Abstract
Measures to suppress inflammatory reactions are taken to prevent fibrous encapsulation of implants. It is proposed in this study that tissue engineered scaffolds that can slowly release anti-inflammatory drugs can help reduce inflammatory reactions around implants. Chitosan and chitosan cross-linked with different concentrations of pectin were made into films and porous scaffolds. Results seen from Fourier-transform infrared spectra and thermal gravimetric analysis showed that polyelectrolyte complexation took place between chitosan and pectin units. As the amounts of pectin added to chitosan increased (0%, 0.5%, 1%, and 2%) the scaffolds became more wettable (contact angle decreased from 81° to 76°), less swellable (swelling ratio decreased from 35% to 30%), and less capable of releasing pentoxifylline (PTX) (release efficacies decreased from 93% to 83%). Higher degrees of pectin cross-linking made the scaffolds more resistant to compression (Young’s modulus increased from 2.4 kPa to 3.7 kPa) and more favorable for initial cell attachment (percentage of attached cells increased from 55% to 67%). In vitro tests showed that, with the reduction of PTX release rates, PTX became more effective in inhibiting TNF-α and IL-6 production from activated macrophages. This investigation has demonstrated that the changes in the basic drug release properties of chitosan scaffolds were proportional to the amount of pectin added. The changes could help improve the effectiveness of PTX.
Introduction
Chitosan is a natural polymer that has been studied as a material for sustained drug release (CitationIllum et al., 2001; CitationBhattarai et al., 2009) and for tissue engineered scaffolds (CitationMachado et al., 2007; CitationRichardson et al., 2008). It is readily available, biocompatible, and bioresorbable. Pectin is a natural cross-linker and has been used to cross-link chitosan beads and membranes to modify their physical and chemical properties as well as biocompatibility (CitationKim et al., 2003; CitationBernabé et al., 2005; CitationGhaffari et al., 2007). Pectin, an anionic polysaccharide, will form polyelectrolyte complex with chitosan, a cationic polysaccharide, through ionic attraction between NH3+ of chitosan and COO− of pectin (CitationMeshali & Gabr, 1992; CitationKim et al., 2003; CitationBernabé et al., 2005).
Surgical procedures to insert implants result in inflammation around implants. Anti-inflammatory medicines have been added to implants to reduce adverse inflammatory reactions in surrounding tissues to improve the success rate of implant surgeries (CitationMa et al., 2008; CitationRevell, 2008; CitationJu et al., 2009). Despite the common practice of adding medicines to tissue regeneration scaffolds, often only qualitative studies have been done on the drugs’ effects on cellular/tissue performances (CitationHou et al., 2007; CitationTakahashi et al., 2007; CitationMa et al., 2008). There is a lack of quantitative studies on porous scaffolds’ drug release properties and on how release rates of the target drugs can change the drugs’ potency. The medicines in use have varying strengths and concentrations, which affect how consistently they perform and any adverse effects they may cause. It is important to obtain quantitative information on the scaffolds’ properties related to drug release and how these changes affect the subsequent responses of the target cells.
Pentoxifylline (PTX) is an anti-inflammatory drug that has been known to inhibit immune cells from producing TNF-α, IL-1, and IL-6 (CitationBessler et al., 1986; CitationBruynzeel et al., 1998; CitationCostantini et al., 2009) and to treat/prevent fibrosis (CitationBerman & Duncan, 1989; CitationBerman et al., 1992; CitationPardakhti et al., 2009). PTX is known to exert its effects through phosphodiesterase inhibition and acts through both protein kinase A dependent and independent pathways (CitationCostantini et al., 2009). PTX was found to be able to decrease oxidative stress during inflammation and to suppress superoxide production of macrophages (CitationBessler et al., 1986; CitationPardakhti et al., 2009).
In vitro results have shown that pectin has good biocompatibility and is osteogenic (CitationKokkonen et al., 2008; CitationMishra et al., 2008). Despite the fact that pectin possesses the potential to be a biocompatible cross-linker, literature on the characterizations of pectin-cross-linked tissue engineered scaffolds as drug delivery carriers is very limited. It was examined in this test how degrees of pectin cross-linking altered chitosan scaffolds’ properties and PTX release efficacies. Also, the correlation between PTX release rates and its effectiveness in reducing TNF-α and IL-6 from macrophage cells was established.
