Abstract
The aim of the present study was to develop a sumatriptan succinate transdermal system for applying migraine treatments efficiently and easily. For this system polyvinyl alcohol was employed as a matrix and Azone® was added as a permeability enhancer. The physical characteristics, mechanical properties, and in vivo bioadhesion of the systems were evaluated, as was in vitro permeation across porcine skin. A uniform distribution of the drug in the matrix was observed, and moisture uptake values were constant. With regard to mechanical parameters, occlusive layer inclusion made the system more resistant, and no significant differences were detected with respect to other systems. Although Azone® reduced the bioadhesivity of the systems, adherence to skin was maintained 24 h after application. Permeation studies showed that the systems formulated with Azone® provided the highest permeability profiles for sumatriptan succinate.
Introduction
Triptans are a family of tryptamine-based drugs that were introduced in the 1990s for the treatment of migraine (CitationBelvis et al., 2009; CitationBigal et al., 2009). This therapeutic group includes sumatriptan, which is a potent and selective agonist of 5-hydroxytryptamine sub-type 1 receptor that is usually prescribed for the treatment of migraine attacks with or without the presence of an aura (visual disturbances, usually halos or flickering lights, which precede an attack) (CitationTfelt-Hansen et al., 2000). Sumatriptan is commercially available as sumatriptan succinate in the following forms: oral tablets containing 25, 50, or 100 mg of the drug, which should be swallowed whole with liquid; nasal spray, packaged in single-dose bottles containing either 5 or 20 mg of the drug; and injections, containing 6 mg/0.5 mL of the drug, which can be administered beneath the skin with an autoinjector. These pathways have an absolute bioavailability of ∼ 14%, 15%, and 96%, respectively (CitationScott, 1994).
Considering the low bioavailability after oral and intranasal administration, due principally to pre-systemic metabolism and incomplete absorption, and the inconvenience associated with parenteral administration, a pharmaceutical form of transdermally-administrated sumatriptan succinate has obvious benefits for the treatment of migraine sufferers (CitationBarry, 1983; CitationChien, 1987).
Transdermal administration is advantageous in that it avoids gastrointestinal incompatibility and first pass metabolism and the consequent reduction of the bioavailability of the drug. Moreover, reducing the frequency of dosing would improve patient compliance (CitationBerti & Lipsky, 1995; CitationTanner & Marks, 2008). For these reasons, it is an aim to obtain transdermal systems that employ sumatriptan succinate.
Recently, we have reported an innovative transdermal system consisting of a bioadhesive, monolaminated system with the usual components of transdermal patches condensed in one unique layer. It is manufactured using a lamination technique and its composition includes polyvinyl alcohol (PVA) as a matrix. It is characterized by the fact that it is not adhesive in a dry state but becomes bioadhesive in the presence of water (CitationPadula et al., 2004).
Different studies were performed in order to include sumatriptan succinate in this new transdermal system and others similar. To develop the formulations, diverse polymers were studied as a matricial support for the drug (CitationFemenía-Font et al., 2006a; CitationBalaguer-Fernández et al., 2007). In the first approach (CitationFemenía-Font et al., 2006a) the enhancers PEG 600 and Transcutol® were added in the formulation based on PVA, and the effect of occlusion was tested by means of covering the films with parafilm®. The results obtained demonstrated that occlusion produced a marked increment in the transport of sumatriptan succinate across the skin. In the second approach an occlusive layer was added to the systems, Methylcelullose, PVP (Polyvinylpirrolidone), and a mixture of PVP-PVA were used to prepare the polymeric matrixes and the systems incorporated Azone® as enhancer. The transdermal systems prepared were well accepted by the volunteers; nevertheless, sumatriptan transdermal flux was not considered enough in order to achieve the pharmacological goal. Since it was observed that the polymer had a great influence on transdermal drug flux in order to optimize the formulation some changes were considered necessary. The aim of this work was to prepare and characterize physical and biopharmaceutically transdermal systems that incorporated sumatriptan succinate from different sources, prepared with PVA, Azone®, and the adhesive Plastoid® E 35 H.
