Abstract
The aim of this study was to evaluate the ability of TMC, with different degrees of quaternization, to increase insulin absorption in vivo following nasal and rectal administration in rats. Two batches of TMC with different degrees of quaternization (TMC-L, 12.3% quaternized and TMC-H, 61.2% quaternized) and chitosan hydrochloride were administered intranasally (0.25 and 0.5% w/v) and rectally (0.5% w/v) with insulin (4 IU/kg body weight), at a pH of 4.40 and 7.40, in rats. Blood samples were taken over a period of 2 h for measurement of blood glucose levels and plasma insulin levels. Local toxicity evaluation was done by histological examination of the nasal and rectal epithelia. At pH 4.40 all these polymers were able to increase nasal and rectal insulin absorption, compared to the control groups. However, at a pH of 7.40, only TMC-H was able to increase the nasal and rectal absorption of insulin. These results relate to the insolubility of chitosan hydrochloride at neutral pH values, while the charge density of TMC-L is still too low for any significant interaction at pH 7.40. Histological evaluation of the nasal and rectal eptihelia shows no changes in the morphology of the cells after exposure to these polymers. Only slight congestion of the nasal submucosa was observed and all these polymers led to a mild increase in mucus secretion at pH 4.40. Highly quaternized TMC proves to be a potent absorption enhancer in vivo, especially at neutral pH values where chitosan salts are ineffective.
Introduction
The absorption enhancing properties of chitosan and chitosan salts, such as chitosan glutamate and chitosan hydrochloride, has been well documented in the past few years (CitationArtursson et al., 1994; CitationBorchard et al., 1996; CitationSchipper et al., 1996; Citation1997; CitationLeußen et al., 1997; CitationFelt et al., 1998; CitationIllum, 1998; Kotzé et al., Citation1999a; CitationAvadi et al., 2005). Very significant is their ability to increase the absorption of various peptide drugs both in vitro and in vivo. The in vitro transport of the peptide 9-desglycinamide, 8-L-arginine vasopressin (DGAVP), was increased significantly after administration with chitosan glutamate at pH 5.60 across intestinal epithelial cells (Caco-2 cell monolayers) (CitationLueßen et al., 1997). Nasal administration of insulin in a chitosan solution had a higher transmucosal flux and was shown to be an effective absorption enhancer across nasal mucosa (CitationYu et al., 2004). Similarly, the intra-duodenal application of buserelin and chitosan hydrochloride in a gel formulation at pH 6.70 increased the absolute bioavailability of buserelin from 0.1 ± 0.1% to 5.1 ± 1.5% in in vivo experiments in rats (Leußen et al., 1996), while the nasal administration of insulin with chitosan glutamate at pH 4.40 resulted in a pronounced reduction in blood glucose levels in rats and sheep (CitationIllum et al., 1994). Chitosan oligomers have been investigated for intestinal absorbtion of insulin and the absorbtion of insulin in the jejunum was significantly improved in the presence of chitosan hexamer (CitationGao et al., 2007; Citation2008). These increases in peptide drug absorption could be attributed to the effect of chitosan on the integrity of the epithelial tight junctions (CitationArtursson et al., 1994; CitationIllum et al., 1994; CitationBorchard et al., 1996; Leußen et al., 1996; Citation1997; CitationSchipper et al., 1996; Citation1997; CitationFelt et al., 1998; CitationIllum, 1998; Kotzé et al., Citation1999b; CitationThanou et al., 2001). Chitosan opens the tight junctions to allow paracellular transport of large hydrophilic compounds. This interaction with the tight junctions is believed to be an interaction of the positively charged amino group on the C-2 position of chitosan, with negatively charged sites on the cell surfaces and tight junctions (CitationArtursson et al., 1994; CitationSchipper et al., 1996; Citation1997; Kotzé et al., Citation1999b). Recent studies have also emphasized the applicability of chitosan microspheres or nanoparticles for peptide delivery (CitationWang et al., 2006; CitationZhang et al., 2008; CitationSonaje et al., 2009; CitationWei et al., 2010).
However, chitosan is a weak base and a certain amount of acid is required to transform the glucosamine units into the positively charged, water-soluble form. It has been shown that chitosan is ineffective as an absorption enhancer in neutral environments (Kotzé et al., Citation1999a). At neutral pH values, the chitosan molecules lose their charge and precipitate from solution. Therefore, possibilities for the potential use of chitosan in more neutral and basic environments such as those found in the nasal and rectal cavities and large intestine and colon are limited.