Materials and methods
All chemicals were purchased from Sigma (MO), unless specified otherwise.
Preparation of chitosan and pectin-crosslinked chitosan solutions
The final contents of chitosan and alginate in the solutions used to make film/scaffolds are listed in . Chitosan (96% deacetylation, C&B Co., Taiwan) was dissolved in 2 v/v% acetic acid solution. Pectin (Acros Organics, NJ) was dissolved in deionized (DI) water. Chitosan and pectin solutions were stirred together for 1 h before they were further processed into films and scaffolds.
Table 1. Concentrations of chitosan and pectin in the solutions used to make films and scaffolds.
Making films and scaffolds
Films was prepared by coating 1 mL of polymer solution on the bottom of a polystyrene petri dish (diameter = 3 cm) and drying at room temperature. The polymer coatings were neutralized in 0.5 N NaOH for 30 min, rinsed with DI water three times, dehydrated with a series of ethanol solutions (20%, 50%, 70%, and 100%), and then air-dried.
A scaffold was prepared by putting 2 mL of polymer solution in a glass tube and freezing it at −20°C overnight. The frozen sample was then lyophilized in a freeze-dryer (RVT4104, Savant, NY). The neutralization and dehydration processes of the scaffolds were the same as those described for film preparation above.
Films were used for the contact angle measurement and cell attachment tests. The rest of the tests were done on scaffolds.
Thermo-gravimetric analysis (TGA) (n = 4)
Dried scaffolds were cut into small pieces (∼ 10 mg) and put into a TGA instrument (Thermal analyst 2000, TA Instruments, DE, USA). The sample chamber was flushed with nitrogen gas before and during the test (60 mL/min). Scaffolds were heated from 25°C to 600°C at 10°C/min, and their weights were recorded over time.
Fourier Transform Infrared Spectrum (FTIR) (n = 3)
Scaffold specimens were ground, mixed with KBr, and pressed into tabs. The specimens were scanned from 4000 cm−1 to 400 cm−1 in a FTIR machine (Spectrum Gx, PerkinElmer, MA) with a resolution of 4 cm−1. Transmittance of signature peaks of chitosan and pectin were identified.
Contact angle (n = 4)
Five microliters of DI water was placed on each polymer film. Static contact angles were measured using a contact angle meter (CA-D, Kyowa Interface Science Co., Japan).
Scaffold morphology by scanning electron microscope (SEM) (n = 3)
Dried scaffolds were sliced in liquid nitrogen and sputter-coated with gold before being placed in the vacuum chamber of a SEM (Hitachi S-3000H, Japan). Micrographs of the scaffold surfaces were taken. Six pores were randomly selected from each micrograph, and three micrographs from different locations of each scaffold were used to calculate the pore size of the scaffold.
Swelling ratio (n = 3)
After measuring the diameters (D0) of each dry scaffold (D ∼ 1 cm, h (thickness) ∼ 0.2 cm), three scaffolds of each kind of polymer were placed in phosphate buffered saline (PBS, pH = 7.4) and their diameters were measured with a vernier caliper at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 15, 20, 30, 40, 50, 60, 90 min (Dt). The swelling ratio at each time point was calculated as [(Dt − D0)/D0] × 100%.
Release efficacy of pentoxifylline (PTX) (n = 3)
Each dry scaffold disc (D ∼ 1 cm, h ∼ 0.2 cm) was loaded with 50 μL PTX (20 mg/ml) and left to dry at room temperature overnight. Ten scaffold discs were placed in each beaker filled with 100 mL of PBS, three beakers (n = 3) for each polymer. All beakers were placed on a shaker (50 rpm) during the test. At each designated time point, 100 μL of PBS was sampled and the same volume of PBS was replenished. The absorbance of PTX at 274 nm was measured (Gene Quant 1300 spectrophotometer, GE Healthcare, NJ) and converted to concentrations (Mt (μg/mL)) with a standard curve. The release efficacy of the PTX was calculated as [(Mt/00 μg/mL)] × 100%. After the PTX in the 10 scaffolds was released, the final PTX concentration in each beaker was 100 μg/mL.