The physical parameters (system morphology and moisture uptake), mechanical parameters, and in vivo adhesivity of the systems developed were characterized. In addition, in vitro drug permeation profiles across pig ear skin were evaluated.
Materials and methods
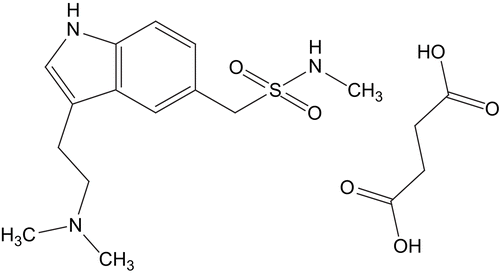
Sumatriptan succinate (Eur. Ph. monograph 1573) is chemically designated as 3-[2-(dimethylamino) ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1). The empirical formula is C14H21N3O2S•C4H6O4 and its molecular weight is 413.5. It is a drug highly soluble in water. Its structure is represented in . Two sources of sumatriptan succinate were considered: sumatriptan succinate powder, generously donated by GlaxoSmithKline (UK), and Imigran® Intranasal 20 mg, also obtained from GlaxoSmithKline. The excipients of the commercial source of the drug are potassium dihydrogenphosphate, anhydrous disodium phosphate, sulfuric acid, sodium hydroxide, and water.
The polyvinyl alcohol 83400 employed as a matrix was obtained from Nipon Ghosei (Osaka, Japan). Eudragit® E100, used to prepare the adhesive Plastoid® E 35 H, was supplied by Rofarma (Gaggiano, Milan, Italy). Lauric acid and glycerol, the other components of the Plastoid® formulation, were obtained from Laboratorios Guinama S.L. (Alboraya, Valencia). Adipic acid was purchased from Sigma-Aldrich Co. (UK).
Sorbitol was purchased from Sigma Aldrich Co. (St. Louis, MO). The chemical enhancer Azone® (1-dodecyl-azacycloheptan-2-one or laurocapram) was obtained from Netqem (Durham, NC).
The occlusive layer employed, Scotchpack™ Backing 9733, was generously donated by 3M™ (USA). HEPES (N-[2-Hydroxiethyl] piperazine-N’-[2-ethanesulfonic acid]) and NaCl were provided by Sigma-Aldrich Co. Ammonium di-hydrogen phosphate (96–102%, w/w) and ortophosphoric acid (85–88%, v/v) were purchased from Panreac Química S.A. (Barcelona, Spain). Acetonitrile was obtained from Análisis Vínicos (Tomelloso, Spain).
All other chemicals used were of analytical grade. Ultrapure water was employed in the preparation of solutions.
The pig ear skin required for in vitro transdermal diffusion experiments was acquired from a local slaughterhouse immediately following the death of the animal.
Preparation of sumatriptan transdermal systems
Six polymeric matrix systems containing the same concentration of sumatriptan succinate were formulated via a lamination method (CitationNicoli et al., 2006; CitationPadula et al., 2009).
First, Plastoid® 35 H was obtained according to the Degussa protocol: Eudragit® E100, lauric acid, and adipic acid are added to hot water (temperature range, 78–82°C). The mixture was stirred, maintaining the temperature at 80°C in order to obtain a clear dispersion. Afterwards, glycerol was added. The mixture was then poured into plastic bottles and maintained at room temperature.
Simultaneously, the PVA was prepared (20%, w/w) by 24 h hydration of the polymer in the appropriate volume of hot water (60–70°C). Then the Plastoid® 35 H was added to the polymeric mixture, which was continuously stirred with a magnetic stirrer bar.
Sumatriptan succinate was dissolved in water and then sorbitol, the plasticizer, was added to the mixture. Azone® was also added when required. Finally, the dispersion including the drug was incorporated into the adhesive polymeric mixture.
The compositions of the different formulations are displayed in , which shows the final percentage of each component in the wet mixtures (% w/w) of the transdermal systems we prepared. Sumatriptan succinate constituted 2.4% (w/w) of the systems into which it was incorporated, while Azone® constituted 5% (w/w). The systems prepared without sumatriptan succinate were identified as PVA/PVA-Azone® (including/not including the chemical enhancer in the composition, respectively). Systems including sumatriptan succinate obtained from an intranasal dispositive (Imigran®) were referred to as PVA-SMT/PVA-SMT-Azone® (without or with the chemical enhancer, respectively) and PVA-SMT powder/PVA-SMT powder-Azone® (without or with the chemical enhancer, respectively).