It has been shown that the partially quaternized derivative of chitosan, N-trimethyl chitosan chloride (TMC), was also able to increase the paracellular transport of several hydrophilic compounds (Kotze et al., Citation1997b; Citation1998) and peptide drugs such as DGAVP (Mw 1412), buserelin (Mw 1300), and insulin (Mw 5778) in Caco-2 cell monolayers (Kotzé et al., Citation1997a; CitationSadeghi et al., 2008; CitationYin et al., 2009). TMC has also been shown to be an effective absorption enhancer of the peptide drug sCT in vivo administered nasally and orally to rats (CitationDu Plessis et al., 2010). TMC has proven to be very soluble over a wide pH range (pH 1–9) up to 10% w/v concentrations, even at degrees of quaternization as low as 10% (CitationKotzé et al., 1998). Furthermore, it was found that the charge density of TMC, as determined by its degree of quaternization, plays an important role in its ability to act as an absorption enhancer in vitro, especially in neutral environments. Highly quaternized TMC (60%) is able to increase the permeation of [14C]-mannitol across Caco-2 cell monolayers 31–48-fold at 0.05–1.5% w/v concentrations at a pH of 7.40, whereby low quaternized TMC (12%) and chitosan hydrochloride do not show any increases in the transport rate of this hydrophilic marker at pH 7.40. It was concluded that the charge density of highly quaternized TMC is sufficient for interaction with the negative charged sites on the cell membranes and tight junctions, while the charge density of the low quaternized TMC is still too low for any significant interaction (Kotzé et al., Citation1999b; CitationThanou et al., 2000). Various studies have proven the applicability of TMC nanoparticles in peptide delivery (CitationMi et al., 2008; CitationSadeghi et al., 2008).
The aim of the present investigation was to evaluate the effect of the degree of quaternization of TMC (low and high) on the in vivo absorption of insulin, after nasal and rectal delivery in rats at different pH values (pH 4.40 and 7.40), and to compare the effect of these polymers with the absorption enhancing ability of chitosan hydrochloride.
Materials and methods
Materials
Two batches of N-trimethyl chitosan chloride (TMC), one with a low degree of quaternization (TMC-L: 12.3% quaternized) and one with a high degree of quaternization (TMC-H: 61.2% quaternized), were synthesized from sieved fractions (< 500 μm) of chitosan (degree of acetylation ca. 7%, Mw 148,000 Da) (Pronova Biopolymer, Drammen, Norway) based on methods described previously (CitationDomard et al., 1986; CitationSmith et al., 1992; CitationSieval et al., 1998). The degree of quaternization of these polymers was calculated from 1H-NMR spectra (CitationSieval et al., 1998). Chitosan hydrochloride (SEACURE CL 210) was kindly provided by Pronova Biopolymer. Crystalline biosynthetic human insulin (24.5 IU/mg) was a gift from Lilly Research Laboratories (Indiana, USA). Hemaglucotest sticks (Glucostix®) were purchased from Boehringer Ingelheim Pharmaceuticals (Randburg, South Africa), and ImmunoChemTM Coated Tube Insulin-125I Radioimmunoassay (RIA) Kits were obtained from ICN Pharmaceuticals (California, USA). All other reagents and materials used were of analytical grade.
Preparation of polymer formulations
For nasal studies, stock solutions (1.0 and 0.5% w/v) of chitosan hydrochloride, TMC-L, and TMC-H were prepared in 14.65 mM phosphate buffer of pH 7.40. Because of the insolubility of chitosan hydrochloride at pH 7.40 (only soluble at pH values lower than 6.6), the resulting polymer preparation was used as a dispersion. A stock solution of insulin (80 IU/ml), containing 0.5% w/v albumin, was prepared in a similar way. Albumin was added to minimize possible adsorption of insulin on plastic and glassware used in preparing the different formulations. This stock solution of insulin was diluted in equal parts with the respective stock solutions of the polymers to obtain a final insulin concentration of 40 IU/ml in 0.25 and 0.5% w/v concentrations of the individual polymers. The pH of these test formulations was readjusted to 7.40 if necessary with 0.1 M NaOH. For experiments at pH 4.40 the pH of the respective formulations was adjusted with 0.1 M HCl. In a similar way to as described above, test formulations containing 4 IU/ml insulin in 0.5% w/v concentrations of the individual polymers were prepared at pH 4.40 and 7.40 for rectal studies.
Test formulations (control solutions and polymer solutions/dispersions) were prepared freshly for each day on which animal experiments were performed immediately before administration. This was deemed necessary to maintain insulin solubility and integrity.
Rat absorption studies
All investigations using experimental rats adhered to the ‘Principles of Laboratory Animal Care’ (NIH publication # 85-23, revised 1985) and were approved by the Local Institutional Animal Experimentation Ethical Committee. Healthy male Sprague Dawley rats with a body weight of 240–260 g were fasted for 12 h, but water was supplied ad libitum. Anesthesia was induced and maintained with mixtures of halothane in medical oxygen. The apparatus for this procedure consisted of two 5-l plastic bags, containing 2 or 4% halothane in medical oxygen, each connected to one end of a three-way valve. A rubber jacket fitted to the remaining end was fitted securely over the head of the rat to supply one of the two halothane mixtures as needed. The artery carotis communis was cannulated for blood sampling and fluid replacement.
