Abstract
Topical application of the drugs at the pathological sites offers potential advantages of delivering the drug directly to the site of action. The main aim of this work was to formulate and evaluate Miconazole nitrate (MN) loaded solid lipid nanoparticles (SLN) for topical application. MN-loaded SLN were prepared by modified solvent injection method and characterized for shape, surface morphology, particle size, and drug entrapment. These solid lipid nanoparticles were spherical in shape with smooth surface and possessed mean average size of 206.39 ± 9.37 nm. In vitro drug release of MN-loaded SLN-bearing hydrogel was compared with MN suspension and MN hydrogel; MN-loaded SLN-bearing hydrogel depicted a sustained drug release over a 24-h period. Tape stripping experiments demonstrated 10-fold greater retention with MN-loaded SLN-bearing hydrogel as compared to MN suspension and MN hydrogel. The in vivo studies were performed by infecting the rats with candida species. It was observed that MN-loaded SLN-bearing hydrogel was more efficient in the treatment of candidiasis. Results indicate that MN-loaded SLN-bearing hydrogel provides a sustaining MN topical effect as well as quicker relief from fungal infection.
Introduction
Solid lipid nanoparticles (SLN) have been established as an alternative particulate carrier system by various research groups (CitationSiekmann & Westesen, 1992; CitationAlmeida et al., 1997). Recently, increasing attention has been focused on these SLN as colloidal drug carriers combining advantages of polymeric nanoparticles, fat emulsions, and liposomes, but simultaneously avoiding some of their disadvantages (CitationBoltri et al., 1993). During the past several years, SLN began to act as a topical carrier not only for pharmaceutical molecules, but also for cosmetic products. Compared with conventional carriers such as cream, tincture, and emulsion, SLN combine their advantages such as controlled release, in vivo good toleration, and protection of active compounds (CitationMuller et al., 2000; CitationSylvia et al., 2003). Especially, SLN can favor drug penetration into the skins (CitationJenning et al., 2000; CitationWissing & Muller 2003), maintain a sustained release to avoid systemic absorption (CitationZur Muhlen et al., 1998), act as a UV sunscreen system (CitationWissing & Muller, 2002a), and reduce irritation (CitationMaia et al., 2000; CitationSivaramakrishnan et al., 2004). It was used for topical delivery of several drugs including clotrimazole, flurbiprofen, prednicarbate, and betamethasone 17-valerate, and SLN also was reported to have skin targeting potential (CitationMaia et al., 2000; CitationSivaramakrishnan et al., 2004; CitationJain et al., 2005; CitationSong & Liu, 2005). SLN was found to have skin targeting which can result in a high accumulation of podophyllotoxin in the skin (CitationChen et al., 2006).
The advantage of SLN is that the lipid matrix is made from physiological lipids, which decrease the danger of toxicity. Lipid and surfactants used in SLN have approved status, e.g. GRAS status (Generally Recognized as Safe) due to their low toxicity, or they are already used as excipients in cosmetics or pharmaceuticals. The small size of the lipid particles ensures close contact to the stratum corneum and can increase the bioadhesive and occlusive properties, which is a desired requirement for topical application. Due to their solid lipid matrix, controlled release from these carriers is possible. This becomes an important tool when it is necessary to supply the drug over a prolonged period of time, to reduce systemic absorption, and when drug produces irritation in high concentrations. As a result of film formation after topical application, occlusive properties have also been reported for SLN (CitationWissing & Muller 2001; Citation2002b).
Miconazole nitrate (MN), one of the broad-spectrum anti-fungal agents, is a weak base (pKa = 6.7) characterized by relatively high molecular weight and melting point. The poor water solubility (1.03 µg/ml) of MN reduces its efficacy for many therapeutic applications (CitationPedersen et al., 1993). MCZ is usually employed at 2% (w/w) in topical suspensions for the treatment of dermatophytoses, superficial mycoses, and mixed infections, or as an oral gel for the treatment of Candidal infections (CitationPeira et al., 2008).
The main aim of our investigation was to develop and evaluate an MN-loaded SLN-bearing hydrogel for topical delivery of MN. The entrapped drug in lipid nanoparticles can facilitate localized delivery of the drug and improve local availability by means of a controlled release pattern which can advance the anti-fungal activity and skin tolerability of miconazole nitrate.
Materials and methods
Materials
Medley Pharmaceutical Ltd. (Mumbai, India) generously supplied anti-fungal drug miconazole nitrate (MN) as a gift sample. Soya lecithin and Dialysis bag (mol. cut-off weight 12,000) were procured from Himedia Ltd. (Mumbai, India). Carbopol 934 (hydrogelling agent) was obtained from S.D. Fine Chem. Ltd. (Biosar, India). Tristearin (solid lipid) was purchased from Colorcon Asia Pvt. Ltd. (Mumbai, India). Methanol (HPLC grade) was purchased from Sigma (St. Louis, MO). Other chemicals were of analytical grade.