Table 1. Composition (%, w/w) of the different wet mixtures used to prepare the sumatriptan succinate transdermal systems.
The mixtures obtained were laminated at 600 µm on a single layer, which acted as an occlusive liner (Scotchpack™ 9733 backing layer), and were dried at room temperature, in the dark.
The systems were subsequently sealed individually in aluminum pouches until they were evaluated.
Moisture uptake studies
To calculate moisture uptake, a uniformly-sized piece (1 cm2) of each transdermal system was kept in a desiccator at room temperature for 24 h, and then weighted and exposed to a relative humidity of 75% (100 mL of saturated solution of sodium chloride) in a desiccator until its weight became constant (n = 3) (CitationUbaidulla et al., 2007).
The water absorption capacity of the systems was calculated as the increase of weight after the assay (equation 1):
Results were statistically compared by means of an ANOVA test (with a post-hoc test T3 Dunnet Test, p < 0.05).
Polarized light microscopy
The external morphology of the sumatriptan succinate transdermal systems before their application to the skin was analyzed using polarized light microscopy (Labophot II Nikon, Tokyo, Japan).
Measurement of mechanical properties
Two parameters—force and elongation at the point at which the systems broke—were studied as mechanical properties in order to obtain an approximate estimation of the resistance and elasticity of the different systems evaluated.
First, these mechanical parameters were determined in order to compare the effect of sumatriptan succinate in the systems, obtained either from a commercial source (Imigran®) or in the form of a powder. The effect of the transdermal enhancer Azone® and the application of the occlusive liner to the systems were simultaneously studied.
The mechanical properties of the sumatriptan systems were evaluated using an electronic dynamometer (Acquati, Milano, Italy) equipped with a 5 Kg load cell. A 10 × 100 mm strip of each system (free from air bubbles and physical imperfections) was held between the two clamps of the dynamometer for analysis. During measurement, the systems were pulled at a rate of 30 mm/s.
The two parameters, force and elongation at the point at which the systems broke, were then measured. Measurements were performed three times for each system. The tensile strength and elongation at breaking point were calculated as (CitationPeh K et al. 2000) (equation 2 and 3):
The statistical analysis of the parameters obtained was performed using an ANOVA test (and post-hoc T3 Dunnet Test, p < 0.05).
Measurement of in vivo bioadhesive strength
Transdermal systems are not adhesive in the dry state but become bioadhesive when applied to wet skin. The application of these systems to moist skin allows their adhesion following seconds of pressure.
The bioadhesive strength of the sumatriptan transdermal systems that included an occlusive layer was evaluated in vivo employing a modification of the Standard Test Methods (ASTM)-Quick Stick Test adhesion test methods (PSTC-5) (CitationGlenview, 1976). A 25 × 70 mm strip from each system was applied to the dorsal side of a hand of three volunteers after wetting the skin with 200 µL of water (the final contact area was 7.5 cm2). All volunteers included in the study were previously informed and provided their signed consent. To fix the systems a slight pressure was applied. After 30 min of application, the strips were fixed to the upper clamp of an electronic dynamometer provided with a 5 Kg load cell and pulled at the rate of 300 mm/min.
To evaluate bioadhesion, the work of adhesion and the peak force of detachment were assessed. Work of adhesion was calculated from the area under the force-distance curve and peak detachment force was defined as the maximum force required for detaching the system from the skin. Data collection and calculation of adhesion parameters were performed using the Scope V 3.5 software. The results of work of adhesion and peak detachment force were statistically compared using an ANOVA test (and post hoc Scheffé Test, p < 0.05).
In vitro permeation studies
Vertical Franz-type diffusion cells with a diffusion area of 0.6 cm2 were employed for in vitro diffusion experiments (DISA, Milan, Italy). For transdermal studies skin was excised from pig ear (outer side) and then dermatomed (600 µm) using an Aesculap-Wagner dermatome C. GA 176 (B. Braun surgical S.A., Barcelona, Spain) and packed and stored at −80°C until use. Samples were defrosted and placed on the cell with the stratum corneum facing the donor compartment.
