Abstract
Prednisolone acetate (PA)-loaded microspheres were prepared by the spray-drying technique using different polymer (1% and 2%) and drug concentrations (10% and 20%). To obtain the optimum formulation, a three-factor two-level (23) design was employed. The independent variables were polymer molecular weight, polymer concentration, and theoretical drug loading. Responses were the particle size, percentage of encapsulation efficiency, and the t50% release. The best formulation was prepared with 20% of PA and 1% of chitosan with medium molecular weight showing relative good yield of production (48.0 ± 6.7%) and encapsulation efficiency (45.7 ± 0.3%), and released the drug at a constant rate in 11 days.
Introduction
Coronary Artery Disease (CAD) is the leading cause of death in the western world in both men and women (CitationMann & Davies, 2002; CitationBurt & Hunter, 2006). It is caused by atherosclerosis, which is the narrowing of the arteries that supply blood and oxygen to the heart (CitationGrech, 2003). Several approaches have been used in its treatment. Among the options, Percutaneous Transluminal Coronary Angioplasty (PTCA) with or without stenting has become a common practice for treating coronary arterial stenosis (CitationPires et al., 2005; CitationBurt & Hunter, 2006). However, PTCA has a major limitation, restenosis, which is the maladaptive response of the arteries to the injury during the stenting procedure, and this response causes the re-narrowing of the artery (CitationKhan & Patravale, 2005; Citationvan der Hoeven et al., 2005). The restenosis rates in patients who received stent implantation are 30–50% (CitationKhan & Patravale, 2005). Numerous pharmacological agents that have demonstrated potential to reduce restenosis in animal models have failed in systemic treatment due to the low drug concentrations at the restenosis site (CitationTanabe et al., 2004). Mechanical approaches for preventing restenosis have proved insufficient. Therefore, localized drug delivery seems to be the most effective approach for preventing restenosis (CitationChorny et al., 2000; CitationSousa et al., 2003; CitationHunter, 2006). The concept of Drug Eluting Stents (DES) is also appealing since it facilitates drug delivery to the site of the narrowing and represents an ideal platform for drug delivery (CitationSousa et al., 2003). These devices can provide mechanical scaffolding to prevent vessel collapse, while the contained drugs inhibit the proliferation and inflammation of the arterial cells involved in the processes of the restenosis (CitationWinslow et al., 2005).
Bare metallic stents were designed only for mechanical scaffolding. DES usually consists of the metallic stent structure and the polymeric coating layer, which acts as a reservoir for the contained drug. The polymeric coating layer enables the prolonged and controlled release of the drug to the surrounding tissues of the vessel, while the metallic structure provides vessel integrity (CitationWaksman, 2002; CitationRanade et al., 2004). Clinical studies have stated that, with the two action mechanisms, DES reduced the restenosis rates to 1–3% using several active pharmacological agents. However, the polymer is the critical material in DES. The polymer layer has to release the drug with control and over an extended time period for continuous effect (CitationSousa et al., 2003; CitationWinslow et al., 2005) and also degrade after it has served its purpose and released the drug. Biocompatibility and biodegradability properties of the polymers play an important role in defining the overall performance of the DES.
Chitosan is a very well known polymer for its hydrophilic, biocompatible, and biodegradable properties, possessing low toxicity and side effects. In the pharmaceutical area, chitosan has been used recently for drug delivering systems such as microspheres (CitationArıca et al., 2002). Prednisolone acetate (PA) is a glucocorticoid that has several functions, including inhibition of inflammatory responses and cell proliferation. PA is a synthetic derivative of hydrocortisone and is insoluble in water. PA was selected as a model drug for its anti-inflammatory effects and solubility properties similar to the commonly used drugs for preventing restenosis.
In this study, our group developed microspheres using chitosan with low and high molecular weight prepared by spray-drying technique and loaded with PA. The objective of our study was to optimize a formulation to be used in the cardiovascular DES studies; hence factorial design was chosen as the suitable statistical model for experimental design. During the optimization process of the microsphere formulations, a three-factor two-level (23) factorial design was used. The effects of variables such as polymer molecular weight, polymer concentration, and theoretical drug loading on the characteristics of the microspheres were evaluated. A factorial design is a rapid technique that makes it possible to study the effects of main factors and formulation variables on the characteristics of microspheres. At the same time, this statistical technique shows whether an interaction exists between the main factors, and, if so, whether the interaction affects the test parameters. Factorial design also reduces the number of required experimental runs to determine the optimum formulation. This design is suitable for the response surfaces and constructs a second order polynomial model, thus helping to optimize a process using a small number of experimental runs.
