Abstract
The purpose of this study is to develop novel intestinal specific drug delivery systems with pH-sensitive swelling and drug release properties. Acryloyl ester of 5-[4-(hydroxy phenyl) azo] salicylic acid (HPAS) as an azo derivative of 5-amino salicylic acid (5-ASA) was prepared under mild conditions. The HPAS was covalently linked with acryloyl chloride, abbreviated as APAS. Cubane-1,4-dicarboxylic acid (CDA), linked to two 2-hydroxyethyl methacrylate (HEMA) groups, was the cross-linking agent (CA). Methacrylic-type polymeric prodrugs were synthesized by free radical copolymerization of methacrylic acid, poly(ethyleneglycol monomethyl ether methacrylate), and APAS in the presence of cubane cross-linking agent. The effect of copolymer composition on the swelling behavior and hydrolytic degradation were studied in simulated gastric (SGF, pH 1) and intestinal fluids (SIF, pH 7.4). The composition of the cross-linked three-dimensional polymers was determined by FTIR spectroscopy. The hydrolysis of drug–polymer conjugates was carried out in cellophane membrane dialysis bags containing aqueous buffer solutions (pH 1 and pH 7.4) at 37°C. Detection of the hydrolysis product by UV spectroscopy shows that the azo prodrug (HPAS) was released by hydrolysis of the ester bond located between the HPAS and the polymer chain. Drug release studies showed that the increasing content of MAA in the copolymer enhances hydrolysis in SIF. These results suggest that pH-sensitive systems could be useful for preparation of a muccoadhesive system and controlled release of HPAS as an azo derivative of 5-amino salicylic acid (5-ASA).
Introduction
Although oral delivery has become a widely accepted route of administration of therapeutic drugs, the gastrointestinal tract presents several formidable barriers to drug delivery. Colonic drugs delivery has gained increased importance not just for the delivery of the drugs for the treatment of local diseases associated with the colon but also for its potential for the delivery of proteins and therapeutic peptides. To achieve successful colonic delivery, a drug needs to be protected from absorption of the environment of the upper gastrointestinal tract (GIT) and then be abruptly released into the proximal colon, which is considered the optimum site for colon-targeted delivery of drugs. Colon targeting is naturally of value for the topical treatment of diseases of the colon such as Chron’s disease, ulcerative colitis, colorectal cancer, and amebiasis. The various strategies for targeting orally administered drugs to the colon include covalent linkage of a drug with a carrier, coating with pH-sensitive polymers, formulation of time released systems, exploitation of carriers that are degraded specifically by colonic bacteria, bioadhesive systems, and osmotic controlled drug delivery systems (CitationSaffran et al., 1986; CitationBrøndsted & Kopecek, 1991; Gombotz & Pettite, Citation1995; CitationBaldwin & Saltzman, 1998; CitationChiu et al., 1999; CitationRubinstein, 2000; CitationKim & Peppas, 2002; CitationChourasia & Jain, 2003; CitationBajpai & Saxena, 2004; CitationMahkam, 2004). One strategy for targeting orally-administered drugs to the colon includes covalent linkage between drug and pH-sensitive hydrogel in such a manner that upon oral administration the moiety remains intact in the stomach and small intestine. 5-Aminosalicylic acid (5-ASA) is useful for localized chemotherapy of inflammatory bowel disease (IBD), but this drug is likely to be absorbed or degraded in the stomach and small intestine before reaching the colon sites.
The colon is known to be a reductive medium in which azo groups can be cleaved with formation of the corresponding amines. The metabolic reduction of azo compounds is considered an important detoxification route (CitationWalker & Ryan, 1971; CitationChung et al., 1976; CitationBrown, 1981; CitationRaffi et al., 1990). This opportunity for reductive degradation of azo compounds by the intestinal microflora was exploited to prepare low molecular and macromolecular prodrugs of the anti-inflammatory agent 5-aminosalicylic acid and p-aminophenol (CitationGoldman & Peppercorn, 1975; CitationBrown et al., 1983; CitationKopeckova & Kopecek, 1990).