The transdermal systems of sumatriptan succinate were placed in the donor compartment. They were applied to the surface of the pig skin using a previously described procedure (skin was pre-wetted with 15 µL/cm2 of water) (CitationPadula et al., 2003). The receptor compartment (4 mL volume) was filled with HEPES/NaCl (20/150 mM) saline buffer solution (pH 7.4) and thermostated at 37 ± 0.1°C. The solution was continuously stirred to prevent boundary layer effects.
The experiment lasted 56 h, during which samples (200 µL) were taken from the receptor chamber at pre-determined time intervals. The volume of sample removed was replaced with the same volume of buffer solution pH 7.4.
Prior to the in vitro experiments a portion of each transdermal system (1 cm2) was extracted by shaking in saline buffer (pH 7.4) to determine the initial concentration of sumatriptan succinate. At the end of the experiments, transdermal systems were peeled off and also shaken in saline buffer (pH 7.4) to determine the residual concentration of the drug. The sumatriptan succinate retained in the skin was extracted through immersion for 12 h in 2 mL of the saline buffer (pH 7.4) (CitationFemenía-Font et al., 2005a).
High pressure liquid chromatography (HPLC) was used to determine the amount of sumatriptan succinate present in the samples (CitationFemenía-Font et al., 2005b). Analysis was performed at room temperature. A Waters 600 Controller equipped with a quaternary pump that included a diode-array detector (Waters 996 Photodiode Array Detector, Barcelona, Spain) was employed for the analysis. An analytical reverse-phase Kromasil C-18 column (4 × 250 mm) (Análisis Vínicos, Tomelloso, Spain) was also employed. The mobile phase consisted of a mixture of ammonium di-hydrogen phosphate water solution (0.05 M, pH 3.3) and acetonitrile (84:16, v/v) at a flow rate of 1 ml/min. The wavelength of detection was 282.7 nm. Aliquots of 50 µL were injected.
The partition parameter (KH) and diffusion (D/H2) of the drug were determined by fitting Fick’s second law of diffusion equation to the amounts of sumatriptan accumulated in the receiver compartment of the diffusion cells as a function of time. The permeability coefficient was the product of KH and D/H2, and the steady-state flux (Jss) was determined as the product between the permeability coefficient (data not shown) and the concentration of the drug in the system. Fittings were performed using WinNonlin 5.0.1 Software (Pharsight Corp). The Jss values obtained were compared using the one-way ANOVA, followed by the Scheffe post-hoc test.
Results and discussion
Sumatriptan succinate is highly soluble in water (water solubility at 20°C = 101 mg/mL), and the partition coefficient of the sumatriptan base in n-octanol/water (Po/w = 0.65) is very low. Due to its characteristics, we selected a hydrophilic matrix to prepare the transdermal systems. We prepared different sumatriptan succinate transdermal systems with polyvinyl alcohol (). All the systems contained sorbitol as a plasticizer, methacrylate copolymer as an adhesive agent, and Scotchpack™ 9733 backing as an occlusive liner. Azone (5%, w/w) was incorporated into some of the systems as a percutaneous enhancer. All of the transdermal systems were thin, durable, flexible, perfectly adaptable to skin irregularities, and rapidly and uniformly adhered to the skin for a reasonable period of time. All were easy to apply and remove.
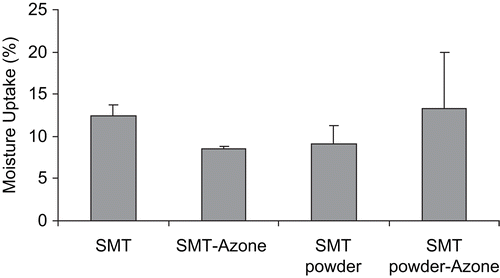
The moisture uptake values of the different transdermal systems were calculated and are shown in . Statistical analysis showed that neither the source of drug nor addition of the chemical enhancer to the system were factors in the increase of water capture (ANOVA, T3 Dunnet, p < 0.05). As expected, the use of different sources of sumatriptan succinate did not affect the uptake of moisture. Even the inclusion of Azone® did not produce changes in the values obtained with respect to the corresponding control. The mean value of moisture uptake obtained was 10.81 ± 2.36%.