The prepared microspheres were intended to be used in biodegradable stent formulations which were developed by our group (CitationSarisozen et al., 2006). This approach allowed us to develop stent formulations that aimed to prevent restenosis with two mechanisms: the first being the stent structure, to prevent the elastic recoil and remodelling of the artery; and the second being PA, to inhibit the neointimal hyperplasia. Entrapping PA in chitosan microspheres was expected to extend the PA release and prolong the effect on the stent site by preventing the proliferation of the vascular cells.
Experimental
Materials
PA was a generous gift from Abdi Ibrahim Drug Company (Istanbul, Turkey). Chitosan, low (CL) and medium (CM) viscosity, average molecular weight (Mw) 150,000 and 400,000 Da, respectively, were purchased from Fluka Chemicals (Germany). Lactic acid (85%, v/v) was from Fischer Chemicals (New Jersey). Ethyl alcohol (96%) was from EGAS Chemicals (Istanbul, Turkey). Sodium lauryl sulfate (SLS) was from Merck Chemicals (Darmstadt, Germany), and sodium azide was purchased from Sigma Chemicals Co. (St. Louis, MO). All chemicals used were of pharmaceutical grade.
Experimental design
A three-factor, two level (23) factorial design was used for the optimization procedure. In the present investigation, polymer molecular weight, polymer concentration, and theoretical drug loading were selected as independent variables (); t50% release, percentage of encapsulation efficiency, and particle size were chosen as responses. Factorial design was used for evaluation of the effects of formulation parameters on the responses given above.
Table 1. Coded units of 23 factorial design.
A 23 factorial design was implemented with three main factors: polymer molecular weight, polymer concentration, and theoretical drug loading. Eight formulations were developed using these designed set of experiments.
Preparation of microspheres
Chitosan microspheres were prepared by spray-drying technique (CitationArıca et al., 1994). Briefly, 1% (w/v) and 0.5% (w/v) chitosan aqueous solutions were prepared by dissolving the two different chitosan free bases (low and medium Mw) in 1% (v/v) lactic acid solution. PA was dissolved in the required amount of ethyl alcohol and added to the chitosan solutions so that PA weight corresponded to 10% (w/w) and 20% (w/w) of the weight of the chitosan in two solutions. The resulting solutions were mixed thoroughly using an ultraturrax (IKA, Staufen, Germany) for obtaining homogeneous solutions before spraying.
Eight microsphere formulations were prepared with the 23 factorial design. The final dispersions were sprayed by Büchi 190 Mini Spray Dryer (Büchi, Flawil, Switzerland) with a standard 0.5 mm nozzle under the following conditions: inlet temperature was 130 ± 3°C, outlet temperature 75 ± 3°C, pump rate 5 mL/min, aspirator setting adjusted to 100%, and spray flow rate 600 Nl/h. The prepared microsphere formulations containing PA in different concentrations are presented in . Dried microspheres were collected and the production yields were measured by percent weight of microspheres obtained with respect to the initial amounts of drug and polymer. Each experiment was done in triplicate.
Table 2. Codes and formulation parameters of the prepared microspheres.
Morphological characterization
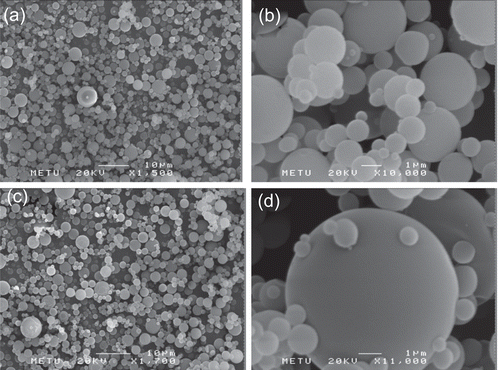
Microsphere morphology was evaluated by scanning electron microscopy (SEM, Jeol, JSM 6400, Japan). The dried samples were gold sputted-coated before observation by SEM at 20 kV.