Compression (n = 4)
Dry scaffolds (D ∼ 1 cm; h ∼ 1.2 cm) were soaked in PBS overnight and excess liquid was removed before testing. A Universal Micro-tribometer (model UMT-2, CETR, CA) was used to obtain stress–strain curves. The speed of crosshead was 0.3 mm/s, and a 10 N load cell was used. The test stopped at 80% strain. The slope of the initial linear section of the stress–strain curve was used to estimate the Young’s modulus.
Cell attachment (n = 4)
The bottom of each well of a 24-well tissue culture polystyrene (TCP) plate (BD Biosciences, CA) was coated with 200 μL of polymer solution and processed as mentioned earlier. The TCP surface served as positive control. Coated films were disinfected with 70% ethanol solution, exposed to UV briefly, and were rinsed with sterile PBS to remove residual ethanol. Osteoblasts (7F2, ATCC, VA) (3 × 105cells/mL) were seeded onto the films (1 mL/well). Cells were cultured in α-MEM (Invitrogen, CA) without serum. The culture was maintained in a cell culture incubator (SCA-165DS, ASTEC, Japan) at 95% humidity, 37°C, and 5% CO2. At designated time points, unattached cells were counted to back calculate the number of attached cells.
In vitro test of anti-inflammatory effects during controlled release (n = 4)
Before this test was performed, cell viability in the presence of PTX and scaffolds were assayed by the MTT(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) method (CitationLin & Lu, 2009). The viabilities of macrophages cultured in medium with 100 μg/mL of PTX reduced to 92% of that of the control (no PTX added) after 6 h. The presence of blank scaffolds in the cell culture medium did not alter the viabilities of macrophage cells, both non-activated and activated, compared to controls (no scaffold present). The amounts of TNF-α and IL-6 released from non-activated macrophage cells (see method below) cultured with blank scaffolds were negligible and those released from activated macrophage cells cultured with the scaffolds were similar.
C1 and C1P2 scaffolds were used in the test to compare the effect of different PTX release rates on macrophage cells. Each scaffold (D ∼ 1 cm, h ∼ 0.2 cm) was disinfected with 70% ethanol and rinsed with sterile PBS. After PBS was carefully removed, 50 μL of PTX (2 mg/mL) was added to each scaffold and air-dried. Macrophage cells (RAW 264.7, ATCC, VA) cultured in DMEM + 10% fetal bovine serum + 100 U/mL penicillin + 100 μg/mL streptomycin (all from Invitrogen, CA) were seeded onto 24-well TCP plates at 1 × 105/cm2 and allowed to settle overnight in a cell culture incubator. The cell culture media was then removed and cells were divided into different test groups based on the contents in the culture media (1 mL) (see ). After all PTX was released from each scaffold, the final concentration of PTX in each well was 100 μg/mL. Lipopolysaccharide (LPS) was used to activate macrophage cells to release inflammatory factors (CitationLin & Bumgardner, 2004).
Table 2. Conditions used in tests of anti-inflammatory effects of PTX release on macrophage cells in vitro.
Six hours later, tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in the media were measured using commercial kits (Mouse TNF-alpha ELISA OptiEIA, BD Bioscience, CA, and Mouse IL-6 ELISA DuoSet, R&D, MN).
Statistical analysis
All data is presented as average ± standard deviation. Statistical analysis was conducted using two-way ANOVA with tukey tests (SigmaStat 3.5 for Windows, Systat Software, IL). Difference between pectin-cross-linked chitosan and chitosan was declared when p < 0.05.
Results and discussion
TGA
The decomposition temperatures of all polymer samples are listed in . The decomposition temperatures of all three pectin-cross-linked chitosan scaffolds fell between those of chitosan (306°C) and pectin (240°C). The higher the amount of pectin in the scaffold there was, the closer its decomposition temperature was to that of pectin (CitationWendlandt, 1986). The absence of the event typical of chitosan (300°C) after pectin addition indicated complexation between the two polymers (CitationGhaffari et al., 2007).
