Abstract
Nano-emulsions are innovative colloidal systems characterized by high kinetic stability, low viscosity, and optical transparency, which make them very attractive in many dermatological applications. Furthermore their small size seems to favor the topical administration of actives which scarcely cross the skin. In the light of these interesting features, the present study was aimed to the evaluation, in vitro and in vivo, of glycyrrhetic acid (GA) release through the skin from the nanoemulsion system. GA-loaded nanoemulsion (GAN) was prepared by phase inversion temperature (PIT) method, and was characterized in order to determine mean droplet size and its stability during a well-defined storage period. Further Cryo-TEM studies were performed to obtain information regarding nanoemulsion structure. The GA release pattern from nanoemulsion was evaluated in vitro, to determine its percutaneous absorption through excised human skin (stratum corneum and epidermis, SCE), and in vivo evaluating GA topical anti-inflammatory activity on healthy human volunteers by the UVB-induced erythema model. Nanoemulsions prepared by PIT method showed a mean droplet diameter of 210 nm that drastically changed during a storage of 5 weeks at room temperature. In vitro and in vivo evidence showed that the nanoemulsion system significantly increased the transdermal permeability of GA in comparison to a control O/W emulsion (GAO/W) containing the same amount of active compound.
Introduction
In recent years much attention has focused on developing alternative drug delivery, nanoscale in particular, in order to improve the bioavailability of poorly water-soluble or hydrophobic compounds which could lead to greater efficacy (CitationTorchilin, 2007; CitationPuglia et al., 2008).
Nanoemulsions are innovative colloidal systems endowed with interesting features such as high kinetic stability, low viscosity, and optical transparency, which make them very attractive in many pharmaceutical applications. These innovative nano-scale carriers are not only kinetically stable but also long-term physically stable (with no apparent flocculation or coalescence) and therefore unique and sometimes referred to as ‘approaching thermodynamic stability’ (CitationSakulku et al., 2009).
Nanoemulsions are non-equilibrium systems and cannot be formed spontaneously. They may be produced either by high-energy emulsification methods (e.g. high-pressure homogenization) or by low-energy emulsification methods (e.g. the phase inversion temperature (PIT) method) (CitationShinoda & Saito, 1968). Although high-energy emulsification methods allow great control of the droplet size and large choice of composition, low-energy emulsification methods are interesting because they take advantage of the energy stored in the system to promote the formation of small droplets.
Recently the preparation of nanoemulsions has seen a real explosion in research activity, partly due to dermal and cosmetic applications. In fact, due to their small size, they can penetrate through the ‘rough’ skin surface, enhancing the penetration of substances and favoring the topical administration of actives which scarcely cross the skin (CitationAlves et al., 2007; CitationKhandavilli & Panchagnula, 2007; CitationShakeel et al., 2009).
Glycyrrhetic acid (GA) is an ingredient used in pharmaceutical and cosmetic fields as a lenitive and anti-reddening agent (CitationCapella & Finzi, 2003), showing anti-ulcer, anti-tumor, and anti-viral properties too (CitationArase et al., 1997; CitationFarina et al., 1998; CitationRossi et al., 2003). Recently, GA has been used in therapy as a topical anti-inflammatory agent both to strengthen the skin activity of hydrocortisone through the inhibition of 11β-hydroxysteroid dehydrogenase (CitationTeelucksingh et al., 1990) and to exert a weak adrenocorticoid-like activity (CitationCapella & Finzi, 2003). Notwithstanding these interesting features, GA shows some unsuitable physicochemical properties (scarce stability, poor water solubility, etc.) that drastically influence its percutaneous absorption profile.
Numerous strategies have been studied with the aim of modifying the pharmacokinetic profile of GA and to obtain a more rational therapeutic use of this active compound (CitationInoue et al., 1989; CitationUm et al., 2003; CitationTakahashi et al., 2004). Among these strategies, the synthesis of GA derivatives has been widely used (CitationInoue et al., 1989; CitationUm et al., 2003; CitationPuglia et al., 2006). Recently our research group has synthesized and evaluated interesting polyoxyethylene esters of GA and an appreciable and sustained in-vivo topical anti-inflammatory activity of some derivatives compared with the parent drug was observed (CitationPuglia et al., 2006).