Differential scanning calorimetry (DSC) analyses
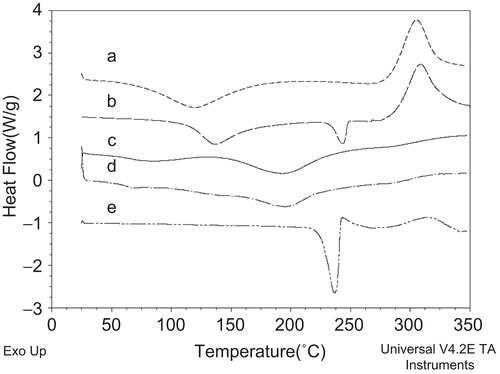
Thermal analyses were performed with a DSC Q100 apparatus (TA Instruments, IL). The calibration of the instrument was done with indium. The analyses were carried out on PA, chitosan free bases, blank microspheres, and PA-loaded microspheres. Samples were accurately weighed (5–10 mg) into the aluminum standard DSC pans and sealed. All samples were heated from 25 to 350°C at a heating rate of 10°C/min in nitrogen atmosphere (flow rate 50 mL/min).
Size distribution of microspheres
Particle sizes of the prepared microspheres were measured by a SympaTec HELOS (HO728, Germany) particle size analyzer. Microspheres were first suspended in isopropyl alcohol using an ultrasonic bath then magnetically stirred in the instrument during the measurements. The given results are the d50 values of the formulations, averages of three runs and standard deviations.
Encapsulation efficiency
Encapsulated PA was determined by adding the microspheres (10 mg) to 10 mL of ethyl alcohol and then placing them in an ultrasonication bath for 1 h at room temperature. The mixtures were centrifuged at 10,000 rpm for 5 min and the extracted solutions were filtered through a 0.45 µm syringe filter. The PA content in the chitosan microspheres was determined spectrophotometrically at 242 nm (Agilent 8453 UV Spectrophotometer, Agilent Comp., California, USA).
Linearity was studied by analyzing eight standard solutions covering the range of 2.5–20 μg/mL. Repeatability was determined at five points of the calibration curve (2.5, 5, 10, 15, and 20 μg/mL, n = 6). Intra-day and inter-day variability was determined by analyzing the standard solutions (5, 10, 15, and 20 µg/ml) within 1 day and on six different days (n = 6), respectively. The limit of detection of the method was 1 µg/ml. The method showed good linearity in the range of concentrations worked (2.5–20 µg/ml). Repeatability, intra-day and inter-day variability were evaluated for the precision of the method. In all of the cases the variation coefficents were determined below 2%. The components of the microspheres did not interfere with PA at this wavelength. The total amount of PA was calculated from the aliquots of each extract. The encapsulation efficiency was calculated (CitationFilipovic-Grcic et al., 2003). All analyses were carried out in triplicate.
where PAa is the actual PA content of the weighed microspheres and PAt is the theoretical PA content of the microspheres.
In vitro drug release studies
In vitro drug release profiles were obtained by incubating the microspheres in the release medium. Five milligrams of drug-loaded microspheres were weighed in the Eppendorf tubes and 1.5 mL of isotonic phosphate buffer saline (PBS) at pH 7.4 containing 0.5% (w/v) SLS and 0.05% (w/v) sodium azide were added into each tube. The tubes were kept under continuous shaking (50 rpm) in a water bath (Memmert, Germany) at 37°C. At regular time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 12, 24, 36, 48, 56, 72, 96, 120, 144, 168, 192, 216, 240, and 264 h), the Eppendorf tubes were taken and centrifuged at 10,000 rpm for 5 min. The PBS was removed, filtered through a 0.45 µm filter, and PA was quantified spectrophotometrically (Agilent 8453 UV Spectrophotometer, Agilent Comp.) at 247 nm. Afterwards, the same volume of fresh PBS was replaced to continue the release study. For each formulation, six runs were performed. In vitro release results were evaluated kinetically. The data were fitted to three kinetic models (zero-order, first-order, and the Higuchi models) and m (slope) and n (intercept) values for each kinetic model are given in .