Table 3. The decomposition temperatures, contact angles, swelling ratios, initial cell attachments (1.5 h after cell seeding), and compressional Young’s moduli of chitosan and pectin cross-linked chitosan films/scaffolds.
FTIR
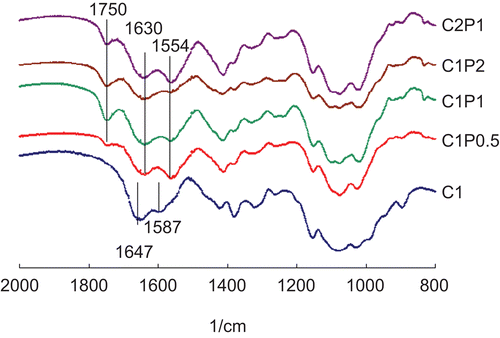
Chitosan’s amino group had typical absorbance at 1587 cm−1 and 1647 cm−1. After being cross-linked with pectin, three peaks appeared at 1554 cm−1, 1630 cm−1, and 1750 cm−1 (). The peak at 1750 cm−1 is characteristic of C=O vibration of the COOH group in pectin (CitationKim et al., 2003; CitationBernabé et al., 2005). Peak 1630 cm−1 is assigned to amide I and asymmetric NH3+ deformation. Peak 1630 cm−1 indicates the formation of ionic bonds between chitosan’s amino groups and pectin’s carboxyl group (CitationGhaffari et al., 2007). Peak 1554 cm−1 is assigned to amide II overlapping with amine and protonated amine (CitationLawrie et al., 2007). The intensity of the peak at 1554 cm−1 decreased as the concentration of pectin increased.
Contact angle
As the amount of pectin in chitosan increased to higher concentrations (from C1 to C1P1 and C1P2), the contact angles of the cross-linked chitosan decreased (). The addition of pectin introduced carboxylate group (O=C-O) to the surface, which attracted water through hydrogen bond formation, and increased the surface wettability. Increases in chitosan concentration from C1P1 to C2P1, on the other hand, raised the contact angle (p < 0.05).
Scaffold morphology (SEM)
Based on the observations of SEM micrographs (), the pores appeared to be larger in pectin-cross-linked chitosan scaffolds (C1P0.5, C1P1, C1P2; average diameter = 288 μm) than in pure chitosan scaffolds (C1; average diameter = 173 μm). The morphology of the porous structures of the scaffolds was similar as the amount of pectin increased. Increases in chitosan concentration resulted in smaller pores. The average pore size of the scaffolds decreased from 288 μm (C1P1) to 170 μm (C2P1).
Figure 2. Representative SEM micrographs of (a) C1 (1% chitosan), (b) C1P0.5 (1% chitosan and 0.5% pectin), (c) C1P1 (1% chitosan and 1% pectin), (d) C1P2 (1% chitosan and 2% pectin), (e) C2P1 (2% chitosan and 1% pectin) scaffolds. Scale bar = 500 μm.
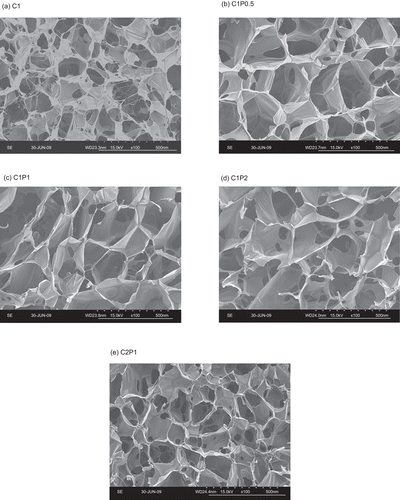
The viscosity of chitosan-pectin solution in slightly acidic environments decreases as the concentration of pectin increases, and decreases in the solution’s viscosity increases the porosity and pore size of the freeze-dried scaffolds (CitationGhaffari et al., 2007). When the amount of chitosan increased, the solution became more viscous, and hence resulted in smaller pores.
