was inserted in the femoral vein and 0.2 mL blood samples were collected right before the anesthetic injection (0 min) and at 15, 30, 45, 60, 120, 180, 240, 300, 360, and 420 min after the anesthetic injection. These intervals were defined to obtain 10 samples between the baseline (0 min) and ∼ 4-times the t1/2 (half-life) of mepivacaine (1.5 h) (CitationCovino & Vassalo, 1976). This geometric progression scheme is commonly used and supplies information about drug disposition (CitationBourne, 1995). Samples were transferred into heparinized tubes and the separated plasma was stored at −70°C.
LC–MS/MS assay: Apparatus and chromatographic conditions
A Waters® HPLC system (2795) coupled to a Micromass Quattro Premier XE triple stage quadrupole mass spectrometer, equipped with API electrospray source was used. Separations were performed using a C18 polaris 5 µm, 50 mm × 2 mm i.d. The mobile phase was 80% acetonitrile and 20% water (with 0.1% formic acid). The total run time was 3.0 min. The mass spectrometer was run in the positive mode (ES+) and set for multiple reaction monitoring (MRM). The full-scan single-mass spectrum and the daughter ion-mass spectrum for mepivacaine and Venlafaxine (internal standard; IS) were (m/z) 247.5 > 150.5 and 278.1 > 215.31, respectively. The data were integrated using the MassLynx 4.1 (Waters®) software. The WinNonlin 5.2 computer program (Pharsight, USA) was used to calculate the pharmacokinetic parameters. Quality control samples (QC) prepared by mixing drug-free plasma with appropriate volumes of working solutions were used to validate the method.
Plasma sample preparation
The extraction procedure was performed by transferring 80 µL of plasma to test tubes, followed by the addition of 25 µL of IS working solution. The samples were vortexed for 1 min and 50 µL of 1 M NaOH were added followed by 1 min vortexing; 1000 µL of hexane/ethyl acetate (1:1, v/v) was added to each tube. After centrifrugation (5 min), 800 µL of the organic phase were removed to clean tubes and the solvent was evaporated under nitrogen flow (N2) at 37°C. Each sample was then diluted in 100 μL of mobile phase, agitated for 2 min, and transferred to LC-MS/MS system vials, for further injection (5 μL).
Local toxicity study: Surgical procedure
Slightly general anesthesia was induced by an intraperitoneal injection of sodium thiopental solution (40 mg/kg), before the administration of the local anesthetic formulations. The animals were divided into four groups and received 0.1 mL of one of the following formulations: MVC2%EPI-2% mepivacaine with 1:100,000 epinephrine; MVC3%-3% mepivacaine; MVCLUV-liposomal 2% mepivacaine; and LUV-mepivacaine-free liposome.
The animals received one of the formulations in the oral mucosa of the upper right first molar, as previously described by CitationCereda et al. (2008). The same amount of saline solution (NaCl 0.9%) was administered in the left side as control. Animals were sacrificed under anesthesia (urethane 1 g/kg and alpha-chloralose 50 mg/kg) 6 h, 24 h, and 4 days after treatment, and the maxilla bones along with soft tissues were removed. Before the administration of the formulations, their pH values were measured with a pHmeter ORION®, model 290A, with a microelectrode LAZAR BNC.
Histological analysis
The samples were fixed with Bowin solution during 24 h and with 10% formalin solution for 48 h, and decalcified with EDTA titriplex (Merck KGaA). Five cross-sections (6 µm thick, 40 μm deep) were obtained from each animal sample. The cross-sections were stained with hematoxylin and eosin. The cross-sections were submitted to qualitative analysis in order to evaluate the intensity of the leucocitary infiltration and/or any area of necrosis. The cross-sections were photographed by a photomicroscope (Zeiss-AXIOSKOP2-PLUS). The analyzed region was the site of the injection and it enclosed the connective tissue in the most internal portion of the anterior maxillary fornix. A preliminary pilot study conducted using methods developed in previous studies (CitationScheib & Garner, 2004; CitationShipper et al., 2005) allowed the use of a qualitative score of the local tissue inflammation. The score was defined based on the following descriptions: (1) no infiltrate; (2) minimal infiltrate; (3) mild infiltrate; (4) severe infiltrate; and (5) severe infiltrate with necrosis areas (CitationCereda et al., 2008).