Figure 2. Percentage of moisture uptake from sumatriptan succinate transdermal films. Data are mean ± SD (n = 3). SMT: sumatriptan succinate.
To demonstrate the uniform distribution of the drug in the polymer matrix (PVA), the surface morphology of the sumatriptan succinate transdermal systems was studied using a polarized light microscope. shows an example of the uniform distribution of sumatriptan succinate in the PVA polymer matrix used to prepare the transdermal systems. All the systems had the same appearance.
Figure 3. Polarized light microscopy photograph of transdermal film containing sumatriptan succinate (Imigran®). System includes Azone® and an occlusive layer (original magnification × 20).
On the other hand, we measured tensile measure strength and elongation at breaking point as an indication of the strength and elasticity of the systems. The mechanical properties, force, and elongation of the sumatriptan succinate transdermal systems are represented in and , respectively. Tensile strength (traction breaking force) was in the range of 0.2–0.8 N/mm2. As can be observed in , the presence of an occlusive liner produced a significant increment of the tensile strength values obtained with the different systems with respect to controls (ANOVA, T3 Dunnet, p < 0.05). The occlusive liner made the systems more resistant to breaking, and in fact proved to be the most resistant of the layers. The highest tensile strength value corresponded with the transdermal system prepared with sumatriptan succinate (Imigran®) and the transdermal enhancer Azone® (p > 0.05). Elongation at breaking point (elasticity) of the various systems was in the range of 42–50%. The inclusion of an occlusive layer did not produce significant differences between the systems whose composition included sumatriptan succinate (ANOVA, T3 Dunnet, p > 0.05).
Figure 4. Tensile strength values (N/mm2) obtained for each of the polyvinyl alcohol transdermal films prepared. SMT: sumatriptan succinate.
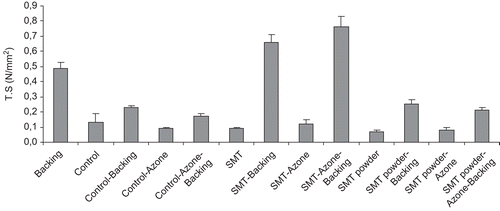
Figure 5. Elongation at breaking point values (%) obtained for each of the polyvinyl alcohol transdermal films prepared. SMT: sumatriptan succinate.
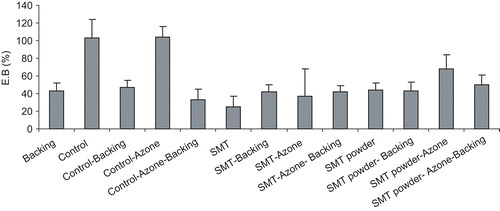
shows the values of the bioadhesive measurements obtained: peak detachment force (N) and work of adhesion (mJ). All the studies performed showed that the presence of sumatriptan succinate in the systems did not affect skin adhesion. Addition of the transdermal enhancer Azone® produced changes in skin–system contact that reduced the adhesivity of the system. Even so, these systems remained attached to the skin surface after 24 h of application.
Table 2. Peak detachment force (N) and work of adhesion (mJ) for the different sumatriptan succinate transdermal systems (mean ± SD).