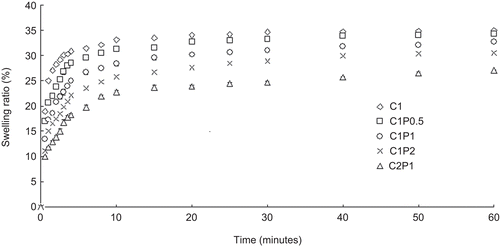
Swelling ratio
The scaffolds quickly absorbed water and swelled up to 70–90% of their maximum swelling ratios in the first 4–8 min (). The C1 scaffolds swelled the fastest and had the highest swelling ratio, followed by C1P0.5, C1P1, C1P2, and C2P1, which swelled the slowest and had the smallest swelling ratio. The swelling ratios of all scaffolds reached a constant value after 60 min. shows the swelling ratios of the scaffolds at 60 min in PBS. Increases in the amount of pectin and chitosan (i.e. from C1P1 to C2P1) reduced the scaffolds’ swelling ratios.
More pectin resulted in more cross-linking with chitosan as well as a higher polymer mass in the polymer network. The more compact the polymer network was, the less likely the network was able to stretch in water and to swell. The higher wettability from adding pectin allows for faster wetting of the scaffolds, but does not necessarily result in higher swelling ratios (CitationKim et al., 2003).
Release efficacy
As the ratios of pectin in the scaffolds increased, the scaffolds’ release efficacies decreased (). C2P1 had the lowest release efficacy and the least initial PTX burst of all the samples.
Figure 3. The pentoxifylline release efficacies of 1% chitosan (C1), 1% chitosan and 0.5% pectin (C1P0.5), 1% chitosan and 1% pectin (C1P1), 1% chitosan and 2% pectin (C1P2), and 2% chitosan and 1% pectin (C2P1) scaffolds.
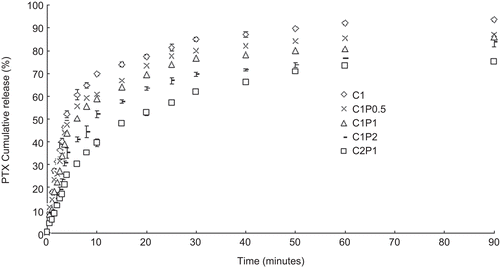
The release efficacy of a hydrogel is mostly influenced by diffusion and swelling (CitationPasparakis & Bouropoulos, 2006). Cross-linking reduces the diffusion efficiency in a hydrogel (CitationPasparakis & Bouropoulos, 2006) as well as the swelling ratio. This can also be seen in our results, as the hydrogel’s release efficacy went down after cross-linking. The higher polymer density also prevented effective diffusion of PTX within the polymer network.
PTX diffused faster within the voids (pores) and more slowly through the polymer networks. Diffusion through the polymer is the rate-determining step. Adding pectin to chitosan not only cross-linked chitosan, but also added more polymer mass to the polymer networks. The more compact networks slowed down the PTX diffusion within.
The scaffolds were placed in a beaker filled with 100 mL of PBS moving at 50 rpm. The moving buffer solution accelerated the PTX mass transfer at the scaffold/buffer interface and sped up the PTX release process. It is reasonable to expect that these scaffolds released PTX faster than those in vivo. As a bone or muscle implant in vivo, an implant would be surrounded in an environment where the drug is carried away by body fluid (flow rate ∼ 2 mL/100 mL min (CitationDeng et al., 2005)).
The 2C1P scaffolds released ∼ 70% of their PTX in 1.5 h during this test. Acute inflammation after surgery lasts anywhere from hours to a couple of days. The PTX release from 2C1P scaffolds made in this investigation may, to a certain degree, be feasible for suppressing initial acute inflammation after surgery. However, a more cross-linked scaffold with a lower release efficacy would be more practical for in vivo applications.
Compression
Young’s modulus is an indication of a materials’ stiffness. The Young’s moduli of chitosan scaffolds increased when the concentrations of pectin increased (). This is due to both the effects of cross-linking and increases in polymer mass. CitationSubramanian and Lin (2005) reported higher Young’s moduli of chitosan scaffolds after they were cross-linked with 1,4 butanediol diglycidyl ether. CitationBartkowiak and Hunkeler (2000) found that a polymer with higher molecular mass and concentration in the solution makes stiffer hydrogel beads.