Nasal studies
The test formulations of insulin (40 IU/ml) in the respective polymers solutions were administered at a dose of 100 μl/kg body weight in the left nasal cavity of the rats to receive a final insulin concentration of 4 IU/kg body weight. The test formulations at pH 4.40 contained insulin and 0.25 or 0.5% w/v concentrations of chitosan hydrochloride, TMC-L, and TMC-H. At pH 7.40 the test formulations contained insulin with 0.5% w/v concentrations of the respective polymers. Control experiments with insulin doses of 4 IU/kg body weight without any absorption enhancers, at pH 4.40 and 7.40, were run in a similar way as described above. To exclude the possibility of ionic interactions between insulin (pKa 5.4) and TMC, special care was taken to note that clear solutions were administered. Six rats were used for each experiment. Blood samples (400 μl) were collected at various time intervals up to 2 h post-administration.
Rectal studies
Test formulations of insulin (4 IU/ml) at pH 4.40 and 7.40, with and without 0.5% w/v concentrations of chitosan hydrochloride, TMC-L and TMC-H, were administered rectally to the rats with a syringe fitted to a blunt needle at a dose of 1000 μl/kg body weight through a suitable rubber plunger fitted into the rectum of the rats. Clear solutions were administered to exclude possible ionic interactions between insulin and TMC. Six rats were used for each treatment. Blood samples (400 μl) were collected at various time intervals for 2 h post-administration.
Histological studies
Two rats were prepared as described above, without cannulation of the artery carotis communis, for each nasal and rectal formulation administered. After 60 min post-administration of the different nasal and rectal formulations with 0.5% w/v concentrations of the respective polymers (without insulin), different cuts were made from the nasal and rectal epithelia and decalcified with an 8% formic acid solution. Specimens were embedded and cuts of 4 μm thickness were made with a sliding microtome. The cuts were examined by light microscopy for changes to the nasal and rectal epithelia. Histopathological changes in both the mucosal and epithelial tissues were investigated. The formation of lesions and increased mucus production, if any, were determined visually and microscopically by the animal pathologist.
Determination of blood glucose and plasma insulin concentrations
The blood glucose levels of the rats were measured immediately after blood samples were collected. A single drop of blood was put on a hemoglucotest stick and it was left for 30 s before the blood was blotted with a soft tissue paper. The stick was placed in a Glucometer® II reflectance meter (Boehringer Ingelheim Pharmaceuticals, Randburg, South Africa) and a reading in mmol/L was obtained after 20 s. Plasma insulin concentrations were determined by a radioimmunoassay (RIA) method with an ImmunoChemTM Coated Tube Insulin-125I RIA kit. Results were expressed in m IU/l.
Data analysis and statistical evaluation
Averages and standard deviations for the blood glucose levels (mmol/l) and plasma insulin concentrations (m IU/l) measured were calculated for each nasal and rectal treatment. The area under the individual plasma insulin concentration-time curves from 0–120 min was calculated with the trapezoidal rule for statistical analysis. Statistical analyses between groups were performed using Student’s t-test and one-way analysis of variance (ANOVA). p-values of 0.05 or less were considered to indicate statistically significant differences.
Results
Nasal absorption of insulin
The effect of the insulin formulations with chitosan hydrochloride, TMC-L, and TMC-H, at pH 4.40 and 7.40, on the absorption of insulin from the nasal cavity of rats is shown in . show the plasma insulin concentration-time curves for the 0.25 and 0.5% w/v concentrations of the different polymers at pH 4.40, while represent similar results for 0.5% w/v concentrations of these polymers at a pH of 7.40. Nasal administration of insulin alone (4 IU/kg body weight) resulted in no increase in plasma insulin concentrations. The plasma insulin concentration remains low and stable throughout the 2 h study period. Similarly, no decrease in blood glucose levels could be found (results not shown). These results clearly indicate that insulin alone is not absorbed across the nasal epithelia and that a low pH (4.40) does not alter the permeability of the nasal epithelia, at least for the study period of 2 h.
Figure 1. Plasma insulin concentration-time curve after nasal administration of chitosan hydrochloride, TMC-L and TMC-H at a concentration of 0.25% w/v (a) and at a concentration of 0.5% w/v (b) at pH 4.40 and at 0.5% w/v at a of pH7.4 (c). Each point represents the mean of six experiments.