Unfortunately the use of new chemical entities synthesized from an original compound is not always tolerated (especially in the cosmetic field), whilst the use of ‘safe vehicles’ to increase drug (or active compounds in general) therapeutic efficacy is preferable.
Therefore, the objective of this study was to assess the ability of nanoemulsion obtained by PIT method in increasing GA in vitro and in vivo percutaneous absorption in comparison to an O/W emulsion evaluated as reference form. Nanoemulsion were prepared and characterized by photon correlation spectroscopy (PCS) to determine their droplet size. Further Cryo-TEM studies were performed to obtain information regarding their structure.
Materials and methods
Materials
Emulgin B2 (Ceteareth-20), Emulgade SE-PF (Glyceryl stearate/Ceteareth-20/Ceteareth- 12/Cetearyl alcohol/Cetyl palmitate), Cetiol CC (Dicaprylyl carbonate), and Cetiol LC (Caprylic capric acid of saturated fatty alcohols C12-18) were obtained from Cognis S.p.A (Como, Italy). 18-β-glycyrrhetic acid (glycyrrhetic acid) was a gift of A.C.E.F. S.p.A. (Piacenza, Italy). Transcutol CG (Ethoxydiglycol) was obtained from Gattefossè (Milan, Italy). Carbopol 934P® (CTFA: Carbomer), BFGoodrich (Cleveland, OH). All other materials were of analytical grade.
Production of GA formulations
The composition of GA nanoemulsion (GAN) is reported in . Nanoemulsion was produced following a method reported elsewhere (CitationIzquierdo et al., 2004), adding glycyrrhetic acid (0.5%) to the oil phase. GAN samples were rapidly brought to 25°C (under manual stirring), using an ice-bath to achieve fast cooling.
Table 1. GA nanoemulsion composition.
A further GA-loaded topical formulation was prepared as control form (GAO/W) for in vitro and in vivo studies. The oil phase of control formulation was composed by: PPG–15 stearyl ether (9 g); isohexadecane/PPG–15 stearyl ether (5 g); and GA (0,5 g). Steareth 2 (4 g); steareth 21 (3 g); stearic acid (2.5 g); cetylstearylic acid (2.1 g); and xanthan gum (0.3 g) were used as surfactants and structurizing agents. The O/W emulsion was prepared by slowly adding the aqueous phase (73.6 ml) to the oil phase and blending of surfactants under continuous agitation: the phases were kept to 70°C. This mixture was stirred until it was cool, thus forming the emulsion formulation.
Droplet size determination
Droplet size analysis of the nanoemulsions was performed by photon correlation spectroscopy (PCS) by using a Zetamaster (Malvern Instrument Ltd, Sparing Lane South, Worcs, UK) equipped with a solid state laser having a nominal power of 4.5 mW with a maximum output of 5 mW 670 nm. Analyses were performed using a 90° scattering angle and at 20 (± 0.2)°C. Samples were prepared by diluting 10 μl of nanoemulsion with 2 ml of deionized water previously filtered through a 0.2 μm Acrodisc LC 13 PVDF filter (Pall-Gelman Laboratory, Ann Harbor, MI).
Cryo-TEM analyses
The TEM was operated at an acceleration voltage of 200 kV. Zeroloss filtered images (DE = 0 eV) were taken under reduced dose conditions (∼ 100–1000 e/nm2). All images were recorded digitally by a bottom mounted CCD camera system (Ultrascan 1000, Gatan, München, Germany) combined and processed with a digital imaging processing system (Digital Micrograph 3.10 for GMS 1.8, Gatan, München, Germany). To prepare the sample one drop of the aqueous solution was put on a hydrophilized (home-made equipment, Biozentrum Basel) lacey carbon coated copper grid (Plano GmbH, Wetzlar, Germany), where most of the liquid was removed with blotting paper, leaving a thin film stretched over the lacey holes. The specimens were instantly shock vitrified by rapid immersion into liquid ethane cooled at ca. 90 K by liquid nitrogen in a temperature-controlled freezing unit (Zeiss Cryobox, Zeiss NTS GmbH, Oberkochen, Germany). The temperature was monitored and kept constant in the chamber during the whole sample preparation steps. After freezing a specimen, remaining ethane was removed using blotting paper. The specimen was inserted into a cryo-transfer holder (CT3500, Gatan, München, Germany) and transferred to the TEM instrument. Examinations were carried out at ca. 90 K.