Table 3. Results of release studies of developed microsphere formulations.
Statistical analysis
The experimental data were analyzed by the response regression procedure using the following polynomial equation:
where y is the measured response, β0 is the arithmetic mean response, β1 is the main effect of polymer molecular weight (x1), β2 is the main effect of polymer concentration (x2), and β3 is the main effect of theoretical drug loading (x3), β12, β13, β23, and β123 are the interactions of the main factors, and e is the error term.
The statistical analysis of the obtained results was done by the corresponding analysis of variances of the selected experimental design, in order to determine the regression significance, its adjustment to the model, and the significance of the coefficients of the polynomial terms. This statistical study was done using the SPSS® 13.0 software.
Results and discussion
Preparation of microspheres and percent yield value
The objective of the present study was to optimize a formulation of biodegradable microspheres loaded with PA to incorporate into future cardiovascular stent studies. The studied independent variables were polymer molecular weight, polymer concentration, and theoretical drug loading. PA-loaded chitosan microsphere formulations were successfully prepared by spray-drying technique.
Spray-drying is a high yield production method for preparing microparticulate systems (He et al., Citation1999a; CitationMartinac et al., 2002; CitationDesai & Park, 2005). The spray-drying technique for encapsulating drugs in biodegradable polymer matrices has been evaluated by several authors (He et al., Citation1999a; CitationGuinchedi et al., 2002; CitationAgnihotri et al., 2004; CitationDesai & Park, 2005). Spray-drying is a one step procedure which produces small microspheres with smooth surface and spherical shape. This technique also avoids the use of organic solvents, thus resulting in a safer production. Microparticle size is an important factor affecting the formation of homogeneous dispersion of microparticles in the polymer matrices. Smaller microparticles can be more easily dispersed in the other polymers. Spray-drying technique facilitates production of microspheres generally with particles sizes smaller than 10 µm, which is considered more suitable for drug administration and for dispersing the microspheres into the different polymeric matrices.
shows the results of the production yields of microspheres, which were up to 70% (most formulations had yields of more than 40%), and these results seemed to reflect a relatively good efficiency of the preparation method. The yield value was significantly affected by the amount of chitosan concentration used in the microsphere preparation (p < 0.05). The higher polymer concentration resulted in increased microsphere production yield for both types of chitosan polymers ().
Table 4. The characteristics of the PA-loaded chitosan microspheres.
Even that given production yield results seemed to limited, there are studies in the literature that showed similar values (CitationGiunchedi et al., 2002; CitationMartinac et al., 2005; CitationGavini et al., 2006). The lower yields could be explained by aspiration of the small microspheres and attached polymer droplets in the spraying wall of the spray-dryer, as also reported by CitationGiunchedi et al. (2002) and CitationGavini et al. (2006).
The molecular weight of chitosan polymer used for formulations affected the production yields (p < 0.001). Chitosan microspheres prepared using low Mw polymers gave higher production yields than the medium Mw polymers at all concentrations. These results can be attributed to the fact that higher Mw chitosan solutions form more viscous solutions than those with low Mw. Thus, medium Mw chitosan solution droplets could be attached to a greater extent inside the main chamber of the spray-drying apparatus (CitationDesai & Park, 2005).
The high and low levels of chitosan polymer solutions were chosen for preparation and development of the microspheres for the following reasons. Chitosan concentrations were determined by the viscosity of the polymers. It was observed that concentrations of polymer solutions higher than 1% (w/v) clogged the nozzle of the spray-dryer, and the resulting microspheres had non-homogeneous particle size distribution. Concentrations of polymer solutions lower than 0.5% (w/v) had very low production yield (lower than 10%). The reason for not using high Mw chitosan was the very high viscosity of the polymer solution (>2200 mPa.s). As seen in , we determined that when the theoretical drug loading and polymer concentration increased, the production ended with higher yields for both polymer molecular weights, as indicated in the literature (CitationGiunchedi et al., 2002; CitationHuang et al., 2002).