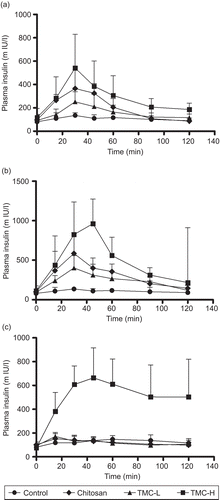
However, when insulin was administered in the chitosan hydrochloride, TMC-L and TMC-H formulations at a pH of 4.40, a sharp and rapid increase in plasma insulin concentrations, peaking at 30 min, were found (). These increases found in the plasma insulin concentrations related very well with blood glucose levels which were reduced by 28, 32, and 27%, respectively, for chitosan hydrochloride, TMC-L, and TMC-H at 0.25% w/v concentrations and 38, 20, and 19% at 0.5%w/v concentrations of the respective polymers.
Compared with the plasma insulin concentration obtained 30 min post-administration in the formulation containing only insulin, administration of insulin with a 0.25% w/v concentration of chitosan hydrochloride resulted in a 2.7-fold increase in the baseline plasma insulin concentration at the same time point, while TMC-L and TMC-H increases the baseline plasma insulin concentration 1.9-fold and 4.0-fold, respectively. At 0.5% w/v concentrations of these polymers the baseline plasma insulin concentration was increased 4.3-, 2.9-, and 6.1-fold for chitosan hydrochloride, TMC-L, and TMC-H, respectively. After 30 min the plasma insulin concentrations started to decrease towards the initial levels that were generally reached after 2 h.
These results are indicative of a polymer concentration-related absorption of insulin. Apparently, it also seems that TMC-H is the most effective polymer in enhancing nasal insulin absorption, followed by chitosan hydrochloride and TMC-L. However, no statistical significant differences between the effect of 0.25 and 0.5% w/v concentrations of the respective polymers could be found. Similarly, no difference between the effect of the different polymers, at both concentrations, could be found. The reason for this result may be the relative high standard deviations founded throughout the study.
In contrast to the results obtained at pH 4.40, only TMC-H (0.5% w/v) was able to increase the nasal absorption of insulin in the formulations with a pH of 7.40. (). Neither chitosan hydrochloride nor TMC-L (0.5% w/v) was able to increase the nasal absorption of insulin at a pH of 7.40. In agreement with the results found at pH 4.40, administration of insulin with a 0.5% w/v concentration of TMC-H resulted in a sharp and rapid increase in the plasma insulin concentration, peaking at 45 min post-administration. This is in agreement with a 48 ± 13% reduction in blood glucose levels observed at 45 min post-administration of the TMC-H formulation. At this time point the plasma insulin concentration was increased 4.8-fold compared to the baseline concentration at the same time. However, as shown in the plasma insulin concentration remains high after 45 min post-administration and only a slow decrease towards the initial insulin levels was found over the remaining 2-h study period.
Rectal absorption of insulin
The effect of the insulin formulations with and without 0.5% w/v concentrations of chitosan hydrochloride, TMC-L, and TMC-H at pH 4.40 and 7.40 on the absorption of insulin from the rectal route in rats is shown in . depicts the plasma insulin concentration-time curves for the different polymers at pH 4.40 and 7.40, respectively. Administration of formulations with insulin (4 IU/kg body weight) alone, at pH 4.40 and 7.40, resulted in no increase in plasma insulin concentrations. The plasma insulin levels remains low and fairly stable over the 2-h study period. Accordingly, no decreases in blood glucose levels were found. From these results it is concluded that insulin alone is not absorbed across the rectal epithelia and that a low pH of 4.40 does not alter the permeability of the rectal epithelia for insulin, at least for the study period of 2 h.
Figure 2. Plasma insulin concentration-time curve after rectal administration of chitosan hydrochloride, TMC-L and TMC-H (0.5% w/v) at pH 4.40 (a) and pH 7.40 (b). Each point represents the mean of six experiments.
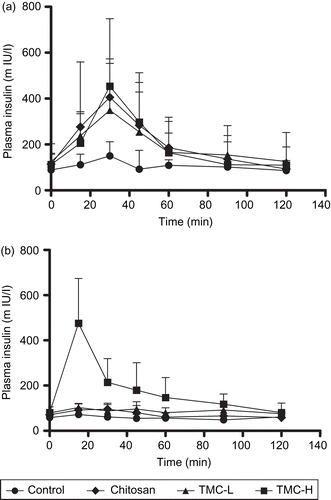
When insulin was administered in the formulations with chitosan hydrochloride, TMC-L, and TMC-H at a pH of 4.40, a sharp and rapid increase in plasma insulin concentrations was found, peaking at 30 min post-administration (). This related well with blood glucose levels which were reduced by 38% (chitosan hydrochloride), 22% (TMC-L), and 22% (TMC-H). Compared with the plasma insulin concentration 30 min after administration of insulin alone, administration of insulin with a 0.5% w/v concentration of chitosan hydrochloride resulted in a 2.7-fold increase in the baseline plasma insulin concentration, while TMC-L and TMC-H increased the baseline plasma insulin concentration 2.3- and 3.0-fold, respectively, at the same time point. After 30 min the plasma insulin concentrations started to decrease towards their initial levels that were generally reached 90 min after administration of the different formulations.