The images were codified and two individuals blinded to treatment conditions evaluated the images according to the qualitative score previously described. The replication of the classification method between the two examiners was calculated by the intra-class correlation test for continuous data.
Statistical analysis
The plasmatic mepivacaine concentrations were analyzed by one-way ANOVA and the Tukey-Kramer test (post-hoc) considering each period of time separately (α = 0.05). The pharmacokinetic parameters (Cmax, Tmax, AUC0–420, AUC0–∞, t1/2) were analyzed using the Kruskal-Wallis test and Student-Newman-Keuls as post hoc test (α = 0.05). The results obtained in each time interval (6 h, 24 h, and 4 days) were compared considering each group and considering the control side. Data were analyzed with the Kruskal-Wallis test considering each group (inter-group analysis). The tissue reaction was also analyzed by Wilcoxon paired test considering treated and control sides (intra-group analysis). The level of significance was set at 5%.
Results and discussion
Pharmacokinetic study
The calibration graph for mepivacaine was generated with increasing amounts of the drug standards. A good response over the range of 1.0–1000.0 ng/mL was shown and presented linearity with high correlation coefficient (r = 0.99972). The precision and accuracy of the method were assessed by determination of seven concentrations in three independent series of spiked serum samples. The accuracy and precision were calculated to be from 92.45–105.5% and from 0.4–9.8%, respectively. The 95% confidence intervals were 92.97–106.83% considering accuracy and 6.84–7.03% for precision. In addition, the limit of quantification (LOQ) was set as the lowest measurable concentration with acceptable accuracy and precision. The LOQ for mepivacaine was set to 1 ng/mL.
shows the graph of mean plasma concentrations vs time after injecting MVC2%EPI, MVC3%, and MVCLUV. reports the median (lower and upper quartiles) values of the pharmacokinetic parameters obtained after intra-oral injections of the tested formulations. MVC3% induced higher plasma concentrations than MVCLUV after intra-oral injection for periods of time up to 240 min (p < 0.05). After this period, no statistically significant difference was observed between the two formulations (p > 0.05). MVC3% also induced higher plasma concentrations than MVC2%EPI at 15, 120, 180, and 240 min (p < 0.05). Statistical differences were not observed for plasmatic concentrations between MVC2%EPI and MVCLUV for all periods of time (p > 0.05) ().
Figure 1. Graph of mean plasmatic concentration vs time after injecting MVC2%EPI (1:100,000); MVC3%, and MVCLUV.
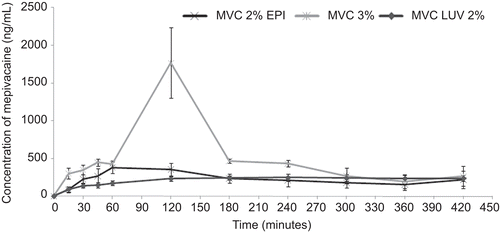
Table 1. Pharmacokinetic parameters of mepivacaine from intra-orally administered formulations.
Regarding the pharmacokinetic parameters, MVCLUV induced higher AUC0–∞ and t1/2 values when compared to MVC3% (2.2- and 10-times, respectively) and MVC2% EPI (12- and 15-times, respectively), as observed in (p < 0.01). However, MVC3% showed statistically different (p < 0.001) Cmax values compared to MVCLUV.
In the present study, mepivacaine analysis was discontinued at 420 min since the blood collection period could not be prolonged due to the volume of blood collected at each time point (0.4 mL). However, the analysis demonstrated that the plasma concentrations obtained with aqueous mepivacaine solution were higher than those obtained with the liposome formulation, similar to the results obtained by CitationBoogaerts et al. (1993b).
Determining drug concentration in biological fluids provides the fundamental information needed for the development of new drug forms (CitationAltun et al., 2004), such as liposome preparations of local anesthetics. Moreover, pharmacokinetic determinations are fundamental in clinical practice (CitationAdams et al., 1989). Although there are several methods for quantifying drug components, liquid chromatography with mass spectrometry (LC-MS/MS) is considered the technique of choice for biological fluid analysis (CitationCobb & Andersson, 2005). The LC-MS/MS system used in this study is one of the most utilized analytical tools for determining pharmacokinetic parameters of a drug with high precision and reliability (CitationKorfmacher, 2005).