Encapsulation efficiency
The values for encapsulation efficiency of PA in chitosan microspheres ranged between 4.6 ± 0.5% and 45.7 ± 0.3% (), and were significantly influenced (p < 0.001) by the two studied factors polymer concentration and drug loading. On the other hand, chitosan molecular weight alone did not seem to affect the encapsulation yields (p > 0.05). Very low encapsulation efficiency values were observed for CL0.5PA10 and CM0.5PA10 coded formulations due to the low polymer concentration and drug loading. For low and medium Mw chitosan, higher encapsulation efficiencies were obtained when high polymer concentration (1% w/v) were used. Keeping polymer concentration constant and increasing the drug loading resulted in increased encapsulation efficiencies. Also high polymer concentrations appeared to allow more drugs to be entrapped in the polymer matrix. On the other hand, for low Mw chitosan microsphere formulations, drug loading did not seem to be effective at high polymer concentration (p > 0.05). Medium Mw chitosan microspheres showed higher encapsulation efficiencies when compared to those with low Mw. The highest encapsulation efficiency was obtained from the CM1PA20 formulation, with a value of 45.7 ± 0.3%, as given in .
Morphological characterization
All microsphere formulations prepared had a spherical shape and non-porous surface. Chitosan molecular weight, polymer concentration, and drug loading did not seem to affect the surface properties of the microspheres (). Furthermore, no free drug crystals were observed on the surface of the microspheres.
Size distribution of microspheres
As shown by particle size analyses, particle sizes ranged from 5.10 ± 0.03 to 11.65 ± 0.03 µm for PA-loaded microspheres. Entrapment of PA at high level increased the particle size of the microspheres. Measurements showed that medium Mw chitosan microspheres possessed smaller particles size than those with low Mw. The distributions of the particle sizes were narrow, as described by He et al. (Citation1999b). Similar particle size results have been observed by several authors when using spray-drying technique for preparation of chitosan microspheres (CitationGanza-Gonzales et al., 1999; CitationGiunchedi et al., 2002; CitationGavini et al., 2006).
Differential scanning calorimetry (DSC) analyses
According to the DSC thermograms, PA presented an endothermic peak at 237°C due to its melting and degradation. Both of the chitosan polymers showed broad endotherms in the range of 45–150°C corresponding to a loss of residual water and a characteristic peak at 305°C. The spray-drying process and dispersion of drug into the polymer matrix led to the amorphization of PA and due to this process the endothermic melting peak disappeared (CitationFilipovic-Grcic et al., 2000; CitationTakahashi et al., 2005). From the results obtained, it could be concluded that the drug dispersed into the microspheres. was chosen as an example and illustrates the thermogram of the formulation prepared with 20% (w/v) of PA and 1% (w/v) of polymer (high level drug loading and polymer concentration) of the experimental design.
Experimental design
In our study, particle size measurement of the microspheres showed that increase in the polymer concentration decreased the particle sizes for low level drug loading, as seen in . The fitted equation relating the response of mean particle size of the microspheres consisting of chitosan low and medium Mw is shown in equation (3).
As seen in equation (3), there was a negative relationship between the polymer concentration and particle size at low level of drug loading. In some of the articles, it was stated that the relationship between the theoretical drug loading and particle size could be negative (He et al., Citation1999a); thus, in research, higher theoretical drug loading percentage resulted in smaller microspheres at low level drug loading. In addition, there are also studies showing that there was no effect of polymer type and drug loading on the particle size. As CitationMartinac et al. (2002) and CitationGavini et al. (2006) described in the literature, chitosan Mw, polymer composition, and polymer/drug ratio did not affect the particle sizes of the microspheres.
Another response evaluated was the encapsulation efficiencies of the microspheres. The equation of this response was shown in equation (4).
The equations show the positive relationship between the polymer concentration and encapsulation efficiencies. This means that increased polymer concentration increased the encapsulation efficiencies of the microspheres. The same positive relationship can be seen in the theoretical drug loading and encapsulation efficiency. For the encapsulation efficiency response, the polymer concentration effect is more significant at low level drug loading. At the high level loading (20%), the encapsulation efficiency values were higher but the results were less affected by polymer concentration. The drug loading values for all formulations show a variation between a minimum of 4.6 ± 0.5% to a maximum of 45.7 ± 0.3%. The data showed that the encapsulation efficiencies were affected by polymer concentration and theoretical drug loading factors, but not affected by polymer molecular weight. The highest drug loading percentage was obtained from the CM1PA20 formulation, which consisted of high level polymer concentration and high level of theoretical drug loading (p < 0.001). CitationGanza-Gonzales et al. (1999) reported encapsulation efficiencies between 63–72% of microspheres prepared by spray-drying technique using chitosan polymer. CitationDesai and Park (2005) also developed the spray-dried medium Mw chitosan microspheres, and reported encapsulation efficiencies of 45–58%, which are similar to our results.