In contrast to the results obtained at pH 4.40, only TMC-H was able to increase the rectal absorption of insulin at pH 7.40 (), reaching the maximum insulin concentration ∼ 22.5 min post-administration. This is in agreement with a 29 ± 7% reduction in blood glucose levels observed at 22.5 min post-administration of the TMC-H formulation. Compared with the plasma insulin concentration 22.5 min after administration of insulin alone, administration of insulin with a 0.5% w/v concentration of TMC-H resulted in a 6.7-fold increase in the baseline plasma insulin concentration. After 22.5 min the plasma insulin concentration started to decrease towards the initial level found with the administration of insulin alone. Neither TMC-L nor chitosan hydrochloride was able to increase the absorption of insulin at pH 7.40.
Histological evaluation of nasal and rectal epithelia
summarizes the histological effects found on the nasal and rectal submucosa and epithelia, as well as observations made in the nasal and rectal lumen, after exposure to chitosan hydrochloride, TMC-L, and TMC-H (0.5% w/v concentrations). The effects of chitosan hydrochloride on the nasal and rectal epithelia after 60 min exposure, both at pH 4.40 and 7.40, were negligible. No cell loss was observed and cilia were still present on the luminal surface. The nuclei appeared normal in both treated and untreated nasal tissue at both pH values. At a pH of 4.40 the submucosa appeared slightly congested, while a slight increase in mucus production was observed at pH 4.40 and 7.40. The rectal epithelia and submucosa appeared normal and no lesions were found after exposure to chitosan hydrochloride at both pH values. Similar results were obtained after exposure of the nasal and rectal epithelia to TMC-L. At a pH of 4.40 the nasal submucosa was slightly congested, while a slight increase in mucus production was observed at both pH values. The rectal epithelia and submucosa appeared normal and no lesions were found.
Table 1. Histological effects of chitosan hydrochloride, TMC-L, and TMC-H (0.5% w/v) on nasal and rectal epithelia.
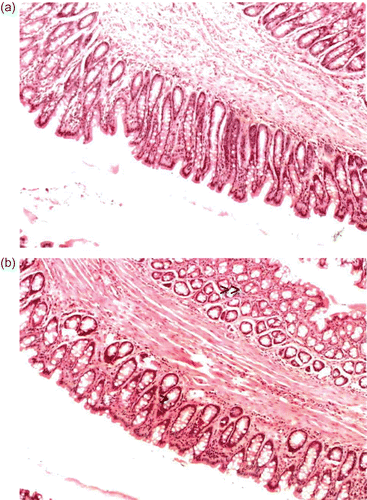
In contrast to the results obtained with chitosan hydrochloride and TMC-L, TMC-H has a more pronounced effect on the nasal and rectal epithelia at pH 4.40 and 7.40. Exposure of a 0.5% TMC-H to the nasal mucosa resulted in mild congestion and edema of the submucosa with very slight flattening of epithelial cells. Evidence of a mild increase in mucus production was found at both pH values. However, these effects did not occur to a large extent and are considered as very mild. Similar results were obtained after exposure of the rectal mucosa to TMC-H, as evident from
Figure 3. The effect of chitosan hydrochloride on rectal epithelia. (a) Untreated rectal epithelia. (b) Rectal epithelia after exposure (60 min) to 0.5% w/v TMC-H at pH 7.40. Note the mild congestion and edema in the submucosa (indicated by the double arrow) and the slight flattening of the epithelial cells (indicated by the arrow).
Discussion
Nasal and rectal drug delivery represents an interesting alternative for the parenteral route of administration of drugs that show poor oral bioavailabilty, such as peptide and protein drugs. However, the absorption of nasally and rectally administered hydrophilic drugs are severely limited by the low permeability of the mucosa to these drugs. Many efforts have been made to enhance the uptake of poorly absorbed drugs. The co-administration of absorption enhancers is one of the most studied approaches (CitationSmith et al., 1992; CitationMackay et al., 1997). However, in most cases the enhancement of drug absorption is accompanied by mucosal injury induced by the enhancer (CitationMuranishi, 1990; CitationTomita et al., 1996; CitationKhafagy et al., 2007). Although the nasal mucosa has the advantage of a large epithelial surface due to the presence of numerous microvilli, its rapid mucociliary clearance mechanism, that can reduce the bioavailability of intranasally administered drugs markedly, may be a major drawback (CitationFelt et al., 1998). A possible strategy to overcome this problem is to retard clearance of the formulation from the nasal cavity and thereby prolong contact between the drug and the mucosa. This can be achieved by employing bioadhesive systems. The good mucoadhesive properties of chitosan (CitationLehr et al., 1992), probably mediated by ionic interaction between a positive amino group of the polysaccharide and the negative residues of sialic acid in mucus, together with its absorption enhancing properties, makes it a promising candidate for the development of nasal and rectal delivery systems. Chitosan is able to swell and form a gel-like layer in an aqueous environment by absorbing water from the mucus layer that is favorable for the interpenetration of the polymer and glucoprotein chains of the mucus. However, the use of chitosan as an absorption enhancer is limited in neutral and basic environments, due to the presence of an uncharged amino group at these conditions. In these regards, chitosan derivatives such as TMC, with a quaternary amino group, might prove useful for enhancing drug absorption at neutral and basic pH values.
