Abstract
Simple and efficient gene transfer to the skin would facilitate many local and systemic gene therapy applications. This study reports a novel approach that allows expression of plasmid DNA in epidermis and hair follicle cells with dimethyl sulfoxide (DMSO) after pre-treatment with depilation and retinoic acid (RA) for the purposes of gene therapy. This study investigated the transdermal efficacy of gene to mouse skin when utilizing DMSO after RA pre-treatment. Retinoic acid pre-treatment can increase the efficiency of transfection. This finding indicates that one can more effectively and much less expensively make use of genes therapy to treat diseases of the hair and skin.
Introduction
Simple and efficient gene transfer to the skin would facilitate many local and systemic gene therapy applications. As the largest organ of the human body, skin provides a painless interface for drug delivery (CitationChen et al., 2006). However, there are many factors that influence the efficient delivery of transgenes to skin. For example, stratum corneum, the outer most layer of human skin, is the major barrier to transdermal gene delivery. Topical skin gene transfer techniques required a DNA carrier. CitationMeykadeh et al. (2005) used liposomes to enhance the transdermal delivery of plasmid DNA in mice. CitationLi and Hoffman (1995) applied a lacZ expression plasmid associated with non-cationic liposome complexes onto mouse skin and detected the lacZ gene product in hair follicles. CitationAlexander and Akhurst (1995) showed β-galactosidase (β-gal) expression in the dermis, epidermis, and hair follicles following topical application of cationic liposome:DNA complexes (CLDC) on to mouse skin previously treated with a depilatory cream.
Dimethyl sulfoxide (DMSO) is the organosulfur compound with the formula (CH3)2SO. It is colourless, odorless, and hygroscopic, and has been shown to promote the permeation of, for example, anti-viral agents, steroids, and antibiotics (CitationWilliams and Barry, 2004). Findings reported by CitationHeckert et al. (2002) showed that naked DNA could be delivered transcutaneously in the chicken using 50% DMSO. CitationKawai and Nishizawa (1984) used DMSO and polycation to enhance the transfection of DNA in vitro. These results provide a foundation that can increased the efficiency of gene to skin by transdermal penetration with DMSO. Although liposome can enhance the transdermal delivery of transgenes, we made use of DMSO:DNA complexes instead of liposome due to the latters higher toxicity (CitationTam et al., 2000) compared with DMSO (CitationRobert and Vignes, 2000).
In general, the major problems with topical skin gene therapy include transient expression and low efficiency of gene transfer (CitationGhazizadeh et al., 1999). So enhancement methods to increase skin permeability were developed. Recent findings showed that penetration enhancers can help to transfer many molecules across the straum comeum, including some biomacromolecules. Retinoic acid (RA) is the oxidized form of Vitamin A compound with the formula C20H28O2, with only partial vitamin A function, which exists in both cis and trans isomeric forms, is the most biologically active metabolite of vitamin A, and is essential for normal development (CitationKummet and Meyskens, 1983; CitationAbu-Abed et al., 2002). It is also reported that the RA is an essential factor for the normal differentiation of epithelial cells (CitationGhezzo and Pegoraro, 1981). Proliferating cells express plasmid DNA more efficiently than quiescent cells (CitationNicolau and Sene, 1982). However, hair follicles only proliferate during anagen, and we have known that the anagen of mouse hair follicles is in the telogen stage of the hair cycle between post-natal days 45–65 (CitationDomashenko et al., 2000). Moreover, several stimuli which include depilation or application of proliferative agents such as phorbol ester or retinoic acid can lead to resting hair follicles entering anagen in a predictable and synchronous manner (CitationChase, 1954; CitationPaus et al., 1990; CitationWilson et al., 1994). Furthermore, the researchers (CitationDomashenko et al., 2000) have demonstrated that the RA can markedly increase the transfection efficiency of gene to skin.