In vitro release
Zero-order, first-order and the Higuchi kinetic models were used to evaluate the in vitro release results. In vitro release data showed that the release of PA from all microsphere formulations followed diffusion control and fitted to Higuchi release kinetic ().
The last response evaluated is the t50% values of the microsphere formulations. The response for the t50% values can be seen in equation (5):
The optimum microsphere formulation was selected considering not only the yield of production and encapsulation efficiency, but also the drug release characteristics including the initial burst. All microsphere formulations prepared released their PA content completely, regardless of the polymer type and drug loading concentrations.
As shown in , polymer concentration had a significant effect (p < 0.001) on the release period extension when polymer concentration was increased. In addition, increased PA loading percent increased the initial burst release and the total release time. Based on the in vitro release studies, CM1PA20 formulation, which demonstrated a prolonged drug release of 11 days, was selected for the future DES studies.
Figure 3. In vitro release profiles of microsphere formulations for low Mw (a) and medium Mw (b) chitosan.
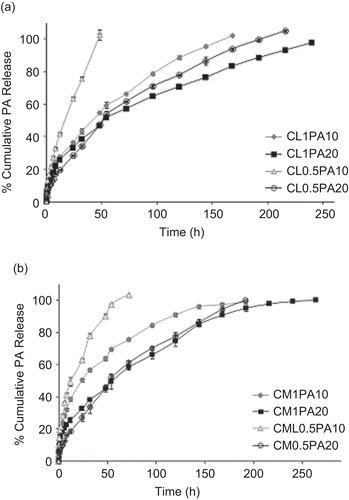
As can be observed from the formulations, PA was released from microspheres prepared with low Mw chitosan within a minimum of 48 (CL0.5PA10 formulation) to maximum of 240 h (). Afterwards, PA was continuously released for ~ 2 days. As shown in , medium Mw chitosan microspheres showed cumulative release profiles between 48 h (CM0.5PA10) and 264 h (CM1PA20). During the microspheres release study, a gel structure was generated and the drug released depended on the gel structure. The drug molecules in the microspheres were either trapped within the polymer or deposited inside the gels. During the release phase, a drug molecule located inside a gel naturally diffuses. A drug molecule located within the network of gels first diffused toward the closest gel layer. At the same time, the internal surface of the gels eroded slowly as a result of its contact with the solvent. This gelling mechanism decreased the drug release from the microspheres (CitationMartinac et al., 2002; CitationDesai & Park, 2005). The formulations prepared with medium Mw chitosan showed slower and extended PA release and also increased t50% values, which are given in . The longest drug release (11 days, t50%: 57.2 ± 9.8 h) was obtained from the CM1PA20 formulation prepared with 20% of PA and 1% of polymer. The experimental values showed a significant effect of the polymer concentration variable for low and medium Mw chitosan microsphere formulations (p < 0.001). This effect is even more significant at low level drug loading. The different release rates of formulations were mainly dependent on the polymer concentration rather than drug loading at low level drug loading. Moreover, at low levels of polymer concentration, polymer molecular weight was not effective on the t50% values. Chitosan molecular weight had a significant effect on the release rate of PA at high levels of polymer concentration. Higher Mw polymer and high concentration of both PA and polymer caused slower drug release. At high levels of drug loading, the drug loading factor is more effective on the t50% response rather than polymer concentration or chitosan molecular weight. There are different in vitro release techniques in the literature for evaluating drug release from chitosan microspheres. CitationDesai and Park (2005) used a dialysis technique while CitationGanza-Gonzales et al. (1999) and CitationGavini et al. (2006) used the USP basket method. The in vitro release technique used in our study was used by CitationFilipovic-Grcic et al. (2000) and the results obtained from our in vitro release study were concordant with those studies.