In this paper we report the synthesis, polymerization, and hydrolytic behavior of acrylic type derivatives of 5-[4-(hydroxy phenyl) azo] salicylic acid (HPAS). HPAS converted to acryloyl ester as a polymerizable vinylic monomer. Free radical cross-linking copolymerization of the resulting monomer (APAS), methacrylic acid (MAA), and poly(ethylene glycol) monomethyl ether methacrylate (PEGMA) produced poly(MAA-co-PEGMA-co-APAS). The swelling characteristics and drug release properties of the hydrogel were investigated as well.
Materials and methods
Materials
Cubane-1,4-bis(methacryloyloxyethyl)carboxylate (CA), 5-[4-(hydroxyl phenyl) azo] salicylic acid (HPAS) and poly(ethylene glycol) monomethyl ether methacrylate (PEGMA) were prepared by the methods described in the literature (CitationMahkam et al., 2000; Citation2004; CitationKim & Peppas, 2003). poly(ethylene glycol) monomethyl ether (PEGME) was purchased from Aldrich (Lyon, France) (Mr = 1000, 2000). Poly2-hydroxyethyl methacrylate (HEMA), acryloyl chloride, methacrylic acid (MAA), and dicyclohexylcarbodiimide (DCC) were purchased from Merck Co., Ltd. (Darmstadt, Germany). 4-dimethylaminopyridine (DMAP) and reagents were obtained from Fluka Co., Ltd. (Tokyo, Japan). Methacrylic acid was purified by distillation under vacuum. Initiation of 2,2′-azobisisobutyronitrile (AIBN) was purified by crystallization from methanol. Enzyme-free simulated gastric fluid (SGF) (pH 1) or simulated intestinal fluid (SIF) (pH 7.4) were prepared according to the method described in the literature (CitationMahkam et al., 2006).
Measurements
1H NMR spectra of the compound were obtained in CDCl3 using a FT-NMR Bruker (400 MHz) spectrometer. FT-IR spectra were recorded using a Bruker rector 22 spectrophotometer. The concentration of HPAS released at selected time intervals was determined on a Philips PU 8620 UV spectrophotometer at the absorption maximum of the free drug in aqueous alkali (λmax =235 nm) using a 1-cm quartz cell. The HPLC apparatus consisted of Bruker LC-21, equipped with a Bruker UV-Vis detector model LC 313 I, Rheodyne loop injector, and a C18 reverse phase column Spherisorb-CN (250 × 4.6 mm id, particle size 5 μm) from Bischoff (Leonberg, Germany). The column used was ODS (C18) and isocratic elution was performed using 50% methanol and 50% buffer containing 0.05 M NH3. The flow-rate and injection volume were 1 mL/min and 100 μL, respectively.
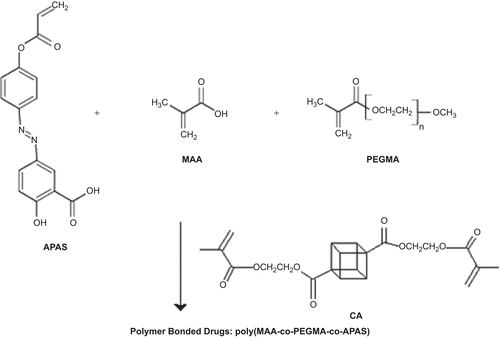
Synthesis of monomer 5-[4-(phenyl acrylate) azo] salicylic acid (APAS)
A mixture of 2 g (7.75 mmol) HPAS and 2.3 g (23 mmol) triethylamine in 50 mL dried THF was treated in a dropwise manner with a solution of 0.7 g (7.75 mmol) acryloyl chloride in 10 mL dried THF under dry argon at −5°C. The precipitate was filtered and the filtrate was evaporated. The residual solid was recrystallized from ethyl acetate:hexane to yield 1.4 g (52 %) of APAS. IR (KBr): 3300-2550 (broadened, -COOH group), 1725, 1680, 1630, 1440, 1250, 1292, 1210, 1150 cm−1. 1H NMR (CDCl3): δ (ppm) 6 (1H, C=CH), 6.3–6.4 (1H, -CH=C), 6.6 (1H, C=CH), 7.3–7.4 (2H, ortho to ester), 7.5 (1H, ortho to -OH), 7.65 (2H, ortho to azo bond), 8.1 (2H, ortho to azo bond), and 11.2 (2H, -OH and -COOH).