In this study, C2P1 had a significantly higher Young’s modulus than C1P2, while the two had a similar polymer mass. SEM micrographs () showed that C2P1 scaffolds had smaller pores than C1P2, and hence a sturdier structure.
Cell attachment
One and half hours after cell seeding, films with higher pectin content (C1P1 and C1P2) appeared to be more favorable for cell attachment than others (p < 0.05) (). Two hours after cell seeding, C2P1 had the lowest percentage of cells attachment (68%) compared to the others, which had ∼ 83% cell attachment.
Cells in this test attached directly to the polymers instead of serum proteins because serum was not added to the cell culture media. Most cells prefer to attach to surfaces with intermediate-to-moderate wettability (CitationHamdan et al., 2006; CitationPashkuleva et al., 2010). In this test, the percentage of cell attachment increased as the wettability increased (or decreased in contact angle) with the addition of pectin (). The addition of pectin brings in the carboxylate groups and increases the surface oxygen content/oxygen functionalities which bind proteins more tightly and promotes cell attachment and growth (CitationSchamberger & Gardella, 1994).
The scaffolds with the same chitosan/pectin proportion but different polymer densities, i.e. C1P0.5 and C2P1, had similar decomposition temperatures, contact angles, and cell attachment rates. A decomposition temperature measures the thermal stability of polymers, which depends on the kinds of polymers in the mixture. Contact angle is an index of hydrophobicity, which affects cells’ affinity to polymers. Differences between the two mixtures showed up in swelling, PTX release, and Young’s modulus. C2P1 had a lower selling ratio, a higher release rate, and a higher Young’s modulus compared to C1P0.5.
Anti-inflammatory effect of PTX slow release
Macrophage cells did not release TNF-α and IL-6 with the presence of scaffolds (). In medium containing 1 μg/ml of LPS (DMEM+LPS), macrophage cells released similar amounts of TNF-α and IL-6 in C1 and C1P2. When cultured in medium that contained both LPS and PTX (DMEM+LPS+PTX(in media)), macrophage cells in both C1 and C1P2 released less TNF-α and IL-6 when compared to DMEM+LPS. There was no significant difference between the two groups. When PTX was released from scaffolds into the culture media (DMEM+LPS+PTX(scaffold release)), the amounts of TNF-α and IL-6 was less than those in DMEM+LPS+PTX(in media) (p < 0.05). Cells in C1P2, which had lower PTX release efficacy than C1, released significantly less TNF-α and IL-6 than those in C1 (p < 0.05).
Figure 4. TNF-α (a) and IL-6 (b) release from macrophage cells cultured in the four conditions listed in . * p < 0.05.
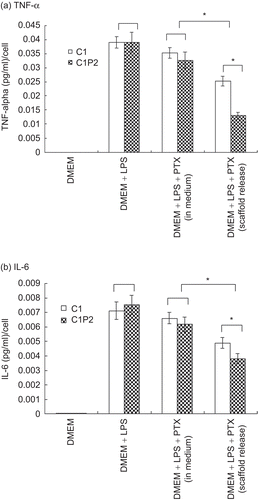
Figure 5. The swelling ratios of 1% chitosan (C1), 1% chitosan and 0.5% pectin (C1P0.5), 1% chitosan and 1% pectin (C1P1), 1% chitosan and 2% pectin(C1P2), and 2% chitosan and 1% pectin (C2P1) scaffolds.
At the dosage of 100 μg/ml, PTX is known to suppress immune cell proliferation (CitationBruynzeel et al., 1998; CitationVukanić et al., 2007). Based on possible PTX metabolic pathways in cells (CitationCostantini et al., 2009), it is speculated that when higher doses of PTX were added directly to the medium with activated macrophage cells, they inhibited macrophage cell proliferation, superoxide production (CitationBessler et al., 1986; CitationVukanić et al., 2007; CitationPardakhti et al., 2009), and TNF-α mRNA and IL-6 expressions. However, when PTX was slowly released into the medium from a scaffold, the concentration of PTX increased gradually to an optimal concentration that PTX could inhibit TNF-α expression but not cell growth (< 10 μg/ml (CitationVukanić et al., 2007)) or superoxide production. CitationKofuji et al. (2004) implanted chitosan beads retaining the anti-inflammatory drug prednisolone (PS) into the backs of mice. They found inflammation significantly reduced after the implantation of chitosan beads when compared with the injection of PS suspension.