In our study, using mice as the experiment model, we investigated whether DMSO can mediate transdermal delivery and expression of DNA with retinoic acid pre-treatment. Our work demonstrated an application of retinoic acid to the skin before DMSO composition application markedly increases the transfection efficiency (CitationDomashenko et al., 2000). These results provide a foundation for subsequent treatment of skin disorders with a topical gene therapy rote route being cheaper and more useful.
Materials and methods
Animals
Mice of 60-days-old were picked out for our experiment. The mice of all studies were approved by the institutional animal care and use committee. In addition, mice were kept under sterile conditions in the core facility of gene engineered mouse of the State Key Laboratory of Biotherapy, Chengdu, Sichuan, PR China.
DMSO and plasmid DNA mixture preparation
We detected whether the DMSO concentration (v/v) influenced the efficiency delivery of gene and the optimum DMSO concentration to promote the efficient transdermal penetration. Formulae added in the spotting compartment were prepared based on the percentage of DMSO in the total volume of 150 µl. Each formula contained 150 µg of pORF-LacZ plasmid. The DMSO concentrations (v/v) were 30% (formula 1), 10% (formula 2), 5% (formula 3), and 0% (formula 4), respectively.
In vitro transfection of mouse skin depends on DMSO composition
The abdominal skin of 60-day-old mice was depilated by depilatory cream (Nair™, Carter-Horner, Mississauga, ON, Canada) for 3–5 min. After pre-treatment with 0.09% physiological saline for 15 min, the abdominal skin was treated with 0.05% retinoic acid mixture (retinoic acid dissolved in 0.09% physiological saline) every other day for 1 week. On the 7th day, mice were anesthetized with chloral hydrate, and we executed the them and harvested their skin. After washing with phosphate buffered saline (PBS), the skin samples were installed in diffusion cells with a diffusion area. There was a spotting compartment and receptor compartment installed separately in both sides of diffusion area. The epidermis face was mounted toward the spotting compartment and the receptor compartment was filled with RPMI1640 medium during the experiment, pEGFP-N1 plasmid (5 µg) was added to the medium as an internal control. Formulae added in the spotting compartment were prepared based on the percentage of DMSO. The medium in the receptor compartment was stirred at 45 rpm during the experiment and the diffusion cells maintained at a constant temperature at 37 ± 0.5°C. At this temperature, the RPMI medium was beneficial to maintain the skin viability. Each formula was run in triplicate.
In vitro PCR analysis
After 24 h, the RPMI1640 medium was adequately passed through the skin and was collected in each receptor compartment. The collection was collected as templates for PCR analysis. Specific primers for lacZ and EGFP were designed, as follows: lacZ (sense) 5′–AAA TCC CGA ATC TCT ATC GT–3′; lacZ (antisense): 5′–AGA CCA GAC CGT TCA TAC AG–3′, amplifying a 912-basepair fragment. EGFP (sense) 5′–CTG ACC CTG AAG TTC ATC TG–3′; EGFP (antisense): 5′–GTG CTC AGG TAG TGG TTG TC–3′, amplifying a 485-basepair fragment.
In vivo transfection of mouse skin
Before transfection, the ear skin of 60-day-old mice was depilated by depilatory cream for 3–5 min. After treatment with 0.09% physiological saline for 15 min, some ears of mice were treated with 20 µl 0.05% retinoic acid mixture (retinoic acid dissolved in 0.09% physiological saline) every other day for 1 week. On the 7th day the mixture (150 µg of pORF-LacZ and 15 µl DMSO, mixed in ddH2O, the total volume is 150 µl) was pipetted topically in 10 µl aliquots to 1 cm2 of ear skin every day for 3 days 4 h after ears was treated with 0.05% retinoic acid mixture. The ears were harvested on the 4th day and processed for β-gal activity.