The present study clearly shows that both chitosan hydrochloride and TMC, with different degrees of quaternization, cause a remarkable increase in the nasal and rectal absorption of insulin at a low pH value. Because of their positive charge, cationic macromolecules such as chitosan and TMC can interact with the anionic components of the glucoproteins on the surface of epithelial cells. Cationic macromolecules can also displace cations from electronegative sites on cell membranes, that require co-ordination with cations for dimensional stability (CitationSiegel & Daly, 1966). It is also known that the interior of the tight junction channel is hydrated and contains fixed negative sites. Changes in the concentration of certain ions in the pore could result in alteration in tight junction resistance, leading to opening of the pore with increased paracellular permeability (CitationMadara et al., 1987). It has been shown that chitosan is able to interact with the opening mechanism of the tight junctions as observed by a decrease in ZO-1 proteins and a change in the cytoskeletal protein F-Actin from a filamentous to a globular structure (CitationArtursson et al., 1994; CitationSchipper et al., 1997). Previous studies confirm that TMC probably acts in a similar way to open the tight junctions, thereby allowing for paracellular transport of hydrophilic compounds (Kotzé et al., Citation1997b; Citation1998).
However, at a neutral pH value chitosan hydrochloride and low quaternized TMC (TMC-L, 12% quaternized) has proven to be ineffective in increasing insulin absorption, whereas highly quaternized TMC (TMC-H, 61% quaternized) was very effective in increasing insulin absorption after both nasal and rectal administration. At a pH of 7.40 chitosan hydrochloride probable exists in a more coiled configuration with no protonated amino groups for interaction with the cell surfaces or tight junctions. With TMC-L, the charge density has not reached the threshold concentration to induce interaction with the anionic components of the glucoproteins at the surfaces of the cells or with the fixed negative charges within the aqueous tight junction pores. Additionally, the attached methyl groups may partially shield the positive charge from any significant interaction with the cell membranes or tight junctions. TMC-H, on the other hand, has a much higher proportion of quaternary amino groups that is sufficient to interact with anionic components on the cell membranes or the negative sites within the tight junctions. Whether a degree of quaternization of 60% of TMC is the optimum degree of quaternization, for absorption enhancement in neutral and basic environments, is still unknown. Further investigation has to establish if there is a direct relationship between the degree of quaternization of TMC, its efficacy as an absorption enhancer in neutral and basic environments, and the local toxicity profile. Although there may be a possibility of ionic interactions between insulin (pKa 5.4) and TMC, it is most unlikely to occur mainly due to size consideration and configuration aspects of both molecules. Both molecules are macromolecular compounds that may exclude paracellular transport of possible formed complexes.
Exposure of the rat nasal and rectal mucosae to solutions of chitosan hydrochloride, TMC-L, and TMC-H in concentrations of 0.5% failed to cause major changes in the morphology of these mucosae as compared to the controls. These observations indicate that the cell membranes remain undamaged and functionally intact after exposure to these polymers. This is in agreement with previous studies about morphological effects of chitosan on nasal epithelium (CitationAspden et al., 1995; Citation1996; Citation1997; CitationIllum, 1998; CitationHaffejee et al., 2001). Results obtained where Caco-2 cell monolayers were tested with the propidium iodide nucleic stain for their viability after 4 h of exposure to TMC (40 and 60% quaternized) in concentrations up to 1% w/v, showed that the monolayers excluded the nucleic stain, demonstrating that TMC does not effect the cell viability (CitationThanou et al., 2001). Various studies have confirmed that TMC is a safe mucoadhesive polymer and absorption enhancer for hydrophilic macromolecules across various mucosal sites (CitationAmidi et al., 2006; Citation2010; CitationVerheul et al., 2008). According to previous studies the cytotoxicity of TMC increases with higher degrees of quaternization and molecular weight (CitationYin et al., 2009; CitationAmidi et al., 2010). In this study TMC-L had less pronounced effects on epithelial cells when compared to TMC-H, but there remains a lack of toxicity in both rectal and nasal mucosae.
In summary, our study shows that the aqueous insolubility of chitosan and chitosan salts such as chitosan hydrochloride prevents these polymers from being effective as absorption enhancers in neutral environments. The degree of quaternization of TMC is demonstrated to play an important role in its ability to act as an absorption enhancer. Highly quaternized TMC proves to be a potent absorption enhancer in vivo as established for nasal and rectal insulin administration in rats, especially in neutral environments where normal chitosan salts are ineffective.