In vitro study
Skin membrane preparation
Samples of adult human skin (mean age 36 ± 8 years) were obtained from breast reduction operations. Subcutaneous fat was carefully trimmed and the skin was immersed in distilled water at 60 ± 1°C for 2 min, after which the stratum corneum and epidermis were removed from the dermis using a dull scalpel blade (CitationKligman & Christophers, 1963). Epidermal membranes were dried in a desiccator at ∼ 25% relative humidity. The dried samples were wrapped in aluminum foil and stored at 4 ± 1°C until use. Previous research had demonstrated the maintenance of the stratum corneum barrier characteristics after storage in the reported conditions (CitationSwarbrick et al., 1982). Preliminary experiments were carried out to assess stratum corneum and epidermis samples for barrier integrity by measuring the in-vitro permeability of [3H]water through the membranes using the Franz cells described below. The value of calculated permeability coefficient (Pm) for [3H]water agreed well with those reported previously (CitationBronaugh et al., 1986).
In-vitro skin permeation experiments
Samples of dried stratum corneum and epidermis were rehydrated by immersion in distilled water at room temperature for 1 h before being mounted in Franz-type diffusion cells supplied by LGA (Berkeley, CA). The exposed skin surface area was 0.75 cm2 and the receiver compartment volume was 4.5 ml. The receptor compartment contained a water–ethanol solution (50:50) to allow the establishment of the ‘sink condition and to sustain permeant solubilization’ (CitationTouitou & Fabin, 1988). Furthermore, the solution was stirred with the help of a magnetic bar at 500 rev/min and maintained at 35 ± 1°C for the duration of the experiments. Approximately 300 mg of GAN and GAO/W forms were placed on the skin surface in the donor compartment and the latter was covered with Parafilm. Each experiment was run in duplicate for 24 h using three different donors (n = 3). At intervals samples (200 μl) of receiving solution were withdrawn and replaced with fresh solution. The samples were analysed for GA content by HPLC as described below.
GA fluxes through the skin were calculated by plotting the cumulative amounts of drug penetrating the skin against time and determining the slope of the linear portion of the curve and the χ-intercept values (lag time) by linear regression analysis. Drug fluxes (μg/cm2/h), at steady state, were calculated by dividing the slope of the linear portion of the curve by the area of the skin surface through which diffusion took place.
In vivo study
Volunteers recruitment
In vivo experiments were performed on a group of 10 volunteers of both sexes in the age range 25–35 years (phototype II and III). They were recruited after medical screening, including the filling out of a health questionnaire, followed by physical examination of the application sites. After they were fully informed on the nature of the study and on the procedures involved, they gave their written consent. The participants did not suffer from any ailment and were not on any medication at the time of the study. They were rested for 15 min prior to the experiments and room conditions were set at 22 ± 2°C and 40–50% relative humidity.
Protocol
Cutaneous erythemas were produced by a UV lamp (UVM-57, UVP, San Gabriel, CA), beaming radiations in the range 290–320 nm. After determining the minimal erythemal dose (MED) for each person, six cutaneous sites (1 cm2) on the center of the forearm were marked and irradiated; the exposition time was twice the MED one. Two skin sites were not further treated and used as control, two ones were treated by 300 mg of GAN formulation and two were treated by 300 mg of GAO/W formulation; the preparations were spread uniformly by means of a solid glass rod and then the sites were occluded for 6 h using Hill Top Chambers (Hill Top Research, Cincinnati, OH). After the occlusion period, the chambers were removed and the skin surfaces were gently washed to remove the formulations and allowed to dry for 15 min. UVB-induced skin erythema was monitored for 52 h by using a reflectance visible spectrophotometer X-Rite model 968 (XRite Inc. Grandville, MI), calibrated and controlled as previously reported (CitationPuglia et al., 2008).