Fabrication of SLN
The solid lipid nanoparticles were prepared by solvent injection method, as reported by CitationGoymann and Schubert (2003). Briefly, tristearin, soya lecithin, and drug (MN) were dissolved in ethanol in a definite ratio and warmed to ∼ 70 ± 2°C. Tween 80 in a definite amount was dissolved in PBS solution (pH 7.4) to prepare aqueous phase. The organic phase, i.e. alcoholic solution containing lipid mixture, was added dropwise with stirring to pre-warmed aqueous solution (70 ± 2°C) with the help of a hypodermic needle. The mixture was then sonicated for varying time periods to obtain nanoparticles with optimum size.
Optimization of different variables for MN-loaded SLN
Various formulation variables, i.e. lipid:soya lecithin (1:1.25–1:0.25%w/w), drug:tristearin (2.5:97.5–10:90%w/w), emulsifier tween 80 concentration (0.1–2.0%w/v), and process variables, i.e. sonication time (2–6 min), which could affect the properties of solid lipid nanoparticles were optimized to get small SLN with maximum drug entrapment. Various parameters were optimized by varying one parameter while keeping others constant () and prepared nanoparticles were studied for their particle size, polydispersity index, and percentage drug entrapment.
Table 1. Composition of various SLN formulations.
Particle size determination
The average particle size and polydispersity index of the lipid particulate dispersions were determined by photon correlation spectroscopy using a Zetasizer (DTS Ver. 4.10, Malvern Instruments, UK). The sample of dispersion was diluted to 1:9 v/v with double distilled water to ensure that the light scattering intensity was within the instrument’s sensitivity range. Double distilled water was filtered through 0.45 µm membrane filters (Pall Life sciences, Mumbai, India) prior to particle size determination.
Particle morphology (TEM)
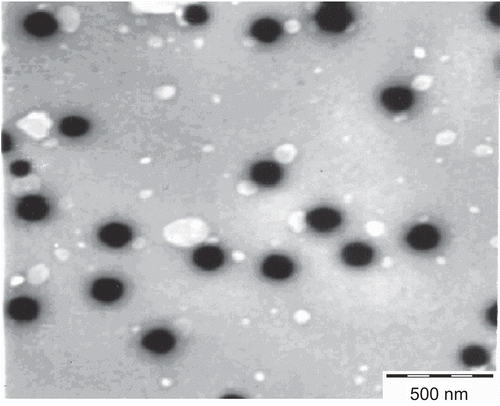
A transmission electron microscope was used as a visualizing aid for particle morphology. The sample (10 µL) was placed on the grids and allowed to stand at room temperature for 90 s. Excess fluid was removed by touching the edge with filter paper. All samples were examined under a TEM (Philips Morgagni 268, Eindhoven, The Netherlands) at an acceleration voltage of 100 kV.
Surface morphology (SEM)
A scanning electron microscope was used as a visualizing aid for surface morphology. The samples for SEM were prepared by lightly sprinkling the SLN powder on a double adhesive tape, which was stuck on an aluminium stub. All samples were examined under a scanning electron microscope (LEO 435 VP, Eindhoven The Netherlands) at an acceleration voltage of 30 kV.
Drug entrapment efficiency
SLN dispersion was ultracentrifuged for 1 h at 55,000 rpm (ALC Centrifugette 4206). The supernatant was used for MN analysis by HPLC and the quantity of free drug was determined. The encapsulated amount of MN was calculated by subtracting the free amount of MN from the total amount in the dispersion. Entrapment efficiency (%EE) was calculated by the following equation, where Wi is the amount of initial drug and Wf is the amount of free drug (CitationEvren et al., 2008).
%EE = (Wi − Wf/WI) × 100
Formulation of MN-loaded SLN-bearing hydrogels
First, Carbopol 934 was dispersed in minimum quantity of double distilled water and stored overnight and then neutralized by triethanolamine. After adding propylene glycol and glycerine the mixture was mixed well with high-speed stirrer at 1000 rpm for 5 min. Finally, the MN-loaded SLN dispersions were incorporated into hydrogel and stirred continuous for the next 5 min (CitationZhinan et al., 2005; CitationJoshi & Patravale, 2006).
Determination of spreadability and pH
The spreadability of the hydrogel was determined using the following technique: Hydrogel (500 mg) was placed within a circle of 1 cm diameter pre-marked on a glass plate over which a second glass plate was placed. A weight of 500 g was allowed to rest on the upper glass plate for 5 min. The increase in the diameter due to spreading of the hydrogels was noted. The pH of the MN-loaded SLN-bearing hydrogel was determined using an Equip-tronic Digital pH meter Model EQ 610, standardized using pH 4.0 and 7.0 standard buffers before use (CitationShah et al., 2007).