Preparation of pH-sensitive hydrogels
Polymer bonded drugs (PBDs) were synthesized by radical terpolymerization of MAA, PEGMA, and APAS with specific mol percents of CA (2 and 4%) in a solution of dried dioxane with a variable feed ratio, as shown in . Terpolymerization was carried out in the presence of 2,2′-azobis isobutyronitrile (AIBN) as an initiator (0.01 mol/L) at 60–70°C in a thermostatic water bath. All experiments were carried out in Pyrex glass ampoules sealed off under vacuum. After the desired time (48 h) the precipitated network polymer bonded drug was collected, washed with deionized water for 1 week, and the water was changed every 12 h in order to remove any unreacted monomers. After washing, the samples were dried in air and stored in desiccators until use (). IR (KBr): 3400–2550 (broadened, -COOH group), 1730, 1700, 1600, 1420, 1250, and 1222 cm−1.
Table 1. Composition of copolymers.
Identification of network polymeric prodrugs
The resulting network polymers swell and become soft in solvents such as H2O and most organic solvents without dissolving. FT-IR spectra confirmed the structure of hydrogels containing HPAS. The absorption of ester carbonyl bond of the pendent group appeared at 1700–1730 cm−1. Analysis of the IR spectra shows that with increase of pH from 2 to 8, the composite passes into the anion form, the band at 1700 cm−1 (the stretching vibrations of the carboxylic group) disappears, and in its place new absorption bands appear at 1560 and 1420 cm−1, which are assigned to the stretching vibrations of the carboxylate anion COO−.
Characterization of hydrolysis product
Fifty milligrams of polymer-drug adduct was dispersed in 20 mL of pH 8 buffered solution. The reaction mixture was maintained at 37°C. After 24 h the hydrolysis solution was sampled and neutralized with 1 mol/L hydrochloric acid and the solvent was evaporated in vacuo. The resulting crude product was treated with 10 mL of acetone and heated. The suspension was then filtered and the acetone solution was evaporated under reduced pressure. Samples were measured using HPLC. HPAS was detected at retention time of 3.5 min.
In vitro release studies
The copolymers (50 mg) were poured into 5 mL of SGF (pH 1) or SIF (pH 7.4). The mixture was introduced into a cellophane membrane dialysis bag. The bag was closed and transferred to a flask containing 25 mL of the same solution maintained at 37°C. The external solution was continuously stirred and 3 mL samples were removed at selected intervals. The volume removed was replaced with SGF or SIF. Triplicate samples were used. The sample of hydrolyzate was analyzed by a UV spectrophotometer, and the quantity of HPAS as an azo derivative of 5-ASA was determined using a standard calibration curve obtained under the same conditions.
Swelling
To measure the swelling, pre-weighed dry samples were immersed in various buffer solutions (pH 7.4 and pH 1) at 37°C. After excess water on the surface was removed with the filter paper, the weight of the swollen samples was measured at various time intervals. The procedure was repeated until there was no further weight increase. The degree of swelling was calculated according to the relation:
SW (%) = [(Ws − Wd)/Wd] × 100
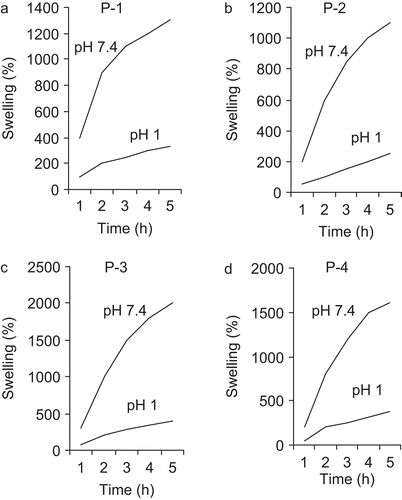