During the anti-inflammatory tests, scaffolds were placed in wells filled with static cell culture medium instead of medium with a steady-state dynamic flow. There were also no enzymes and other types of cells present as it would have been in vivo. The test results may or may not be reproducible in vivo. Whether the drug release efficacy of a scaffold would change the scaffold’s surrounding tissues and cells’ reactions to the drug will need to be determined by further tests.
Summary
This is the first time a porous chitosan scaffold and its pectin-cross-linked forms were studied as drug delivery carriers. Results in this study showed that cross-linking altered the scaffolds’ drug release properties, and the reduced release rate altered the drug’s effectiveness in inhibiting TNF-α and IL-6 release from activated macrophage cells.
Declaration of interest
This project is funded mainly by grants from the National Science Council: NSC 97-2221-E-027-001.
References
- Bartkowiak, A., Hunkeler, D. (2000). Alginate-oligochitosan microcapsules. II. Control of mechanical resistance and permeability of the membrane. Chem Mater. 12:206–12.
- Berman, B., Duncan, M.R. (1989). Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 92:605–10.
- Berman, B., Wietzerbin, J., Sanceau, J., Merlin, G., Duncan, M.R. (1992). Pentoxifylline inhibits certain constitutive and tumor necrosis factor-α induced activities of human normal dermal fibroblasts. J Invest Dermatol. 98:706–12.
- Bernabé, P., Peniche, C., Argüelles-Monal, W. (2005). Swelling behavior of chitosan/pectin polyelectrolyte complex membranes. Effect of thermal cross-linking. Polym Bull. 55:367–75.
- Bessler, H., Gilgal, R., Djaldetti, M., Zahavi, I. (1986). Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J Leukoc Biol. 40:747–54.
- Bhattarai, N., Gunn, J., Zhang, M. (2009). Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 62:83–99.
- Bruynzeel, I., Stoof, T.J., Willemze, R. (1998). Pentoxifylline and skin inflammation. Clin Exp Dermatol. 23:168–72.
- Costantini, T.W., Deree, J., Loomis, W., Putnam, J.G., Choi, S., Baird, A., Eliceiri, B.P., Bansal, V., Coimbra, R. (2009). Phosphodiesterase inhibition attenuates alterations to the tight junction proteins occludin and ZO-1 in immunostimulated Caco-2 intestinal monolayers. Life Sci. 84:18–22.
- Deng, C., Chen, J., Fan, H., Zuang, X. (2005). Effect of flowing speed on bone-like apatite formation in porous calcium phosphate in dynamic RSBF. J Mater Sci. 40:1809–12.
- Ghaffari, A., Navaee, K., Oskoui, M., Bayati, K., Rafiee-Tehrani, M. (2007). Preparation and characterization of free mixed-film of pectin/chitosan/Eudragit RS intended for sigmoidal drug delivery. Eur J Pharm Biopharm. 67:175–86.
- Hamdan, M., Blanco, L., Khraisat, A., Tresguerres, I.F. (2006). Influence of titanium surface charge on fibroblast adhesion. Clin Implant Dent Relat Res. 8:32–38.
- Hou, L.T., Liu, C.M., Liu, B.Y., Chang, P.C., Chen, M.H., Ho, M.H., Jehng, S.M., Liu, H.C. (2007). Tissue engineering bone formation in novel recombinant human bone morphogenic protein 2-atelocollagen composite scaffolds. J Periodontol. 78:335–43.
- Illum, L., Jabbal-Gill, I., Hinchcliffe, M., Fisher, A.N., Davis, S.S. (2001). Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 51:81–96.