In vitro drug release studies
The in vitro drug release profile of MN suspension, MN hydrogel, and MN-loaded SLN-bearing hydrogel were studied using a dialysis bag. Different formulations were taken into a dialysis bag (molecular weight cut-off, 12 KDa, Himedia, India) and placed in a beaker containing 20 ml of mixture of methanol:PBS (pH 6.4) (30:70). Then, the beaker was placed over a magnetic stirrer and the temperature of the assembly was maintained at 37 ± 1°C throughout the study. Samples (1 ml) were withdrawn at definite time intervals and replaced with equal amounts of fresh buffer. The samples were analyzed for drug content using HPLC.
In vitro skin permeation, stripping, and retentivity studies
In vitro skin permeation experiments for MN from different formulations were performed using excised full thickness hairless abdominal skin of rats (Male albino rats, Sprague Dawley; 100–150 g). The skin samples were mounted on modified Franz diffusion cells (Crown Glass Co., NJ) with a surface of 3.14 cm2 and a receptor volume of 10 ml such that the dermal side of the skin was exposed to the receptor fluid (methanol:PBS (pH 6.4), i.e. 30:70) ratio and the stratum corneum remained in contact with the content of donor compartment. Different formulations (MN-loaded SLN-bearing hydrogel, MN hydrogel, and MN suspension) were placed in the donor compartment enabling one to to cover the entire skin surface evenly. The temperature was maintained at 37 ± 1°C. Sampling was done at various intervals up to 24 h and assayed for MN content using the HPLC method.
Franz diffusion cells were then dismantled and skin surface washed 10 times with a cotton swab and stripped using a fixed diameter and length of Scotch Magic Tape. The 10 tape strips were mixed in 2-propanol and 0.1 M HCl (90:10) mixture and shaken to dissolve adhered MN in solvent mixture. Then, the solvent mixture was filtered through a 0.45 µm membrane filter and analyzed for drug concentration using an HPLC method (CitationWissing & Muller 2002a). Further, percentage drug skin deposition after tape stripping was analyzed. The skin was washed 10 times with a cotton swab followed by weighing and homogenizing in methanol. The resulting solution was centrifuged for 10 min at 7000 rpm and supernatant was analyzed for drug using the HPLC method.
Skin-irritation testing
The skin irritation potential of the MN-loaded SLN-bearing hydrogel in comparison to MN hydrogel and MN suspension were evaluated by performing a Draize patch test on male albino rabbits (1.5–2 kg) (CitationDraize et al., 1944; CitationJoshi & Patravale, 2006). The experimental protocol was approved by the Animal Ethical Committee of Dr. H.S. Gour University Sagar, India. Animals were divided into four groups with three animals (rabbits 1.5–2 kg) in each group as follows:
Group I: Control (no drug treatment);
Group II: MN suspension;
Group III: MN Hydrogel; and
Group IV: MN-loaded SLN-bearing hydrogel.
The back of the rabbits were clipped free of hair, 24 h prior to the application of the formulations. Different formulations were applied on the hair-free skin of rabbits by uniform spreading within the area of 4 cm2. The skin was observed for any visible changes such as erythema (redness) at 24, 48, and 72 h after the application of various formulations. The mean erythemal scores were recorded (ranging from 0–4) depending on the degree of erythema as follows: no erythema = 0, slight erythema (barely perceptible-light pink) = 1, moderate erythema (dark pink) = 2, moderate-to-severe erythema (light red) = 3, and severe erythema (extreme redness) = 4.
HPLC analysis of MN
MN content was determined using an HPLC apparatus consisting of a pump (LC 10-AD), a UV detector (SPD-10A), and a data station (Shimadzu, Kyoto, Japan). An Ultra sphere® C-18 column (250 mm × 4.6 mm i.d.) was used. After mixing, the mobile phase, which comprised of methanol:phosphate buffer solution at pH 3.0 (90:10, v/v), was degassed. The eluent was run at a rate of 1.0 ml/min and monitored at 230 nm following injected volumes of 20 μL of sample. The relative retention time was found to be 7.2 min (CitationPeira et al., 2008).