The optimum microsphere formulation (CM1PA20) showed the best encapsulation efficiency (45.7 ± 0.3%) and it released the total amount incorporated in the microspheres. This formulation also presented a relative good yield of production (48.0 ± 6.7%).
The selected formulation showed a slow release rate, with 50% of the incorporated drug released in 57.2 h. A prolonged drug release is important for the treatment of stenosis caused by inflammation responses that implies long-term treatment for several weeks to prevent restenosis. Due to the high loading and its adequate release properties, the selected formulation (20% drug loading. 1% polymer concentration, and medium polymer Mw) could offer a good alternative to successive application, avoiding the risk of insufficient drug concentration in the stenosis area and the systemic side-effects.
The application of a three-factor two-level (23) factorial design appeared to be a useful tool for the characterization and optimization of PA-loaded chitosan microspheres prepared by spray-drying technique. For all the responses there were significantly effective interactions between the main factors which can easily evaluated with given design. Existence of significant interactions made factorial design necessary for making healthy comparisons. The multiple regression analysis of the obtained results led to polynomial equations that adequately describe the influence of the selected variables (drug loading, polymer concentration, and polymer Mw) at different levels on the responses that were under investigation in the present work.
According to the studied factors, the selected optimum formulation was prepared with 20% of PA and 1% of chitosan. The optimum microsphere formulation was selected considering not only the particle size and encapsulation efficiency, but also the drug release rates. The selected formulation released PA at a constant rate for 11 days allowing the treatment of restenosis caused by implantation of a stent.
Acknowledgement
The authors wish to thank Abdi İbrahim Drug Company (Turkey) for the generous supply of Prednisolone acetate. The authors also would like to acknowledge Professor Ergun Karaağaoğlu for supporting/advising statistical analyses.
Declaration of interest
This study was supported by Hacettepe University Research Fund (Project No: 0302301007). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agnihotri, S.A., Mallikarjuna, N.N., Aminabhavi, T.M. (2004). Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Contr Rel. 100:5–28.
- Arıca, B., Çalış, S., Kaş, H.S., Hıncal, A.A. (2002). Chitosan microspheres of ibuprofen: evaluation and in vitro characterization. In: Muzzarelli, R.A.A., Muzzarelli, C., eds. Chitosan in pharmacy and chemistry. Italy: Atec, 71–6.
- Arıca, B., Çetemen, M., Öner, L., Kaş, H.S., Hıncal, A.A. (1994). Microencapsulation of diclofenac sodium by spray-drying process, In: Kaş, H.S., Hıncal, A.A. eds. Minutes, 9th International Symposium on Microencapsulation. Paris, France: Editions de Santé, 136–41.
- Burt, H.M., Hunter, W.L. (2006). Drug-eluting stents: a multidisciplinary success story. Adv Drug Deliv Rev. 58:350–7.
- Chorny, M., Fishbein, I., Golomb, G. (2000). Drug delivery systems for the treatment of restenosis. Crit Rev Ther Drug Car Syst. 17:249–84.
- Desai, K.G.H., Park, H.J. (2005). Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J Microencapsul. 22:179–92.
- Filipovic-Grcic, J., Perissutti, B., Moneghini, M., Voinovich, D., Martinac, A., Jalšenjak, I. (2003). Spray-dried carbamazepine-loaded chitosan and HPMC microspheres: preparation and characterization. J Pharm Pharmacol 55:921–31.
- Filipovic-Grcic, J., Voinovich, D., Moneghini, M., Becirevic-Lacan, M., Magarotto, L., Jalsenjak, I. (2000). Chitosan microspheres with hydrocortisone and hydrocortisone–hydroxypropyl-β-cyclodextrin inclusion complex. Eur J Pharm Sci. 9:373–9.
- Ganza-Gonzalez, A., Anguiano-Igea, S., Otero-Espinar, F.J., Blanco Mendez, J. (1999). Chitosan and chondroitin microspheres for oral-administration controlled release of metoclopramide. Eur J Pharm Biopharm. 48:149–55.
- Gavini, E., Hegge, A.B., Rassu, G., Sanna, V., Testa, C., Pirisino, G., Karlsen, J., Giunchedi, P. (2006). Nasal administration of carbamazepine using chitosan microspheres: in vitro/in vivo studies. Int J Pharm. 307:9–15.