Histochemical assay of β-galactosidase activity
Ear samples were fixed in freshly prepared 2% formaldehyde/0.2% glutaraldehyde in PBS at 4°C for 2–4 h, then washed in three changes of PBS (pH 7.4) at room temperature for 60 min. All fixed tissue was incubated at 37°C overnight in 1 mg/ml X-gal. After being washed in three changes of PBS (PH7.4) at room temperature for 1 h, the tissue samples were fixed in 10% formalin at room temperature for 24 h. They were then washed with running water overnight, and embedded in paraffin. After sections of 5 µm deparaffin, they were washed in two changes of PBS at room temperature for 5 min, and counterstained with nuclear fast red (CitationDomashenko et al., 2000).
Western blot assay of β-galactosidase protein expression
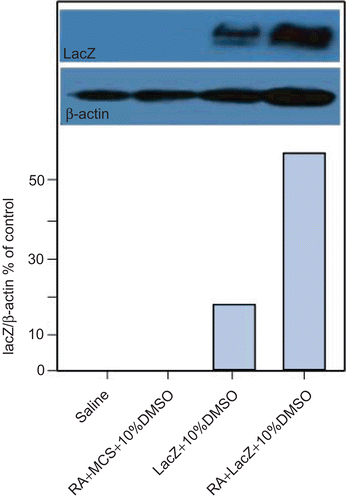
We detected the β-galactosidase protein expression in another ear of mouse. A total of four formulaewere used in the in vivo transdermal study: (1) the optimal formula from the in vitro experiment (10 µg of pORF-LacZ and appropriate amount of DMSO), which was treated with RA; (2) empty carrier (10 µg of pORF-mcs and amount of DMSO), which did not contain LacZ gene compared to pORF-LacZ gene, was treated with RA; (3) 10 µg pORF-LacZ and appropriate amount of DMSO, which was not treated with RA; and (4) saline solution. The total volume of all of the formulations was 10 µl. The tissue samples were immediately put in liquid nitrogen, and the tissue rubbed in mortar. The powder of tissue was divert into a 1.5 ml Ep tube which had RIPA schizolysis liquid with prolease inhibitor. After being put in ice for 3–5 min, the mixture was swirled to make it fully dissolve, and put on ice for 30 min. Then the mixture was centrifuged at 4°C for 20 min, absorbed the top clear liquid and did electrophoresis experiment.
Statistics
Values were shown as mean ± SEM. The significance of differences between all groups were evaluated using one-way ANOVA with a post-hoc Student-Newman-Keuls multiple comparisons test. Statistical analyses were performed using SPSS Software (V13.0, SPSS, USA), and a p-value < 0.05 was considered to be statistically significant.
Results
DMSO optimal concentration analysis in vitro
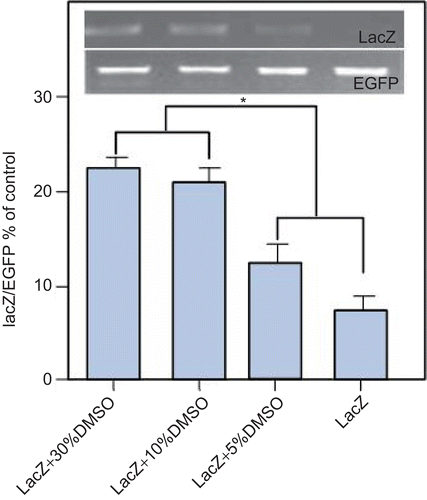
We selected that the DMSO concentration (v/v) as 30% (formula 1), 10% (formula 2), 5% (formula 3), and 0% (formula 4), respectively (). There were remarkable difference in the transdermal efficacy of formula 3 containing 5% compared to the naked plasmid control (formula 4). In addition, formula 2 containing 10% DMSO was similar to 2-fold compared with formula 3 containing 5% DMSO. However, both formula 2 containing 10% DMSO and formula 1 containing 30% DMSO had similar delivery efficacy and transported more pORF-lacZ through the mice skin than formulae 3 and 4. Meanwhile, we verified that formulae 1 and 2 showed the same DNA delivery efficacy, but the concentration of formula 1 containing DMSO was higher than formula 2. So, formula 2 (10% DMSO) was the optimal transdermal delivery formula for further examination in vivo.