- Ju, Y.M., Yu, B., West, L., Moussy, Y., Moussy, F. (2010). A dexamethasone-loaded PLGA microspheres/collagen scaffold composite for implantable glucose sensors. J Biomed Mater Res A. 93:200–10.
- Kim, T.H., Park, Y.H., Kim, K.J., Cho, C.S. (2003). Release of albumin from chitosan-coated pectin beads in vitro. Int J Pharm. 250:371–83.
- Kofuji, K., Akamine, H., Qian, C.J., Watanabe, K., Togan, Y., Nishimura, M., Sugiyama, I., Murata, Y., Kawashima, S. (2004). Therapeutic efficacy of sustained drug release from chitosan gel on local inflammation. Int J Pharm. 272:65–78.
- Kokkonen, H., Cassinelli, C., Verhoef, R., Morra, M., Schols, H.A., Tuukkanen, J. (2008). Differentiation of osteoblasts on pectin-coated titanium. Biomacromolecules. 9:2369–76.
- Lawrie, G., Keen, I., Drew, B., Chandler-Temple, A., Rintoul, L., Fredericks, P., Grøndahl, L. (2007). Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules. 8:2533–41.
- Lin, H., Bumgardner, J.D. (2004). In vitro biocorrosion of Co-Cr-Mo implant alloy by macrophage cells. J Orthop Res. 22:1231–6.
- Lin, H.Y., Lu, K.H. (2010). Repairing large bone fractures with low frequency electromagnetic fields. J Orthop Res. 28:265–70.
- Ma, J.Y., Jiang, W.W., Zhou, Z.T., Li, J.M., Wang, H.Y. (2008). The promoting angiogenesis and anti-inflammation effect of scutellarin on polyglycolic acid scaffold of balb/c mice model. J Asian Nat Prod Res. 10:1147–53.
- Machado, C.B., Ventura, J.M., Lemos, A.F., Ferreira, J.M., Leite, M.F., Goes, A.M. (2007). 3D chitosan–gelatin–chondroitin porous scaffold improves osteogenic differentiation of mesenchymal stem cells. Biomed Mater. 2:124–31.
- Meshali, M.M., Gabr, K.E. (1992). Physical properties of fast-release nonreverting hydrochlorothiazide solid dispersions. Pharm Res. 9:960–2.
- Mishra, R.K., Datt, M., Pal, K., Banthia, A.K. (2008). Preparation and characterization of amidated pectin based hydrogels for drug delivery system. J Mater Sci Mater Med. 19:2275–80.
- Pardakhti, A., Alavi, S.A., Kheshti, N.M., Eshaghi, P., Safaeian, L. (2009). Effect of slow release pentoxifylline and captopril on delayed pulmonary complications of mustard gas in animal models. Tanaffos. 8:41–9.
- Pashkuleva, I., Marques, A.P., Vaz, F., Reis, R.L. (2010). Surface modification of starch based biomaterials by oxygen plasma or UV-irradiation. J Mater Sci-Mater M. 21:21–32.
- Pasparakis, G., Bouropoulos, N. (2006). Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int J Pharm. 323:34–42.
- Revell, P.A. (2008). The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 5:1263–78.
- Richardson, S.M., Hughes, M., Hunt, J.A., Freemont, A.J., Hoyland, J.A. (2008). Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials. 29:85–93.
- Schamberger, P.C., Gardella, J.A. (1994). Surface chemical modification of materials which influence animal cell adhesion—a review. Colloids Surf B Biointerf. 2:209–23.
- Subramanian, A., Lin, H. (2005). Crosslinked chitosan: its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation. J Biomed Mater Res A. 75:742–53.
- Takahashi, Y., Yamamoto, M., Yamada, K., Kawakami, O., Tabata, Y. (2007). Skull bone regeneration in nonhuman primates by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 13:293–300.
- Vukanić, Z.S., Čolić, M., Dimitrijević, M. (2007). Effect of pentoxifylline on differentiation and maturation of human monocyte-derived dendritic cells in vitro. Int Immunopharmacol. 7:167–74.
- Wendlandt, W.W. (1986). Thermal analysis. Hoboken, NJ: John Wiley & Sons.