The spectra obtained for each cutaneous site allowed calculation of the values of erythema index (EI) by the equation:
where R is the reflectance, 540, 560, and 580 are the wavelengths at which there are peaks of adsorption of hemoglobin, n=and 510 and 610 are the wavelengths related to the absorption of melanin.
The EI values of the sites before irradiation were subtracted from the values after irradiation of the same sites, obtaining the ΔEI values. The integrated areas (AUC) of the curves ΔEI vs time define values inversely related to the intensity and the duration of the erythema, and, therefore, evaluate the GA capacity to inhibit the erythema formation. In particular, the efficiency of the GA forms was assessed by the percentage of inhibition of the erythema (PIE):
where C refers to the control sites, and T refers to the other sites studied.
High-performance liquid chromatography
The HPLC apparatus consisted of a Shimadzu LC10 AT Vp (Milan, Italy) equipped with a SPD-M 10 A Vp Shimadzu photodiode array UV detector.
Chromatography was performed using a ODS Hypersil column (particle size, 5 μm; 25 cm · 4.6 mm i.d; Thermohypersil, Bellefonte, PA). GA was determined by HPLC using a mobile phase consisted of water/acetonitrile (10:90). Each sample was filtered prior to injection with a Millex HV13 filter (Waters-Millipore Corporation, Milford, MA) and an aliquot (20 μl) was injected into the HPLC apparatus. The flow rate was set at 1 ml/min and the effluent was continuously monitored at 254 nm. The retention time was 5.2 min.
Statistical analysis
Statistical analysis of in vitro data was performed using the Mann–Whitney U-test. Statistical differences of in vivo data are determined using repeated measure analysis of variance (ANOVA) followed by the Bonferroni-Dunn post-hoc pair-wise comparison procedure.
A probability, p, of less than 0.05 was considered significant.
Results
Characterization of nanoemulsions
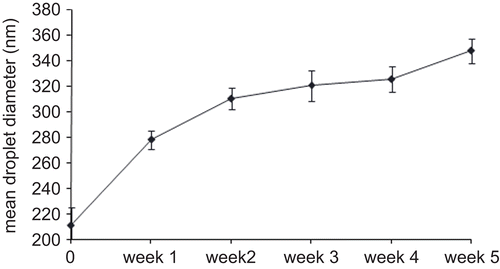
Nanoemulsions prepared by PIT method were characterized by a mean droplet diameter of 210.2 ± 30.1 nm. The stability of GA nanoemulsion produced was assessed by measuring the change in droplet size with time of storage at room temperature (∼ 20°C). As shown in , droplets in the emulsion stored at 20°C growth of ∼ 150 nm with time, during a monitoring period of 5 weeks. Not-loaded nanoemulsion showed a similar trend when submitted to the same storage conditions (data not shown). To obtain further information regarding the internal structure of nanoemulsions, cryo-TEM analyses have been conducted. Results confirm the evidences of PCS analyses and report that the presence of GA does not seem to affect the ultrastructure of the nanoemulsion disperse phase. Similar images were taken for not loaded and loaded nanoemulsions ().
In vitro study
In vitro skin permeation experiments were performed using SCE membranes instead of full-thickness skin, since the dermis in vitro can act as a significant artificial barrier to the absorption of lipophilic compounds.
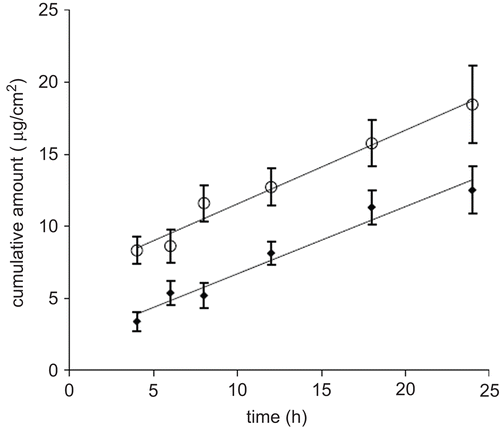
In the plots of the cumulative amounts of glycyrrhetic acid permeated through human SCE membranes as a function of time are shown.