Figure 1. After abdominal skin pre-treatment with depilatory cream, the hair of the rat was treated with RA every other day for 1 week, then the skin was treated with different concentrations of DMSO. At 24-h, RPMI1640 medium in each receptor compartment was collected as templates for PCR analysis. Higher concentration DMSO (10% or 30%) can promote better transdermal efficacy of plasmid DNA than lower concentration DMSO (0 or 5%). The 10% concentration of DMSO was the optimal transdermal delivery formula for further examination in vivo. * p < 0.05. n = 3, bars show mean ± SEM.
Sites of gene expression
We demonstrated that 10% DMSO is the optimal concentration to promote the efficient delivery to the skin (as shown in ). We examined the expression of β-galactosidase activity in mice skin 24 h following last topical application of plasmid DNA using histochemical assay. As shown in , blue spots indicated the sites where β-galactosidase (CitationDomashenko et al., 2000) was selectively taken up and expressed by epidermis and hair progenitor cells in the matrix. Meanwhile, the efficient delivery of gene was higher with pre-treatment with RA compared with DMSO alone(). In addition, no LacZ gene expression in the group of empty vector plasmid and control was found ( and ).
Table 1. Transfection efficiency in mice treated with DMSO complex.
Figure 2. In vivo transfection of mouse hair follicles after depilation (DP) and/or retinoic acid (RA) treatment and topical application of DMSO mixtures. (a–d) show
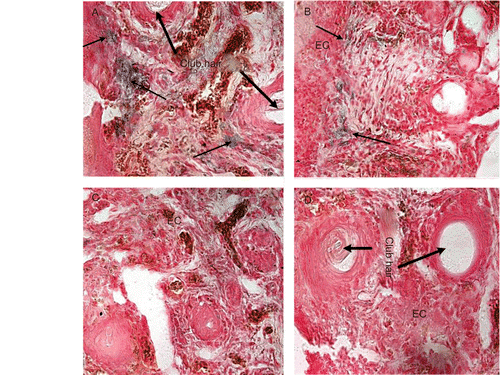
histochemical staining for β-galactosidase expression. (a) RA + LacZ + 10% DMSO; (b) LacZ + 10% DMSO; (c) RA + MCS + 10% DMSO; (d) Saline. EC: epidermis cells. Scale bar = 75 µm(original magnification, ×400).
We also detected expression level of LacZ gene in vivo. The total protein extracted from the ear samples was used for western blot assay. As shown in , lanes 3 and 4 had one band separately; in addition, the expressive consistency of 4 band was higher than 3 band, the results also corresponding with and . The results showed that expression levels of LacZ-10%DMSO-RA-treat mice were much higher than LacZ-10%DMSO-treat mice. Lanes 2 and 1 (MCS-10%DMSO-RA treat mice and saline-treat mice) had no bands were found, the results to correspond with and .
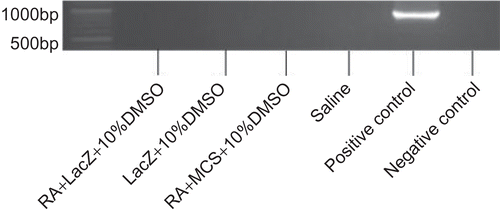
LacZ plasmid DNA was inexistence in blood circulatory system
To confirm whether plasmid DNA existed in the blood circulation system and expression, blood serum was analyzed for the presence of pORF-LacZ. LacZ was not detected in all groups (), which demonstrated that there was no LacZ plasmid DNA existing in the circulating system of mice 24 h after topically applied LacZ plasmid DNA on mouse ear skin.
Discussion
In this study, we have developed a novel method for topical skin gene delivery that utilized retinoic acid (RA) and DMSO as accelerants of gene transferred into skin transdermal penetration.