There are no previous reports that compare the absorption of local anesthetics, free as well as liposome-encapsulated, after an intra-oral injection. Several studies assess the absorption of liposome-ncapsulated local anesthetics such as: epidural in dogs (CitationMashimo et al., 1992), intra-articular in rabbits (CitationHou & Yu, 1997), subcutaneous in rats (CitationYu et al., 2002), and during axillary block in rabbits (CitationBoogaerts et al., 1993b). Despite these methodological differences, the results obtained in the present study are compatible with those obtained by those authors.
CitationHou and Yu (1997) evaluated the pharmacokinetics of aqueous lidocaine and liposome lidocaine after an intra-articular injection in rabbit knees. These authors observed that the liposome drug was absorbed to a lesser extent than non-vasoconstrictor-containing lidocaine, which was similar to the findings of this study. This may have occurred due to the slow release of the anesthesia by the liposomes.
CitationBoogaerts et al. (1993b) compared plasma concentrations of bupivacaine after administration of free bupivacaine and a complex with multilamellar liposomes during axillary block in rabbits. Those animals given liposome bupivacaine had lower plasma levels during the first 10 min and then higher levels after 24 h, demonstrating that the slow release of the anesthetic was responsible for its prolonged action.
CitationMashimo et al. (1992) showed the pharmacokinetics of free and liposome encapsulated lidocaine in dogs after epidural administration. The areas under the curve (AUC) and Tmax were greater in animals given liposome encapsulated lidocaine, contrary to the observations in this study that revealed smaller parameters for liposome formulations compared with free formulations of the local anesthetic. These differences may be explained by the type of local anesthetic agent used in this study as well as the distinct concentrations and vasoconstrictors, since the commercial formulations with mepivacaine used here were similar to those used in clinical dentistry. Moreover, the different route of administration may have contributed towards these differences.
CitationYu et al. (2002) studied the pharmacokinetics of free and liposome bupivacaine after injecting these formulations in rat tails. In this study, the blood was collected from the animals hourly for 480 min. These authors described that Cmax value was 5-times greater for the non-encapsulated anesthetic. In the present study, the Cmax obtained after injecting MVC3% was 7-fold greater than that obtained after injecting MVCLUV. Likewise, CitationYu et al. (2002) obtained smaller AUC values for liposomal formulations. Even if statistical differences were not observed, the area under the curve (AUC0–t) after treatment with MVCLUV was less than half that obtained after injecting MVC3%. This difference is probably not only due to encapsulation of the anesthetic but also due to the different mepivacaine concentrations in these formulations. The results presented in the present study are similar to those observed by CitationYu et al. (2002), showing the liposomal anesthetic preparation maintained a constant plasmatic level when compared with a non-encapsulated common anesthetic without a vasoconstrictor. In this manner, the results obtained by the present study as well as the documented anesthetic efficacy (Citationde Araujo et al., 2004; CitationCereda et al., 2006) indicate a promising future for liposomal formulations of mepivacaine.
Local toxicity evaluation
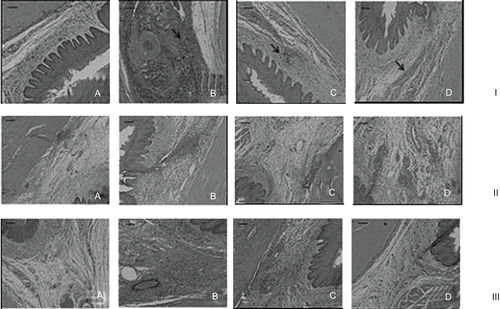
shows transverse sections of the maxilla bones and their surroundings soft tissues 6 h, 24 h, and 4 days after the injections of MVCLUV, MVC3%, MVC2% EPI, or empty liposomal vesicles (LUV). The pH values of each formulation were verified as follows: commercial mepivacaine formulation without vasoconstrictor, 0.9% NaCl, and mepivacaine with vasoconstrictor formulation showed similar pH values of 5.5, 5.8, and 3.8, respectively. The liposomal mepivacaine (7.1) and the mepivacaine free liposome (liposome vesicles) (7.3) had a pH value on the physiologic range. As quality control assay of the histological examination, the intra-class correlation test showed an index of 0.96 (p < 0.0001), indicating agreement between both examiners.