- Guinchedi, P., Juliano, C., Gavini, E., Cossua, M., Sorrenti, M. (2002). Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur J Pharm Sci. 53:233–9.
- Grech, E.D. (2003). Pathophysiology and investigation of coronary artery disease. In: Grech, E.D., ed. ABC of interventional cardiology. London: BMJ Publishing Group, 1–6.
- He, P., Davis, S.S., Illum, L. (1999a). Chitosan microspheres prepared by spray drying. Int J Pharm. 187:53–65.
- He, P., Davis, S.S., Illum, L. (1999b). Sustained release chitosan microspheres prepared by novel spray drying methods. J Microencapsul. 16:343–55.
- Huang, Y.C., Yeh, M.K., Chiang, C.H. (2002). Formulation factors in preparing BTM–chitosan microspheres by spray drying method. Int J Pharm. 242:239–42.
- Hunter, W.L. (2006). Drug-eluting stents: beyond the hyperbole. Adv Drug Deliv Rev. 58:347–9.
- Khan, I.A., Patravale, V.B. (2005). The intra-vascular stent as a site-specific local drug delivery system. Drug Dev Ind Pharm. 31:59–78.
- Mann, M.J., Davies, J.M. (2002). Epidemiology and pathophysiology of coronary artery disease. In: Grech, E., Ramsdale, D.R. eds. Practical interventional cardiology. London: Martin Dunitz Ltd., 1–8.
- Martinac, A., Filipovic-Grcic, J., Barbaric, M., Zorc, B., Vionovich, D., Jalsenjak, I. (2002). Gemfibrozil encapsulation and release from microspheres and macromolecular conjugates. Eur J Pharm Sci. 17:207–16.
- Martinac, A., Filipovic-Grcic, J., Voinovich, D., Perussutti, B., Franceschinis, E. (2005). Development and bioadhesive properties of chitosan-ethylcellulose microspheres for nasal delivery. Int J Pharm. 291:69–77.
- Pires, N.M.M., van der Hoeven, B.L., de Vriesa, M.R., Havekes, L.M., van Vlijmen, B.J., Hennink, W.E., Quax, P.H.A., Jukema, J.W. (2005). Local perivascular delivery of anti-restenotic agents from a drug-eluting poly(ϵ-caprolactone) stent cuff. Biomaterials. 26:5386–94.
- Ranade, S.V., Miller, K.M., Richard, R.E., Chan, A.K., Allen, M.J., Helmus, M.N. (2004). Physical characterization of controlled release of paclitaxel from the TAXUS™ Express2™ drug-eluting stent. J Biomed Mater Res. 71A:625–34.
- Sarisozen, C., Arica, B., Calis, S., Hincal, A.A. (2006). Development of biodegradable polymeric cardiovascular stents and in vitro evaluation. AAPS J. 8:Supplement 2 (S2):T3147.
- Sousa, J.E., Serruys, P.W., Costa, M.A. (2003). New frontiers in cardiology drug-eluting stents: part II. Circulation. 107:2383–9.
- Takahashi, H., Chen, R., Okamato, H., Danjo, K. (2005). Acetaminophen particle design using chitosan and a spray-drying technique. Chem Pharm Bull. 53:37–41.
- Tanabe, K., Regar, E., Lee, C.H., Hoye, A., van der Giessen, W., Serruys, P.W. (2004). Local drug delivery using coated stents: New developments and future perspectives. Curr Pharm Design. 10:357–67.
- van der Hoeven, B.L., Pires, N.M.M., Ward, H.M., Oemrawsingh, P.V., van Vlijmen, B.J.M., Quax, P.H.A., Schalij, M.J., van der Wall, E.E., Jukema, J.W. (2005). Drug-eluting stents: results, promises and problems. Int J Cardiol. 99:9–17.
- Waksman, R. (2002). Drug-eluting stents. From bench to bed. Cardiovasc Radiat Med. 3:226–41.
- Winslow, R.D., Sharma, S.K., Kim, M.C. (2005). Restenosis and drug-eluting stents. Mt Sinai J Med. 72:81–9.