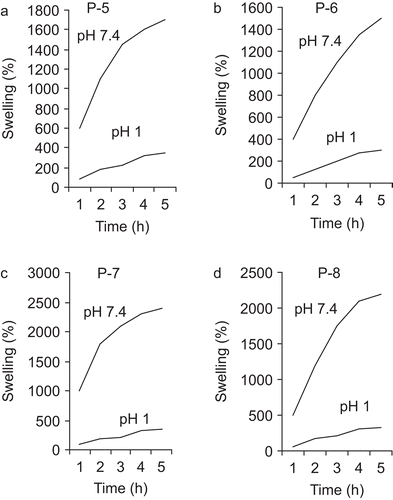
where Ws and Wd represent the weight of swollen and dry samples, respectively. This results from the ionization or deionization of the functional groups in the polymer networks responding to environment pH changes. At pH values lower than the pka of the gel, the carboxylic acid groups of the hydrogel are protonized, forming hydrogen bonding with adjacent oxygens, whereas at pH values higher than the pka of the gel, carboxylic acid groups become ionized, leading to swollen networks due to the electrostatic repulsion between charged groups. Time-dependent swelling behavior of cross- linked terpolymers in pH 1 and pH 7.4 at 37°C are plotted in and . These figures show that swelling increases with time, first rapidly and then slowly, reaching maximum constant swelling (mass equilibrium swelling, MES). In all cases, the swelling weight reached its equilibrium after 5 h. The equilibrium swelling ratio of the hydrogels was a function of the network structure, cross-linking ratio, hydrophilicity, and degree of ionization of the functional groups. As expected, there was a drastic change in the equilibrium swelling ratio of hydrogels between pH 1 and pH 7.4. This sharp transition between the collapsed and the swollen states at pH 1 and pH 7.4 indicates that in pharmaceutical applications where these systems will be used as controlled release carriers, they can swell and collapse rapidly, responding to the physiologically pH change of the human GI tract. Thus, when the drug-incorporated gels reach the upper small intestine, increase of the pH can induce gel swelling immediately, leading to release of drug. The equilibrium swelling was dependent on the content of MAA groups units in copolymer and reticulated degree. With increased cross-linking and an increase in the reticulated degree of the polymer, diffusion of the water in the network’s polymer is reduced and the swelling is slower. The existence of hydrogen-bonding interactions between –COOH groups in the polymer matrix results in a complex structure within the network and so the movement of polymeric segments is restricted. This also accounts for minimum swelling of the gel in a medium of pH 1. However, when the sample is placed in a medium of pH 7.4, the almost complete ionization of –COOH groups present within the polymer network not only increases the ion osmotic swelling pressure to a great extent, but also enhances the relaxation of macromolecular chains because of repulsion among similarly charged –COO− groups. These two factors ultimately result in a greater increase in water uptake.
It was expected that the incorporation of water-soluble polymer such as PEGMA could enhance the extent and rate of drug release. It seems that hydrogen-bonded complexes may form between the PEG and MAA units of the hydrogel under acidic conditions. Consequently, hydrogels exhibit a relatively low degree of swelling in SGF. However, at pH 7.4, hydrogels prepared from PEGMA2000 exhibited a higher swelling ratio than hydrogels with PEGMA1000. This was because hydrogels with PEGMA2000 have several proximate carboxylic groups; thus, there was more electrostatic repulsion between ionized carboxylic acid groups at high pH, thus rendering these networks more swellable.