The flux values of glycyrrhetic acid from nanoemulsion (GAN) or reference emulsion (GAO/W) calculated from the linear segments at the steady-state are 0.60 ± 0.08 μg/ cm2/h and 0.41 ± 0.13 μg/cm2/h, respectively. Statistical analysis revealed a significant difference (p < 0.05) between the steady-state flux values obtained for GAN and GAO/W.
In vivo anti-inflammatory study
GA delivery into the skin following topical application of GAN and GAO/W formulations was indirectly evaluated in vivo by monitoring the effect on UVB-induced erythema. Skin reflectance spectrophotometry was used to determine the extent of the erythema and to assess the GAN and GAO/W inhibition capacity after their preventive application onto the skin. The AUC was determined for each subject plotting ΔEI values vs time. reports AUC0–52 values obtained by treating skin sites, after exposure to UV-B, with either GAO/W or GAN.
Table 2. AUC0–52 values obtained by treating skin sites, after exposure to UV-B, with either GAO/W or GAN.
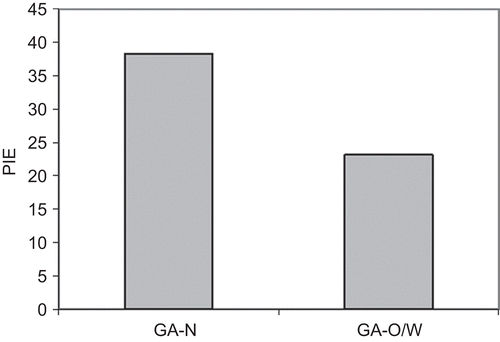
reports the PIE values. GAN formulation proved to be more effective than GAO/W in inhibiting the induced erythema (p < 0.05).
Discussion
The PIT method, employed in the present study, was suitable to obtain nanoemulsions characterized by a droplet diameter ranging from 180–240 nm. PCS results are in agreement with cryo-TEM observations, evidencing that dispersions are characterized by well shaped vesicles. Furthermore, the results evidences that the presence of GA does not seem to affect the ultrastructure of nanoemulsion disperse phase. The phase inversion temperature (PIT) method, introduced by CitationShinoda and Saito (1968), is, among the low-energy emulsification methods, the most widely used in industry (CitationFörster & Rybinski, 1998). It is based on the changes in solubility of polyoxy-ethylene-type non-ionic surfactants with temperature. This type of surfactants become lipophilic with increasing temperature because of dehydration of the polyoxyethylene chains. At low temperature, the surfactant monolayer has a large positive spontaneous curvature forming oil-swollen micellar solution phases which may co-exist with an excess oil phase. The great stability of nanoemulsions is due to a steric stabilization phenomena that prevents the droplets flocculation and hence coalescence.
During the preparation of GA nanoemulsion, we observed that the crucial point of the process was the ‘cooling step’. By rapidly cooling the emulsions kinetically stable forms can be produced with very small droplet size and distribution, but if the ‘cooling step’ is not fast polydisperse coarse emulsions are formed.
The main feature of nanoemulsions is their great stability of droplet suspension. A kinetic stability that lasts for months and is totally unlike the (thermodynamically stable) microemulsions. However, this evidence, reported by others (CitationSolans et al., 2005), should take into account the effect of storage temperature that often acts negatively, increasing nanoemulsion droplet diameter and favoring coalescence phenomena. Our stability study, in fact, demonstrated that during 5 weeks of monitoring at room temperature (∼ 20°C) the droplets in the emulsion grow ∼ 150 nm. In a recent paper, CitationEe et al. (2008) demonstrated that nanoemulsions need to be kept at an optimum storage temperature corresponding to their surfactant concentrations, to remain in the nano-size state. Away from the optimum temperature, any change in temperature will result in an increase in emulsion droplet size and polydispersity due to coarsening and/or coalescence.
In vitro and in vivo results showed that nanoemulsion system significantly increased the transdermal permeability of GA in comparison to a conventional O/W formulation.