Here we made use of DMSO as a penetration enhancer rather than cationic lipids and cationic polymers, which are the most extensively investigated carriers for transdermal gene delivery. In addition, cationic compounds can also have more toxic side-effects in vitro and in vivo (CitationTam et al., 2000) than DMSO (CitationRobert and Vigenes, 2000). For example, when the skin was treated with lipoplexes, it caused the cells to change subsequently, which included cell shrinking, reduction of number of mitoses, and vacuolization in the cytoplasm (CitationLappalainen et al., 1994). Certain proteins such as protein kinase C may also be affected (CitationAberle et al., 1998). Furthermore, liposomal complexes also potentially induce inflammation (CitationNorman et al., 2000), which means the liposome may be unfit for transdermal treatment for inflammatory disease. Compared with cationic compounds, the delivery system with DMSO in our study has less systemic side-effects. In our study, we demonstrated that DNA can be transferred into skin, but we used a concentration of DMSO of 10% rather than 50% (CitationHeckert et al., 2002). At high concentrations DMSO can cause erythema and wheals of the stratum corneum and may denature some proteins (CitationWilliams and Barry, 2004). A foul odor on the breath caused by the metabolite of DMSO also makes people embarrassed. So, although high concentration of DMSO may have a more notable enhancement efficacy, it is beyond the scope of our study. The side-effects of DMSO can be minimized in low concentrations. A former study (CitationRephaeli, 1990) reported that DMSO can promote release of calcium existing in the cells, the viscosity of which is one of the influencing factor to cells differentiation. So this may be one of factors that increase the efficiency of transfection.
The higher transfection efficiency was related to depilation and retinoic acid increased the percentage of follicle cells and epidermic cells in anagen onset (CitationDomashenko et al., 2000). It is possible that the RA promote the epidermal cells differentiation and keep circumstances of lower keratinisation. A former study (CitationGhezzo and Pegoraro, 1981) also has confirmed that the RA can promote the epidermal cells differentiation. However, the mechanism of the effect of retinoic acid on transfection efficiency is further studied.
Sites of plasmid DNA expression in skin were reported in several studies (CitationYu et al., 1999; CitationDomashenko et al., 2000). In the present in vivo study, histochemical staining for β-galactosidase expression showed that the blue product was detected in skin specimens treated with lacZ plasmid in 10% DMSO, mainly located in the epidermis and hair follicle cells. Our results were consistent with the earlier research.
In the study of the plasmid DNA which existed in the blood circulatory system, 24 h after the last application, no target band in agarose gel was found following PCR analysis (), which is in accordance with the results of a former study reported by CitationBadea et al. (2005). The possibility is that the plasmid DNA could not pass through the blood vessel barrier to enter into the blood circulatory system, or the concentration of plasmid DNA in the blood was too low to be detected. The levels of plasmid DNA in serum collected after 8 h seemed very low, and so the levels after 24 h are much lower and may not be detected by general PCR analysis (CitationKang et al., 2004).
The major advantage of topical cutaneous gene delivery is that it can produce efficient transfection and potentially transfect large areas of skin using a non-invasive method. Moreover, it is of potential benefit to treat many dermatoses using therapeutic gene by a transdermal delivery system. Further studies are required to determine the pre-clinical utility of this model system.
Acknowledgements
The authors thank members of the State Key Laboratory of Biotherapy for help and discussion.
Declaration of interest
This study was supported by a grant from the State Key Basic Research and Development Plan (2010CB529900). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aberle, A.M., Tablin, F., Zhu, J., Walker, N.J., Gruenert, D.C., Nantz, M.H. (1998). A novel tetraester construct that reduces cationic lipid-associated cytotoxicity. Implications for the onset of cytotoxicity. Biochemistry. 37:6533–40.
- Abu-Abed, S., MacLean, G., Fraulon, V., Chambon, P., Ppetkovich, M., Dolle, P. (2002). Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26 B1 during murine organogenesis. Mech Devel. 110:173.
- Alexander, M.Y., Akhurst, R.J. (1995). Liposome-medicated gene transfer and expression via the skin. Hum Mol Genet. 4:2279–85.