Finally, sumatriptan succinate permeation through pig ear skin was evaluated in vitro. Even though the permeability of porcine skin differs from that of human skin, this animal model is appropriate for the screening and selection of techniques for enhancing the transdermal delivery of drugs (CitationDick & Scott, 1992; CitationSimon & Maibach, 2000; CitationGodin & Touitou, 2007) and the analysis of the permeation of many drugs including sumatriptan succinate (CitationFemenía-Font et al., 2006b). shows the permeation profiles of sumatriptan succinate obtained in the transdermal diffusion experiments performed. It can be observed that the amount of drug in the receptor compartment differed depending on the preparation of the system. In fact, as can be seen in , the amount of sumatriptan accumulated in the receptor compartment at the end of the experiments was lower in the case of the PVA systems formulated with sumatriptan succinate powder (PVA-SMT powder) than in those in which the drug was obtained from a commercial source (PVA-SMT). In the case of the systems that included the transdermal enhancer Azone®, the final amount of sumatriptan in the receptor compartment also varied depending on the source of the drug, with the commercially-provided version (PVA-SMT-Azone®) resulting in a higher accumulation of drug in the receptor chamber. The steady-state flux values calculated for each formulation (Jss) are listed in . When Azone® (5%, w/w) was incorporated into the systems it produced a statistically significant increase in sumatriptan succinate steady-state flux values with respect to controls (ANOVA, Scheffe test, p < 0.05). Formulations with the chemical enhancer and sumatriptan succinate obtained from the commercial source (Imigran®) exhibited a statistically higher drug flux at steady-state (ANOVA, Scheffe test, p < 0.05). Results demonstrated that the transdermal flux values obtained at steady-state with Imigran® intranasal were higher than those obtained with sumatriptan succinate powder. This could be due to the excipients contained in the Imigran® preparation, which is formulated for intranasal administration (potassium dihydrogenphosphate, anhydrous disodium phosphate, sulfuric acid, sodium hydroxide, and water). It is possible that substances within the formulation produced an additive effect apart from that of the chemical enhancer. Similar findings have been reported in experiments carried out with a drug solution (CitationFemenía-Font et al., 2006b). Furthermore, we observed that higher amounts of sumatriptan were retained in the skin after the transdermal diffusion experiments (), which constituted a depot in the skin when the system was retired.
Figure 6. Permeation profiles of sumatriptan succinate for the transdermal systems: PVA-SMT (•), PVA-SMT-Azone® (○), PVA-SMT powder (▾), PVA-SMT powder-Azone® (Δ). Average values ± SD, n ≥ 4. PVA: polyvinyl alcohol; SMT: sumatriptan succinate.
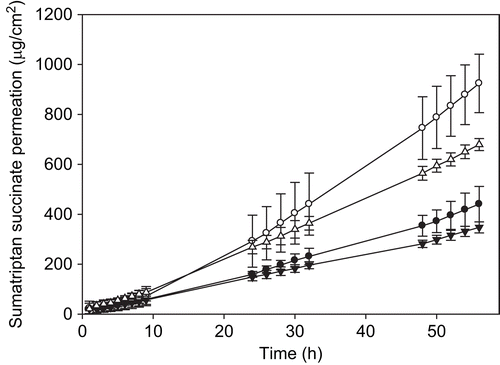
Table 3. Cumulative amount of sumatriptan succinate in receptor compartment at the end of experiments (µg/cm2) (mean ± SD), partition parameter (KH; cm) (mean ± SE), diffusion parameter (D/H2; h-1) (mean ± SE), transdermal flux of sumatriptan succinate across pig skin calculated at the steady-state (Jss; µg/cm2h) (mean ± SD), and sumatriptan succinate retained in the skin (µg/cm2) (mean ± SD).