A possible explanation of these findings could be ascribed to the nanoemulsion’s very small particle size. As reported by others (CitationShafiq et al., 2007), smaller particle size seems to play a critical role in maximizing the interfacial area of encapsulated compound into the aqueous phase. This data is in according with the evidence reported in the literature (CitationKotyla et al., 2008; CitationKuo et al., 2008; CitationSubramanian et al., 2008). Kotyla et al., for instance, demonstrated that the nanoemulsion system increased the bioavailability of transdermally applied delta tocopherol when compared to micron-sized emulsion preparations of this compound.
Notwithstanding the high total surface area of the nanoemulsions plays a fundamental role in increasing drug permeation, in our opinion, other features of nanoemulsions, such as type and concentration of excipients and active pharmaceutical ingredients (API), could contribute as well (CitationCalderilla-Fajardo et al., 2006; CitationYilmaz & Boechet, 2006). Other interesting nanoscale vehicles, in fact, are able to determine a drug penetration enhancement or a drug localizing effect onto the skin that with difficulty could be explained without considering their composition (CitationEsposito et al., 2005; CitationRicci et al., 2005).
Conclusions
Nanoemulsions prepared by the PIT method showed a droplet diameter ranging from 180–240 nm. This value drastically changed during a storage of 5 weeks at room temperature, suggesting the crucial role of temperature in maintaining nanoemulsion stability. In vitro and in vivo evidence showed that the nanoemulsions system significantly increased the transdermal permeability of GA in comparison to a control O/W emulsion containing the same amount of active compound. The obtained results suggest a new opportunity for GA to be employed in a modern topical formulation.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
References
- Alves, M.P., Scarrone, A.L., Santos, M., Pohlmann, A.R., Guterres, S.S. (2007). Human skin penetration and distribution of nimesulide from hydrophilic gels containing nanocarriers. Int J Pharm. 341:215–20.
- Arase, Y., Ikeda, K., Murashima, N., Chayama, K., Tsubota, A., Koida, I., Suzuki, Y., Saitoh, S., Kobayashi, M., Kumada, H. (1997). The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 79:1494–500.
- Bronaugh, R.L., Stewart, R.F., Simon, M. (1986). Methods for in vitro percutaneous absorption studies VII: use of excised human skin. J Pharm Sci. 75:1094–7.
- Calderilla-Fajardo, S.B., Cazares-Delgadillo, J., Villalobos-Garcia, R., Quintanar-Guerrero, D. (2006). Influence of sucrose ester on the vivo percutaneos penetration of octyl methoxycinnamate formulated in nanocapsules, nanoemulsion and emulsion. Drug Dev Ind Pharm. 32:107–13.
- Capella, G.L., Finzi, A.F. (2003). Complementary therapy for psoriasis. Dermatol Ther. 16:164–74.
- Ee, S.L., Duan, X., Liew, J., Nguyen, Q.D. (2008). Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. Chem Eng J. 140:626–31.
- Esposito, E., Cortesi, R., Drechsler, M., Paccamiccio, L., Mariani, P., Contado, C., Stellin, E., Menegatti, E., Bonina, F., Puglia, C. (2005). Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 22:2163–73.
- Farina, C., Pinza, M., Pifferi, G. (1998). Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Farmaco. 53:22–32.
- Förster, T., Rybinski, W.V. (1998). Applications of emulsions. In Binks, B.P., ed. Modern aspect of emulsion science. Cambridge: The Royal Society of Chemistry, 395–426.
- Inoue, H., Mori, T., Shibata, S., Koshihara, Y. (1989). Modulation by glycyrrhetinic acid derivatives of TPA-induced mouse ear edema. Br J Pharmacol. 96:204–10.
- Izquierdo, P., Esquena, J., Tadros, T.F., Dederen, J.C., Feng, J., Garcia-Celma, M.J., Azemar, N., Solans, C. (2004). Phase behavior and nano-emulsion formation by the phase inversion temperature method. Langmuir. 20:6594–8.
- Khandavilli, S., Panchagnula, R. (2007). Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J Invest Dermatol. 127:154–62.
- Kligman, A.M., Christophers, E. (1963). Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 88:702–5.
- Kotyla, T., Kuo, F., Moolchandani, V., Wilson, T., Nicolosi, R. (2008). Increased bioavailability of a transdermal application of a nano-sized emulsion preparation. Int J Pharm. 347:144–8.