- Badea, I., Verrall, R., Baca-Estrada, M. (2005). In vivo cutaneous interferon-gamma gene delivery using novel dicationic gemini surfactant-plasmid complexes. J Gene Med. 7:1200–14.
- Chase, H. (1954). Growth of the hair. Physiol Rev. 34:113–26.
- Chen, Y., Shen, Y., Guo, X. (2006). Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 24:455–60.
- Domashenko, A., Gupta, S., Cotsarelis, G. (2000). Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol. 18:420–3.
- Ghazizadeh, S., Harrington, R., Taichman, L. (1999). In vivo transduction of mouse epidermis with recombinant retroviral vectors: implications for cutaneous gene therapy. Gene Ther. 6:1267–75.
- Ghezzo, F., Pegoraro, L. (1981). Effects of retinoic acid on the fibrinolytic activity of HL60 human promyelocytic leukemia cells. Experientia. 37:425–6.
- Heckert, R.A., Elankumaran, S.., Oshop, G.L., Vakharia, V.N. (2002). A novel transcutaneous plasmid-dimethylsulfoxide delivery technique for avian nucleic acid immunization. Vet Immunol Immunopathol. 89:67–81.
- Kang, M.J., Kim, C.K., Kim, M.Y. (2004). Skin permeation, biodistribution, and expression of topically applied plasmid DNA. J Gene Med. 6:1238–46.
- Kawai, S., Nishizawa, M. (1984). New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 4:1172–1174.
- Kummet, T., Meyskens, F. (1983). Vitamin A: a potential inhibitor of human cancer. Sem Oncol. 10:281.
- Lappalainen, K., Jaaskelainen, I., Syrjanen, K., Urtti, A., Syrjanen, S. (1994). Comparison of cell proliferation and toxicity assays using two cationic liposomes. Pharm Res. 11:1127–31.
- Li, L., Hoffman, R.M. (1995). The feasibility of targeted selective gene therapy of the hair follicle. Nat Med. 1:705–6.
- Meykadeh, N., Mirmohammadsadegh, A., Wang, Z., Basner-Tschakarjan, E., Hengge, U.R. (2005). Topical application of plasmid DNA to mouse and human skin. J Mol Med (Berlin, Germany). 83:897–903.
- Nicolau, C., Sene, C. (1982). Liposome-mediated DNA transfer in eukaryotic cells: dependence of the transfer efficiency on the type of liposome used, and the hostcell stage. Biochim Biophys Acta. 721:185–90.
- Norman, J., Denham, W., Denham, D., Yang, J., Carter, G., Abouhamze, A., Tamahill, CL., Mackay, SL., Moldawer, LL. (2000). Liposome-mediated, nonviral gene transfer induces a systemic inflammatory response which can exacerbate pre-existing inflammation. Gene Ther. 7:1425–30.
- Paus, R., Stenn, R., Link, R. (1990). Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 122:777–84.
- Rephaeli, A. (1990). The role of calcium in differentiation of leukemic cell lines[J]. Cancer Biochem Biophys. 11:119.
- Robert, Vignes. (2000). Dimethyl sulfoxide (DMSO)—A “New” Clean, Unique, Superior solvent. Acs Presentation National Meeting 8/20–8/24.Washington, DC.
- Tam, P., Monck, M., Lee, D. (2000). Stabilized plasmid-lipid particles for systemic gene therapy. Gene Ther. 7:1867–74.
- Williams, A.C., Barry, B.W. (2004). Penetration enhancers. Adv Drug Deliv Rev. 56:603–18.
- Wilson, C., Cotsarelis, G., Wei, ZG., Fryer, E., Margolis-Fryer, J., Ostead, M. (1994). Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation. 55:127–36.
- Yu, W.H., Kashani-Sabet, M., Liggitt, D., Moore, D., Heath, T.D., Debs, R.J. (1999). Topical gene delivery to murine skin. J Invest Dermatol. 112:370–5.