Azone® was the first molecule to be designed specifically as a percutaneus enhancer. It can be used for improving the absorption of topical agents and for optimizing transdermal devices (CitationWalters & Hadgraft, 1993). Is a highly lipophilic agent, with a logP octanol/water of ∼ 6.2, is soluble and compatible with most organic solvents, including alcohol and propylene glycol (CitationPathan & Setty, 2009), and it can be employed with lipophilic and hydrophilic molecules. Its mechanism of action is based on its capacity to alter the organization of the lipid structure of the stratum corneum and augment the diffusion of a drug across the skin (CitationSmith & Maibach, 1995; CitationLópez-Cervantes et al., 2006). The enhancer effect of Azone® depends predominantly on the concentration employed and the lipophilicity of the compound assayed. The number of drugs whose penetration can be increased by Azone® is greater when the enhancer is used at high concentrations (5% w/w), therefore this was the concentration selected to prepare the formulations of this study. The lower the lipophilicity value of the penetrant, the greater the enhancer effect observed. The inverse dependence between the lipophilicity of the penetrant and the efficacy of Azone® could be explained by the fact that Azone® not only exerts a well-known fluidifying action on skin lipids by disrupting their highly ordered structure between corneocites, but also affects the hydration of the stratum corneum (CitationSugibayashi et al., 1992; CitationLópez et al., 2000). The permeation parameters obtained with our systems were calculated and analyzed in order to determine the mechanism of sumatriptan absorption and the effect of Azone®. Both partitioning and diffusion parameters are presented in . The values obtained differed depending on the system in question. The parameter KH, which gives an indication of the partitioning of the permeant between the stratum corneum and the formulation, was 0.02 × 10−4 cm for the systems formulated without the enhancer and increased to 46 × 10−4 cm and 3.97 × 10−4 cm for the systems whose composition included Azone®. In other words, the inclusion of Azone® in a matrix system produced an increment in the partitioning into the stratum corneum. In the case of the systems that were prepared without the enhancer, the small partitioning values may be explained if we bear in mind that the matrix itself is quite hydrophilic, and that the drug does not tend to migrate into the stratum corneum (CitationNicoli et al., 2006). In the case of the diffusion parameter D/H2, its value varied for each formulation. In general, when Azone® was included in the transdermal formulations the diffusion parameter was lower. This indicates that sumatriptan succinate diffusion was negatively influenced by the presence of the penetration enhancer in the system, and that the enhancing effect of the sumatriptan flux produced by Azone® was only due to an increase in the partitioning of the drug in the skin.
When sumatriptan plasma clearance (70 L/h) and the maximum plasma level of the drug required (72 ng/mL) (CitationScott, 1994) are taken into account, the necessary entrance of drug is ∼ 1 µg/h. This value was obtained in vitro with all the systems evaluated, though, obviously, these results need to be verified in vivo.
In conclusion, the present work suggests that transdermal systems for the administration of sumatriptan succinate are viable. Our systems are thin, flexible, transparent, and non-adhesive in a dry state, but bioadhesive in the presence of water, and are maintained on the skin for a reasonable period of time. Although Azone® was found to reduce the adhesivity of the systems, they remained adhered to the skin 24 h after application. Azone® is an effective transdermal absorption enhancer that can be incorporated into transdermal systems that contain sumatriptan succinate. The systems prepared using the polymer matrix of PVA render higher transdermal flux of sumatriptan than those elaborated previously with PVP and PVP-PVA. The drug transdermal flux is comparable to that provided by the methylcellulose systems, but this new transdermal systems prepared adhere better to skin (CitationBalaguer-Fernández et al., 2007).
Acknowledgements
Declaration of interest
The authors wish to thank the Fondo de Investigaciones Sanitarias (Ministerio de Sanidad y Consumo) (PI 060108) and the Universidad CEU Cardenal Herrera for their financial support. The authors also thank Pharsight corp. for the academic license of WinNonlin 5.0.1. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Balaguer-Fernández, C., Femenía-Font, A., del Rio-Sancho S., Merino V., López-Castellano A. (2007). Sumatriptan succinate transdermal delivery systems for the treatment of migraine. J Pharm Sci. 97:2102–9.
- Barry, B.W. (1983). Properties that influence percutaneus absorption. In: Dermatological formulations: percutaneous absorption. Editor: Barry BW. New York: Marcel Dekker, 181–2.
- Belvis, R., Pagonabarraga, J., Kulisevsky, J. (2009). Individual triptan selection in migraine attack therapy. Recent Pat CNS Drug Discov. 4:70–81.
- Berti, J.J., Lipsky, J.J. (1995). Transcutaneous drug delivery: a practical review. Mayo Clin Proc. 70:581–6.
- Bigal, M.E., Krymchantowski, A.V., Hargreaves, R. (2009). The triptans. Expert Rev Neurother. 9:649–59.
- Chien, Y.W. (1987). Development concepts and practice in transdermal therapeutic systems. In: Chien, Y.W., ed. Transdermal controlled systemic medications. New York: Marcel Dekker, 25–81.
- Dick, I.P., Scott, R.C. (1992). Pig ear skin as an in vitro model for human skin permeability. J Pharm Pharmacol. 44: 640–5.
- Femenía-Font, A., Balaguer-Fernández, C., Merino, V., López-Castellano, A. (2006b). Combination strategies for enhancing transdermal absorption of sumatriptan through skin. Int J Pharm. 323:125–30.