Figure 2. Images obtained after 6 h (I), 24 (II) h, and 4 days (III) of the treatment with: (A) MVCLUV; (B) MVC2%EPI; (C) MVC3%, and (D) LUV. Scale bar: 20 µm.
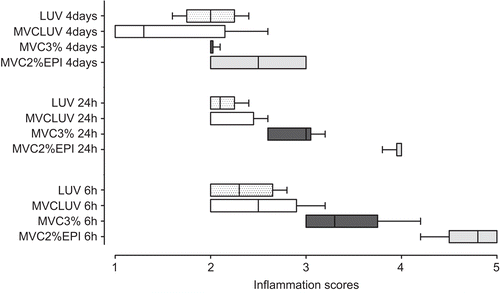
shows the median scores for intensity of the inflammatory reaction after the injection of different mepivacaine formulations (inter-group analysis). At 6 h after injection, MVC2%EPI had higher inflammatory scores compared to MVCLUV (p = 0.0043) and LUV (p = 0.0012). However, MVC3% did not show statistically significant differences from MVC2%EPI (p > 0.05). In addition, no differences were observed between MVCLUV, LUV, and MVC3% (p > 0.05). After 24 h, the results followed the same profile observed at 6 h. However, after 4 days the inflammation scores were higher for MVC2%EPI compared to MVCLUV (p = 0.0096).
Figure 3. Scores of the intensity of the inflammatory reaction (Central line: median; Box: lower and upper quartiles; Whisker: maximum and minimum values) induced by MVC2%EPI; MVC3%, MVCLUV, and LUV.
The right (treated) and the left (control) sides of the maxilla of each animal were compared (intra-group analysis). Considering the intra-group analysis after 6 and 24 h of treatment, the commercial solutions MVC2%EPI and MVC3% showed higher inflammatory reaction scores (p < 0.05) when compared to their controls. Non-significant differences were observed after the administration of MVCLUV and LUV when compared to their controls (p > 0.05). Four days after the treatment, the group treated with MVC2%EPI still had higher scores when compared with the control side (p < 0.05). Non-significant differences were observed after the administration of MVC3%, MVCLUV, and LUV when compared to their controls (p > 0.05). demonstrates the median values of the scores obtained after the treatments and their controls.
Table 2. Median (minimum–maximum limits) of the inflammatory scores for all treatments and their controls 6 h, 24 h, and 4 days after the treatment.
The tissue reaction induced by local anesthetic solutions or by liposomal formulations of local anesthetics was previously evaluated (CitationMalinovsky et al., 1997; CitationRibeiro et al., 2003). However, the present study evaluated the oral tissue reaction to mepivacaine liposomal anesthetic formulation. This local toxicity evaluation is necessary for future use in human subjects. Those studies were performed using polyethylene tubes containing absorbent-paper cones soaked in different anesthetic solutions (lidocaine, mepivacaine, articaine, and bupivacaine), implanted subcutaneously in the backs of rats. According to this previous study, this method could promote a more prolonged contact between local anesthetics and tissues and easily would allow the localization of the implanted area (CitationRibeiro et al., 2003). In the present study, the site of the injection was the oral mucosa close to the upper first molar. This area was used to better simulate the inflammatory reaction in oral sites and it also allowed the use of the upper first molar to locate the injection area. Besides the differences in methodological approaches, both of the studies showed that local anesthetics induce inflammatory reactions.
A 0.9% NaCl solution induced a more intense inflammatory reaction when compared with plain 2% lidocaine solution, probably due to the vasodilator effect of lidocaine, which could cause a rapid absorption and elimination of the anesthetic from the site of the injection (CitationRibeiro et al., 2003). In our study the plain mepivacaine solution evoked more intense inflammatory reactions compared to the 0.9% NaCl solution at 6 and 24 h periods after treatment. The low vasodilator effect of mepivacaine allows longer residence in the site of injection and increases the possibility of tissue irritation, and could be responsible for the more intense inflammatory reaction.