Results and discussion
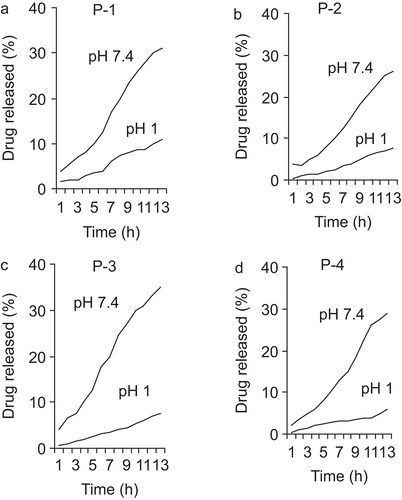
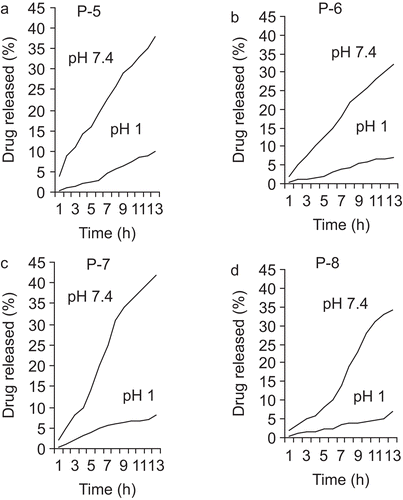
The large intestine may be optimal for drug delivery because of high residence time and low digestive enzymatic activity. To achieve successful colonic delivery, a drug needs to be protected from absorption or the environment of the upper gastrointestinal tract (GIT) and then be abruptly released into the proximal colon, which is considered the optimum site for colon-targeted delivery of drugs. The release profiles of hydrogels at 37°C in SGF and SIF are shown in and . The drug release proceeds more efficiently at a higher pH (SIF). Furthermore, increasing the content of the MAA units in hydrogel composition enhances the rate of drug release. Acids and bases catalyze the hydrolysis of ester bonds in APAS, and the ionization of COOH groups of MAA units’ increases with the basic conditions. Both factors (pH and MAA/PEGMA ratio) increase the swelling of hydrogel, which enhances the rate and extent of drug release in SIF. Drug release studies showed that increasing content of MAA in the copolymer enhances the hydrolysis in SIF but has no effect in SGF. This is because the increase of MAA content in the hydrogels provides more hydrogen bonds at low pH and more electrostatic repulsion at high pH (CitationMahkam, 2005; CitationMahkam & Doostie, 2005).
In general, PEG has good biocompatibility and has been approved for a wide range of biomedical applications (CitationHarris, 1992). Especially, the presence of grafted PEG chains in these hydrogels plays an important role. At low pH, the oxygen groups in the grafted PEG chain form hydrogen-bonded complexes by interacting with carboxylic groups of PMAA. These hydrogen bonds lead to more collapsed polymer networks resulting in protection of drug incorporated in the hydrogels. Moreover, several studies have shown that these grafted PEG chains promote mucoadhesion by chain interpenetration, leading to increased drug absorption through the intestinal wall.
In order to study potential application of PBDs containing azo derivatives of 5-aminosalicylic acid as a pharmaceutically active compound, we have studied the hydrolysis behavior of the polymers under physiological conditions. The degree of hydrolysis of the network polymer containing HPAS as a function of time is shown in and . The order of hydrolysis in this series was significantly affected by polymer composition. It appears that the degree of hydrolysis of network polymers depends on the amount of the MAA units in copolymer and reticulated degree of cross-linking. With increased cross-linking and an increase in the reticulated degree of the polymer, diffusion of the hydrolyzing agents in the network’s polymer is reduced and the hydrolysis rate is slower. On the other hand, the number of carboxylic acid groups along the polymer chain response to decrease and increase hydrophilicity of polymer in pHs 1 and 7.4, respectively. In pH 7.4 solutions with completed ionization and an increase in the hydrophilicity of the polymer, diffusion of the hydrolyzing agents on polymer increased and the hydrolysis rate increased (CitationKopeček et al., 1992). In the colon, HPAS is split at azo bond by the colonic bacteria with the liberation of 5-ASA and p-aminophenol.
Conclusion
As a first part of our developing study about polymeric pro-drugs, we have described the synthesis and properties of the pH-sensitive hydrogels consisting of 5-[4-(hydroxy phenyl) azo] salicylic acid (HPAS) by preparation of polymerizable acrylic derivatives. Since HPAS is undergoing metabolic reduction in the colon the anti-inflammatory agent’s 5-ASA and p-aminophenol are released on the site of action. This type of monomer can easily be copolymerized with various vinyl monomers to improve the hydrolytic behavior by introducing hydrophilic units along the polymer chain. The optimized methods described for synthesis and polymerization procedures can be applied for any drug with a hydroxyl functional group. Based on the great difference in hydrolysis rates at pH 1 and 7.4, these hydrogels appear to be good candidates for colon-specific drug delivery.
Acknowledgements
The office of research vice chancellor Azarbaijan University of Tarbiat Moallem has supported this work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bajpai, S.K., Saxena, S. (2004). Enzymatically degradable and pH-sensitive hydrogels for colon-targeted oral drug delivery. Synthesis and characterization. J Appl Polym Sci. 92:3630–43.
- Baldwin, S.P., Saltzman, W.M. (1998). Materials for protein delivery in tissue engineering. Adv Drug Deliv Rev. 33:71–86.