- Kuo, F., Subramanian, B., Kotyla, T., Wilson, T.A., Yoganathan, S., Nicolosi, R.J. (2008). Nanoemulsions of an anti-oxidant synergy formulation containing gamma tocopherol have enhanced bioavailability and anti-inflammatory properties. Int J Pharm. 363:206–13.
- Puglia, C., Blasi, P., Rizza, L., Schoubben, A., Bonina, F., Rossi, C., Ricci, M. (2008). Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int J Pharm. 357:295–304.
- Puglia, C., Ostacolo, C., Sacchi, A., Laneri, S., Bonina, F. (2006). In-vitro and in-vivo evaluation of oligoethylene esters as dermal prodrugs of 18beta-glycyrrhetic acid. J Pharm Pharmacol. 58:311–9.
- Ricci, M., Puglia, C., Bonina, F., Di Giovanni, C., Giovagnoli, S., Rossi, C. (2005). Evaluation of indomethacin percutaneous absorption from nanostructured lipid carriers (NLC): in vitro and in vivo studies. J Pharm Sci. 94:1149–59.
- Rossi, T., Castelli, M., Zandomeneghi, G., Ruberto, A., Benassi, L., Magnoni, C., Santachiara, S., Baggio, G. (2003). Selectivity of action of glycyrrhizin derivatives on the growth of MCF-7 and HEP-2 cells. Anticancer Res. 23:3813–8.
- Sakulku, U., Nuchuchua, O., Uawongyart, N., Puttipipatkhachorn, S., Soottitantawat, A., Ruktanonchai, U. (2009). Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int J Pharm. 372:105–11.
- Shafiq, S., Shakeel, F., Talegaonkar, S., Ahmad, F.J., Khar, R.K., Ali, M. (2007). Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 66:227–43.
- Shakeel, F., Ramadan, W., Ahmed, M.A. (2009). Investigation of true nanoemulsions for transdermal potential of indomethacin: characterization, rheological characteristics, and ex vivo skin permeation studies. J Drug Target. 17:435–41.
- Shinoda, K., Saito, H. (1968). The stability of O/W type emulsions as functions of temperature and the HLB of emulsifiers: the emulsification by PIT-method. J Colloid Interf Sci. 24:4–9.
- Solans, C., Izquierdo, P., Nolla, J., Azemar, N., Garcia-Celma, M.J. (2005). Nanoemulsions. Curr Opin Colloid Interface Sci 10:102–10.
- Subramanian, B., Kuom, F., Ada, E., Kotyla, T., Wilson, T., Yoganathan, S.,Nicolosi, R. (2008). Enhancement of anti-inflammatory property of aspirin in mice by a nano-.emulsion preparation. Int Immunopharmacol. 8:1533–9.
- Swarbrick, J., Lee, G., Brom, J. (1982). Drug permeation through human skin. I. Effects of storage conditions of skin. J Invest Dermatol. 78:63–6.
- Takahashi, H., Onishi, H., Machida, Y. (2004). Glycyrrhetinic acid-loaded microparticles: liver-specific delivery and therapeutic potential against carbon tetrachloride-induced hepatitis. J Pharm Pharmacol. 56:437–44.
- Teelucksingh, S., Mackie, A.D., Burt, D., McIntyre, M.A., Brett, L., Edwards, C.R. (1990). Potentiation of hydrocortisone activity in skin by glycyrrhetinic acid. Lancet. 335:1060–3.
- Torchilin, V.P. (2007). Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 24:1–16.
- Touitou, E., Fabin, B. (1988). Altered skin permeation of a highly lipo-philic molecule: tetrahydrocannabinol. Int J Pharm. 43: 17–22.
- Um, S.J., Park, M.S., Park, S.H., Han, H.S., Kwon, Y.J., Sin, H.S. (2003). Synthesis of new glycyrrhetinic acid (GA) derivatives and their effects on tyrosinase activity. Bioorg Med Chem. 11:5345–52.
- Yilmaz, E., Boechet, H.H. (2006). Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema—an in vivo study. Int J Pharm. 307:232–8.