Along with the local anesthetic properties, another important factor to consider regarding the inflammatory response was the presence of vasoconstrictors. In fact, the pH of local anesthetics with sympathomimetic amines (epinephrine, norepinephrine, levonordephrin, and phenylephrine) is usually in the pH range of 3.5–5.5 (USP XXII). This is necessary to avoid the oxidation of the sympathomimetic amines (CitationMurakami et al., 1994). In this study, the pH of the 2% mepivacaine with 1:100,000 epinephrine solution was 3.8. This low pH could have contributed for the high levels of inflammatory reaction induced by this solution at 6 and 24 h after administration. Previous studies reported adverse effects associated with the use of acidic solutions. These solutions could cause discomfort and pain during or after the injection, and retard the onset of anesthesia (CitationOikarinen et al., 1975; CitationMurakami et al., 1994; CitationMeechan & Day, 2002; CitationWahl et al., 2002).
Previous studies showed that a single intrathecal injection of plain epinephrine (up to 0.5 mg) is not associated with histological injury in rabbits (CitationOka et al., 2001) or rats (CitationBahar et al., 1984; CitationHashimoto et al., 2001). However, epinephrine worsens histological spinal cord injury when added to 5% lidocaine in rats (CitationHashimoto et al., 2001) or 1–2% tetracaine in rabbits (CitationOka et al., 2001). Thus, it seems that the addition of epinephrine to a potential irritating substance (local anesthetic) worsens the tissue reaction. In our study the injection of 1 µg of epinephrine with 2% mepivacaine produced the most intense inflammatory reaction when compared to the saline control and to the other formulations. The low pH and the prolonged tissue exposure to the local anesthetic caused by the vasoconstrictor action of epinephrine could explain the more intense inflammatory reaction of MVC2%EPI. In addition, the use of vasoconstrictors evokes ischemia, hypoxia, and important cell injury (CitationKumar et al., 2007). This feature could explain the increased toxic effects observed after the injection of the MVC2%EPI formulation. The liposomal formulations, with a pH in the physiologic range, with or without mepivacaine, did not promote an inflammatory reaction higher than the one evoked by the injection of 0.9 % NaCl solution. Histopathology characteristics of the spinal cord of rabbits were evaluated after an intra-cisternal injection of plain and liposomal bupivacaine, both at 0.5%, and bupivacaine free liposomes (CitationMalinovsky et al., 1997). These authors observed that the injection of liposomal bupivacaine did not produce histopathological changes in the spinal cord different from those evoked by the plain solution. Despite methodological differences, our study showed similar results, since the injection of liposomal mepivacaine did not produce an inflammatory reaction more intense than the injection of plain mepivacaine (MVC3%), mepivacaine free-liposomes, and 0.9 % NaCl solution.
Conclusion
Results from our study indicate that 2% liposome-encapsulated mepivacaine reduced the Cmax and prolonged AUC0–∞ and t1/2 compared with 3% plain and 2% vasoconstritor-associated mepivacaine, after intra-oral injection. In addition, liposomal mepivacaine might protect the tissue against local inflammation evoked by plain or vasoconstrictors-associated mepivacaine, supporting its safety and possible clinical use in dentistry. This formulation could be considered an alternative to the use of vasoconstrictor-containing local anesthetic formulations when the vasoactive compound is contraindicated.
Acknowledgements
The authors thank Mrs Valeria Lobo for her assistance in manuscript preparation.
Declaration of interest
The authors thank Cristália Prod. Quím. Farmac. Ltda for the donation of mepivacaine and for the pyrogen tests and FAPESP, Proc 06/00121-9 for the financial support. Giovana R. Tofoli received a fellowship from CNPq. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adams, H.A., Biscoping, J., Ludolf, K., Börgmann, A., Bachmann, M.B., Hempelmann, G. (1989). The quantitative analysis of amide local anesthetics using high pressure liquid chromatography and ultraviolet detection (HPLC/UV). Reg Anaesth. 12:53–7.
- Altun, Z., Abdel-Rehim, M., Blomberg, L.G. (2004). New trends in sample preparation: on-line microextraction in packed syringe (MEPS) for LC and GC applications Part III: Determination and validation of local anaesthetics in human plasma samples using a cation-exchange sorbent, and MEPS-LC-MS-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 813:129–35.
- Bahar, M., Cole, G., Rosen, M., Vickers, M.D. (1984). Histopathology of the spinal cord after intrathecal cocaine, bupivacaine, lignocaine and adrenaline in the rat. Eur J Anaesthesiol. 1:293–7.