- Brøndsted, H., Kopecek, J. (1991). Hydrogels for site-specific oral delivery. Synthesis and characterization. Biomaterials. 12:584–92.
- Brown, J.P. (1981). Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl Environ Microbiol. 41:1283–6.
- Brown, J.P., Mccarraugh, G.V., Parkinson, T.M., Wingard, Jr. R.E., & Onderdonk, A.B. (1983). A polymeric drug for treatment of inflammatory bowel disease. J Med Chem. 26:1300–1307.
- Chiu, H.C., Hsiue, G.H., Lee, Y.P., & Huang, L.W. (1999). Synthesis and characterization of pH-sensitive dextran hydrogels as a potential colon-specific drug delivery system. J Biomater Sci Polym Ed. 10:591–608.
- Chourasia, M.K., & Jain, S.K. (2003). Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 6:33–66.
- Chung, K.T., Flunk, G.E., & Egan, M. (1976). Azo dye reduction by anaerobes. Appl Environ Microbiol. 5:558.
- Goldman, P., Peppercorn, M.A. (1975). Drug therapy: sulfasalazine. N Engl J Med. 293:20–3.
- Gombotz, W.R., & Pettite, D.K. 1995. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 6:332–51.
- Harris, J.M. (1992). Poly(ethylene glycol) chemistry, biotechnical and bomedical applications. New York: Plenum Press.
- Kim, B., Peppas, N.A. (2002). Synthesis and characterization of pH-sensitive lycopolymers for oral drug delivery systems. J Biomater Sci Polym Edn. 13:1271–81.
- Kim, B., Peppas, N.A. (2003). Poly(ethylene glycol)-containing hydrogels for oral protein delivery applications. Biomed Microdev. 3:333–41.
- Kopeček, J., Kopečková, P., Brøndsted, H., Rathi, R., Rihova, B., Yeh, P.Y., & Ikesue, K. (1992). Polymers for colon-specific drug delivery. J Contr Rel. 19:121–30.
- Kopeckova, P., Kopecek, J. (1990). Release of 5-aminosalicylic acid from bioadhesive N-(2-hydroxypropyl) methacrylamide copolymers by azoreductases in vitro. Makromol Chem. 191:2037–45.
- Mahkam, M. (2004). Controlled release of biomolecules from pH-sensitive hydrogels. J Bioact Comp Polym. 19:209–20.
- Mahkam, M. (2005). Using pH-sensitive hydrogels containing cubane as a crosslinking agent for oral delivery of insulin. J Biom Mat Res (Applied Biomaterials). 75B(1):108–112.
- Mahkam, M., Allahverdipoor, M., Mohammadi, R., Ranaeisiadat, S. O., Rashidi, M. R., Davaran, S., Barshan, M., & Ranaei-siadat, S.E. (2006). A designed oral delivery system for insulin. J Bioact Comp Polym. 21:135–48.
- Mahkam, M., Assadi, M.G., Zahedifar, R., Allahverdipoor, M., Doostie, L., & Djozan, J. (2004). Synthesis and evaluation of new linear azo-polymers for colonic targeting. Desig Mono Polym. 7:351–9.
- Mahkam, M., Doostie, L. (2005). The relation between swelling properties and cross-linking of hydrogels designed for colon-specific drug delivery. J Drug Del. 12:343–7.
- Mahkam, M., Sharifi, N., Entezami, A.A. (2000). Regulation of controlled release of ibuprofen from crosslinked polymers containing cubane as a new crosslinking agent. J Bioact Comp Polym. 15:396–404.
- Raffi, R., Franklin, W., Cerniglia, C.E. (1990). Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. J Appl Environ Mivrobiol. 56:2146–51.
- Rubinstein, A. (2000). Natural polysaccharides as targeting tools of drugs to the human colon. J Drug Dev Res. 50:435–9.
- Saffran, M., Kumar, G.C., Savariar, C., Burnham, J.C., Williams, F., & Neckers, D.C. (1986). A new approach to oral administration of insulin and other peptide drugs. Science. 233:1081–4.
- Walker, R., Ryan, A.J. (1971). Some molecular parameters influencing rate of reduction of azo compounds by intestinal microflora. Xenobiotica. 1:483–6.