- Femenía-Font, A., Balaguer-Fernández, C., Merino, V., Rodilla, V., López-Castellano, A. (2005a). Effect of chemical enhancers on the in vitro percutaneus absorption of sumatriptan succinate. Eur J Pharm Biopharm. 61:50–5.
- Femenía-Font, A., Merino, V., Rodilla, V., López-Castellano, A. (2005b). High-performance liquid chromatographic determination of sumatriptan after in vitro transdermal diffusion studies. J Pharm Biomed Anal. 37: 621–6.
- Femenía-Font, A., Padula, C., Marra, F., Balaguer-Fernández, C., Merino, V., López-Castellano, A., Nicoli, S., Santi, P. (2006a). Bioadhesive monolayer system for the in vitro transdermal delivery of sumatriptan. J Pharm Sci. 95:1561–9.
- Glenview, I.L. (1976). Test methods for pressure sensitive tapes; 7th ed. Pressure Sensitive Tape Council. Specifications and Technical Committee. The Council; Glenview, Ill. USA.
- Godin, B., Touitou, E. (2007). Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 59:1152–61.
- Peh, K., Khan, T., Ch’ng, H. (2000). Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci. 3:303–11.
- López, A., Llinares, F., Cortell, C., Herráez, M. (2000). Comparative enhancer effects of Span 20® with Tween 20® and Azone® on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 202:133–40.
- López-Cervantes, M., Márquez-Mejía, E., Cázares-Delgadillo, J., Quintanar-Guerrero, D., Ganem-Quintanar, A., Angeles-Anguiano, E. (2006). Chemical enhancers for the absorption of substances through the skin: laurocapram and its derivatives. Drug Dev Ind Pharm. 32:267–86.
- Nicoli, S., Penna, E., Padula, C., Colombo, P., Santi, P. (2006). New transdermal bioadhesive system containing oxybutynin: in vitro permeation across rabbit ear skin. Int J Pharm. 325:2–7.
- Padula, C., Colombo, G., Nicoli, S., Catellani, P.L., Massimo, G., Santi, P. (2003). Bioadhesive system for the transdermal delivery of lidocaine: in vitro and in vivo behavior. J Control Release. 88:277–85.
- Padula, C., Nicoli, S., Aversa, V., Colombo, P., Falson, F., Pirot, F., Santi, P. (2004). Bioadhesive system for dermal and transdermal drug delivery. Eur J Dermatol. 17:309–12.
- Padula, C., Nicoli, S., Santi, P. (2009). Innovative formulations for the delivery of levothyroxine to the skin. Int J Pharm. 372:12–6.
- Pathan, I.B., Setty, C.M. (2009). Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pham Res. 8:173–9.
- Scott, A.K. (1994). Sumatriptan clinical pharmacokinetics. Clin Pharmacokinet. 27:337–44.
- Simon, G.A., Maibach, H.I. (2000). The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations—an overview. Skin Pharmacol Appl Skin Physiol. 13:229–234.
- Smith, E.W., Maibach, H.I. (1995). Azone®. In: Smith, E.W., Maibach, H.I., eds. Percutaneous penetration enhancers. Boca Raton: CRC Press, 129–30.
- Sugibayashi, K., Nakayama, S., Seki, T., Hosoya, K., Morimoto, Y. (1992). Mechanism of skin penetration-enhancing effect by laurocapram. J Pharm Sci. 81:58–64.
- Tanner, T., Marks, R. (2008). Delivering drugs by the transdermal route: review and comment. Skin Res Technol. 14:249–260.
- Tfelt-Hansen, P., De Vries, P., Saxena, P.R. (2000). Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 60:1259–87.
- Ubaidulla, U., Reddy, M.V., Ruckmani, K., Ahmad, F.J., Khar, R.K. (2007). Transdermal therapeutic system of carvedilol: effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS PharmSciTech. 8: Article 2.
- Walters, K.A., Hadgraft, J. (1993). Azone®: mechanism of action and clinical effect. In: Walters, K.A., Hadgraft, J., eds. Pharmaceutical skin penetration enhancement. Vol. 59. New York: Marcel Dekker, 175–7.