Acknowledgements
The authors wish to thank John Beliën and Alex Sieval for their help in the synthesis of TMC.
Declaration of interest
This study was supported in part by grants from The South African Druggist Group and ELI Lilly Company, South Africa. The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amidi, M., Mastrobattista, E., Jiskoot, W., Hennik, W.E. (2010). Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 62:59–82.
- Amidi, M., Romeijn, S.G., Borchard, G., Junginger, H.E., Hennik., W.E., Jiskoot, W. (2006). Preparation and characterisation of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Contr Rel. 111:107–16.
- Artursson, P., Lindmark, T., Davis, S.S., Illum, L., (1994). Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res. 11:1358–61.
- Aspden, T.J., Adler, J., Davis, S.S., Skaugrud, Ø., Illum, L. (1995). Chitosan as a nasal delivery system: evaluation of the effect of chitosan on mucociliary clearance rate in the frog palate model. Int J Pharm. 122:69–78.
- Aspden, T.J., Illum, L., Skaugrud, Ø. (1996). Chitosan as a nasal delivery system: evaluation of insulin absorption enhancement and effect on nasal membrane integrity using rat models. Eur J Pharm Sci. 4:23–31.
- Aspden, T.J., Mason, J.D., Jones, N.S., Lowe, J., Skaugrud, O., Illum, L. (1997). Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J Pharm Sci. 86:509–13.
- Avadi, M.R., Jalali, A., Sadeghi, A.M.M., Shamimi, K., Bayati, K.H., Nahid, E., Dehpour, A.R., Rafiee-Tehrani, M. (2005). Diethyl methyl chitosan as an intestinal paracellular enhancer: ex vivo and in vivo studies. Int J Pharm. 293:83–9.
- Borchard, G., Leußen, H.L., de Boer, A.G., Verhoef, J.C., Lehr, C., Junginger, H.E. (1996). The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Contr Rel. 39:131–8.
- Domard, A., Rinaudo, M., Terrassin, C. (1986). New method for the quaternization of chitosan. Int J Biol Macromol. 8:105–7.
- du Plessis, L.H., Lubbe, J., Strauss, T., Kotzé, A.F. (2010). Enhancement of nasal and intestinal calcitonin delivery by the novel Pheroid fatty acid based delivery system, and by N-trimethyl chitosan chloride. Int J Pharm. 385:181–6.
- Felt, O., Buri, P., Gurny, R. (1998). Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 24:979–93.
- Gao, X., Wu, B., Zhang, Q., Chen, J., Zhu, J., Zhang, W., Rong, Z., Chen, H., Jiang, X. (2007). Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Contr Rel. 121:156–67.
- Gao, Y., He, L., Katsumi, H., Sakane, T., Fujita, T., Yamamoto, A. (2008). Improvement of intestinal absorption of insulin and water-soluble macromolecular compounds by chitosan oligomers in rats. Int J Pharm. 359:70–8.
- Haffejee, N., Du Plessis, J., Muller, D.G., Schultz, C., Kotzé, A.F., Goosen, C. (2001). Intranasal toxicity of selected absorption enhancers. Pharmazie. 56:882–8.
- Illum, L. (1998). Chitosan and its use as a pharmaceutical excipient. Pharm Res. 15:1326–31.
- Illum, L., Farraj, N.F., Davis, S.S. (1994). Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res. 11:1186–9.
- Khafagy, E., Morishita, M., Onuki, Y., Takayama, K. (2007). Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Deliv Rev. 59:1521–46.
- Kotzé, A.F., de Leeuw, B.J., Leußen, H.L., de Boer, A.G., Verhoef, J.C., Junginger, H.E. (1997a). Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: in vitro evaluation in Caco-2 cell monolayers. Int J Pharm. 159:243–53.
- Kotzé, A.F., Leußen, H.L., de Boer, A.G., Verhoef, J.C., Junginger, H.E. (1999a). Chitosan for enhanced intestinal permeability: prospects for derivatives soluble in neutral and basic environments. Eur J Pharm Sci. 7:145–51.
- Kotzé, A.F., Luessen, H.L., de Leeuw, B.J., de Boer, A.G., Verhoef, J.C., Junginger, H.E. (1998). Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J Contr Rel. 51:35–46.
- Kotzé, A.F., Luessen, H.L., de Leeuw, B.J., de Boer, B.G., Verhoef, J.C., Junginger, H.E. (1997b). N-trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: in vitro evaluation in intestinal epithelial cells (Caco-2). Pharm Res. 14:1197–202.
- Kotzé, A.F., Thanou, M.M., Leußen, H.L., de Boer, A.G.D., Verhoef, J.C., Junginger, H.E. (1999b). Enhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: in vitro evaluation in intestinal epithelial cells (Caco-2). J Pharm Sci. 88:253–257.