- Boogaerts, J.G., Declercq, A., Lafont, N., Benameur, H., Akodad, E.M., Dupont, J.C., Legros, F.J. (1993a). Toxicity of bupivacaine encapsulated into liposomes and injected intravenously: comparison of plain solutions. Anesth Analg. 76:553–5.
- Boogaerts, J.G., Lafont, N.D., Declercq, A.G., Luo, H.C., Gravet, E.T., Bianchi, J.A., Legros, F.J. (1994). Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: a first study. J Clin Anesth. 6:315–20.
- Boogaerts, J.G., Lafont, N.D., Luo, H., Legros, F.J. (1993b). Plasma concentration of bupivacaine after brachial plexus administration of liposome associated and pain solutions to rabbits. Can J Anesth. 40:1201–4.
- Bourne, D.W.A. (1995). Mathematical modeling of pharmacokinetic data. Lancaster: Technomic Publishing Inc.
- Bucalo, B.D., Mirikitani, E.J., Moy, R.L. (1998). Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 24:537–41.
- Cereda, C.M.S., Brunetto, G.B., Araujo, D.R., de Paula, E. (2006). Liposomal formulations of prilocaine, lidocaine and mepivacaine prolong analgesic duration. Can J Anaesth. 53:1092–7.
- Cereda, C.M.S., Tofoli, G.R., Brito-Junior, R.B., de Jesus, M.B., Fraceto, L.F., Araujo, D.R., de Paula, E. (2008). Stability and local toxicity evaluation of a liposomal prilocaine formulation. J Lipos Res. 18:329–39.
- Cobb, Z., Andersson, L.I. (2005). Determination of ropivacaine in human plasma using highly selective molecular imprint-based solid phase extraction and fast LC-MS analysis. Anal Bioanal Chem. 383:645–50.
- Covino, B.G., Vassalo, H.G. (1976). Local anesthetics: mechanisms of action and clinical use. New York: Grune and Stratton.
- Cox, B., Durieux, M.E., Marcus, M.A. (2003). Toxicity of local anaesthetics. Best Pract Res Clin Anaesthesiol 17:111–36.
- de Araujo, D.R., Cereda, C.M.S., Brunetto, G.B., Pinto, L.M.A., Santana, M.H.A., de Paula, E. (2004). Encapsulation of mepivacaine prolongs the analgesia provided by sciatic nerve blockade in mice. Can J Anesth. 51:566–72.
- de Araujo, D.R., Pinto, L.M.A., Braga, A.F.A., de Paula, E. (2003). Formulações de anestésicos locais de liberação controlada: Aplicações Terapêuticas. Rev Bras Anestesiol. 53:653–66.
- Felnerova, D., Viret, J.F., Glück, R., Moser, C. (2004). Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 15:518–29.
- Fraceto, L.F., Pinto, L.M.A., Franzoni, L., Braga, A.A., Spisni, A., Schreier, S., de Paula, E. (2002). Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophys Chem 6:229–43.
- Gesztes, A., Mezei, M. (1988). Topical anesthesia of the skin by liposome-encapsulated tetracaine. Anesth Analg. 67:1079–108.
- Grant, G.J., Bansinath, M. (2001). Liposomal delivery systems for local anesthetics. Reg Anesth Pain Med. 26:61–3.
- Grant, G.J., Barenholz, Y., Bolotin, E.M., Bansinath, M., Turndorf, H., Piskoun, B., Davidson, E.M. (2004). A novel liposomal bupivacaine formulation to produce ultralong-acting analgesia. Anesthesiology 101:133–7.
- Hashimoto, K., Hampl, K.F., Nakamura, Y., Bollen, A.W., Feiner, J., Drasner, K. (2001). Epinephrine increases the neurotoxic potential of intrathecally administered lidocaine in the rat. Anesthesiology 94:876–81.
- Hou, S.M., Yu, H.Y. (1997). Comparison of systemic absorption of aqueous and liposomal lidocaine following intra-articular injection in rabbits. J Formos Med Assoc. 96:141–3.
- Korfmacher, W.A. (2005). Principles and applications of LC-MS in new drug discovery. Drug Discov Today. 10:1357–67.