- Lehr, C., Bouwstra, J.A., Spies, F., Onderwater, J., van het Noordeinde, J., Vermeij-Keers, C., van Munsteren, C.J., Junginger, H.E. (1992). Visualization studies of the mucoadhesive interface. J Contr Rel. 18:249–60.
- Luessen, H.L., de Leeuw, B.J., Langemeyer, M.W., de Boer, A.B., Verhoef, J.C., Junginger, H.E. (1996). Mucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm Res. 13:1668–72.
- Leußen, H.L., Rentel, C., Kotzé, A.F., Lehr, C., de Boer, A.G., Verhoef, J.C., Junginger, H.E. (1997). Mucoadhesive polymers in peroral peptide drug delivery. IV. Polycarbophil and chitosan are potent enhancers of peptide transport across intestinal mucosae in vitro. J Contr Rel. 45:15–23.
- Mackay, M., Phillips, J., Hastewell, J. (1997). Peptide drug delivery: colonic and rectal absorption. Adv Drug Deliv Rev. 28:253–73.
- Madara, J.L., Moore, R., Carlson, S. (1987). Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol. 253:C854–61.
- Mi, F.-L., Wu, Y.-Y., Lin, Y.-H., Sonaje, K., Ho, Y.-C., Chen, C.-T., Juang, J.-H., Sung, H.-W. (2008). Oral delivery of peptide drugs using nanoparticles self-assembled by poly(γ-glutamic acid) and a chitosan derivative functionalized by trimethylation. Bioconjugate Chem. 19:1248–55.
- Muranishi, S. (1990). Absorption enhancers. Crit Rev Ther Drug Carrier Syst. 7:1–33.
- Sadeghi, A.M.M., Dorkoosh, F.A., Avadi, M.R., Weinhold, M., Bayat, A., Delie, F., Gurny, R., Larijani, B., Rafiee-Tehrani, M., Junginger, H.E. (2008). Permeation enhancer effect of chitosan and chitosan derivatives: comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur J Pharm Biopharm. 70:270–8.
- Schipper, N.G., Olsson, S., Hoogstraate, J.A., deBoer, A.G., Varum, K.M., Artursson, P. (1997). Chitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharm Res. 14:923–9.
- Schipper, N.G., Varum, K.M., Artursson, P. (1996). Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res. 13:1686–92.
- Siegel, S.M., Daly, O. (1966). Regulation of betacyanin efflux from beet root by poly-L-lysine, Ca-ion and other substances. Plant Physiol. 41:1429–34.
- Sieval, A.B., Thanou, M., Kotzé, A.F., Verhoef, J.C., Brussee, J., Junginger, H.E. (1998). Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydrate Polym. 36:157–65.
- Smith, P.L., Wall, D.A., Gochoco, C.H., Wilson, G. (1992). (D) Routes of delivery: case studies: (5) Oral absorption of peptides and proteins. Adv Drug Deliv Rev. 8:253–90.
- Sonaje, K., Lin, Y.-H., Juang, J.-H., Wey, S.-W., Chen, C.-T., Sung, H.-W. (2009). In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials. 30:2329–39.
- Thanou, M., Verhoef, J.C., Junginger, H.E. (2001). Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 52:117–26.
- Thanou, M.M., Kotzé, A.F., Scharringhausen, T., Lueßen, H.L., de Boer, A.G., Verhoef, J.C., Junginger, H.E. (2000). Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J Contr Rel. 64:15–25.
- Tomita, M., Hayashi, M., Awazu, S. (1996). Absorption-enhancing mechanism of EDTA, caprate, and decanoylcarnitine in Caco-2 cells. J Pharm Sci. 85:608–11.
- Verheul, R.J., Amidi, M., van der Wal, S., van Riet, E., Jiskoot, W., Hennik, W.E. (2008). Synthesis, characterisation and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials. 29:3642–9.
- Wang, L.-Y., Gu, Y.-H., Su, Z.-G., Ma, G.H. (2006). Preparation and improvement of release behaviour of chitosan micropsheres containing insulin. Int J Pharm. 311:187–95.
- Wei, W., Ma, G.-H., Wang, L.-Y., Wu, J., Su, Z.-G. (2010). Hollow quaternized chitosan micropsheres increase the therapeutic effect of orally administered insulin. Acta Biomaerilia. 6:205–9.
- Yin, L., Ding, J., He, C., Cui, L., Tang, C., Yin, C. (2009). Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 20:5691–700.
- Yu, S., Zhao, Y., Wu, F., Zhang, X., Lu, W., Zhang, H., Zhang, Q. (2004). Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int J Pharm. 281:11–23.
- Zhang, X., Zhang, H., Wu, Z., Wang, Z., Niu, H., Li, C. (2008). Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur J Pharm Biopharm. 68:526–34.