- Kumar, V., Abbas, A., Fausto, N., Mitchell, R. (2007). Robbins basic pathology. Philadelphia: Saunders Company.
- Lafont, N.D., Boogaerts, J.G., Legros, M.D. (1994). Use of liposome associated bupivacaine for the management of a chronic pain syndrome. Anesth Analg 79: 818.
- Lafont, N.D., Legros, M.D., Boogaerts, J.G. (1996). Use of liposome associated bupivacaine in a cancer pain syndrome. Anaesthesia 51:578–9.
- Langerman, L., Grant, G.J., Zakowski, M., Golomb, E., Ramanathan, S., Turndorf, H. (1992). Prolongation of epidural anesthesia using a lipid drug carrier with procaine, lidocaine, and tetracaine. Anesth Analg 75:900–5.
- Malinovsky, J.M., Benhamou, D., Alafandy, M., Mussini, J.M., Coussaert, C., Couarraze, G., Pinaud, M., Legros, F.J. (1997). Neurotoxicological assessment after intracisternal injection of liposomal bupivacaine in rabbits. Anesth Analg 85:1331–6.
- Mashimo, T., Uchida, I., Pak, M., Shibata, A., Nishimura, S., Inagaki, Y., Yoshiya, I. (1992). Prolongation of canine epidural anesthesia by liposome encapsulation of lidocaine. Anesth Analg 74:827–834.
- McLure, H.A., Rubin, A.P. (2005). Review of local anaesthetic agents. Minerva Anestesiol 71:59–74.
- Meechan, J.G., Day, P.F. (2002). A comparison of intraoral injection discomfort produced by plain and epinephrine-containing lidocaine local anesthetic solutions: a randomized, double-blind, split-mouth, volunteer investigation. Anesth Prog 49:44–48.
- Mowat, J.J., Mok, M.J., MacLeod, B.A., Madden, T.D. (1996). Liposomal bupivacaine: extended duration nerve blockade using large unilamellar vesicles that exhibit a proton gradient. Anesthesiology 85:635–43.
- Murakami, C.S., Odland, P.B., Ross, B.K. (1994). Buffered local anesthetics and epinephrine degradation. J Dermatol Surg Oncol. 20:192–5.
- Oikarinen, V.J., Ylipaavalnpemi, P., Evers, H. (1975). Pain and temperature sensations related to local analgesia. Int J Oral Surg. 4:151–6.
- Oka, S., Matsumoto, M., Ohtake, K., Kiyoshima, T., Nakakimura, K., Sakabe, T. (2001). The addition of epinephrine to tetracaine injected intrathecally sustains an increase in glutamate concentrations in the cerebrospinal fluid and worsens neuronal injury. Anesth Analg. 93:1050–7.
- Ribeiro, P.D. Jr., Sanches, M.G., Okamoto, T. (2003). Comparative analysis of tissue reactions to anesthetic solutions: histological analysis in subcutaneous tissue of rats. Anesth Prog. 50:169–80.
- Samad, A., Sultana, Y., Aqil, M. (2007). Liposomal drug delivery systems: an update review. Curr Drug Deliv. 4:297–305.
- Scheib, S.A., Garner, W.H. (2004). Anti-inflammatory effects of topical ocular MAXIDEX administration to rabbits following vitrectomy or lensectomy. Exp Eye Res. 79:893–902.
- Shipper, G., Teixeira, F.B., Arnold, R.R., Trope, M. (2005). Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or resilon. J Endod. 31:91–6.
- Torchilin, V.P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Rev Drug-Discov. 4:145–60.
- Unites States Pharmacopeia XXII. (1995). The national formulary XVII. Rockville.
- Wahl, M.J., Schmitt, M.M., Overton, D.M., Gordon, M.K. (2002). Injection pain of bupivacaine with epinephrine vs. prilocaine plain. J Am Dent Assoc. 133:1652–6.
- Whiteside, J.B, Wildsmith, J.A.W. (2001). Developments in local anesthetics drugs. Br J Anaesth 87:27–35.
- Yu, H.Y., Li, S.D., Sun, P. (2002). Kinetic and dynamic studies of liposomal bupivacaine and bupivacaine solution after subcutaneous injection in rats. J Pharm Pharmacol. 54:1221–7.