In vivo performance studies
Candida albicans (MTCC 183) was used to induce mycosis in experimental animals to study the in vivo performance of the prepared drug delivery system. The study was approved by the Animal Ethical Committee of Dr. H.S. Gour University Sagar, India. Cutaneous Candidiasis was induced on Sprague Dawley albino rats (100–150 g) by slight modification of an earlier reported procedure (CitationMaebashi et al., 1995). Briefly, hairs on the back of the rats were removed using depilatory cream and an area of 3 × 3 cm2 was marked. On the second day the marked skin was slightly abraded with sandpaper and 500 mg of previously prepared Candida Albicans inoculum was applied using a glass rod. Male albino rats (Sprague Dawley; 100–150 g) selected for the study were weighed and divided into four groups, including one control group, with six animals in each group. The different formulations, i.e. MN-loaded SLN-bearing hydrogel, MN hydrogel, and MN Suspension, were applied topically separately once daily for six consecutive days, starting on the day of post-infection to albino rats of respective groups, excluding the animals of the control group. The animals of the control group were infected but did not receive any drug therapy. The rats were continuously observed visually for the changes in texture of skin of the infected area after initiation of treatment. The performance of various groups to alleviate mycosis was compared with that of control after 6 days, skin texture was evaluated, and recovery time was measured (CitationPershing et al., 1994).
Culture studies were done to assess the efficacy of treatment. Six days after the respective treatment, each treated site was wiped thoroughly with a cotton swab containing 70% ethanol. The skin was excised from each treated site, minced with scissors, and homogenized in 4 ml of saline using the tissue homogenizer. A portion of homogenate (each one) was streaked on the solidified growth medium. All plates were incubated at 25°C for 5 days in the incubator. The numbers of CFUs in the agar plates were counted and the logarithm of the number of CFUs per infected site was calculated. An animal was considered fungus positive when more than one fungal colony was counted.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) of the mean and statistical analysis was carried out employing the ANOVA test using the software PRISM (Graph Pad). A value of p < 0.005 was considered statistically significant.
Results and discussion
Solid lipid nanoparticles-based hydrogel formulation was prepared in two steps, first the drug-loaded SLN were prepared using the solvent injection method reported by CitationGoymann and Schubert (2003) and then SLN dispersion was dispersed in carbopol hydrogel. The various formulation and process variables were optimized to obtain nanosized particles with maximum drug entrapment efficiency.
In the present investigation, ethanol was selected as a miscible solvent for the preparation of SLN due to its fairly good dermal acceptability as compared to other solvents. Moreover, ethanol has good solubilizing potential for tristearin at ∼ 70°C. No efforts were made to remove ethanol and surfactants in the SLN dispersion, as all the excipients used in this study, at the concentration present in the dispersion, are acceptable for topical application.
Optimization of different variables
The different variables involved in the preparation of SLN were optimized, including lipid/lecithin ratio, drug/lipid ratio, surfactant concentration, and sonication time to obtain nanosized particles with maximum drug entrapment ().
The lipid ratio (tristearin:soya lecithin) was first optimized by varying the ratio of tristearin:soya lecithin. It was found that on increasing the soya lecithin concentration in the tristearin, the particle size decreased from 425.58 ± 10.5 to 311.24 ± 7.2 nm. The decrease in particle size may be due to the increased surfactant action of soya lecithin with increase in its concentration for covering the fixed amount of lipid concentration. Further increasing the lecithin concentration led to an increase in particle size, possibly due to the major effect of lecithin itself and probably due to formation of liposomal and other structures, as evident from a sharp increase in PDI values ().
Drug:lipid ratio was optimized by varying the drug concentration from of 2.5 to 7.5%. The drug entrapment efficiency increased from 87.62 ± 2.51% to 92.74 ± 2.11%. On further increasing the concentration of drug to 10%, the drug entrapment efficacy decreased. Further, there was no significant increase in particle size on increasing drug concentration up to 7.5% (p > 0.005). On further increasing the concentration of drug to 10% a significant increase in particles size was observed (p < 0.005). This could be due to the saturation of lipid matrix with the drug above 7.5% drug concentration and reduction of surfactant effect of PC. Because of depletion of surfactant effect of PC due to high concentration of drug, the particle aggregation might occur, leading to an abrupt increase in particle size of SLN ().
On increasing the concentration of Tween 80 from 0.1–2% w/v a decreased particle size was observed. This may be due to the decrease of the surface tension between organic and aqueous phases that possibly allows the formation of initially smaller solvent droplets at the site of solvent injection and causes the decreased particle size. Moreover the emulsifier also helps to stabilize the newly generated surfaces and particle aggregation (CitationGoymann & Schubert, 2003). However, the PDI values decreased up to 0.5% w/v Tween 80 concentration and further addition of Tween 80 led to a sharp increase in PDI values possibly due to irregular size reduction with high Tween 80 concentration (1 or 2%). Hence, the formulation D31 was taken further for optimization of sonication. In the case of increasing sonication time from 4 to 6 min, no significant difference in size was observed (p > 0.005), whereas PDI and entrapment efficiency changed significantly (p < 0.005). Hence, D311 was considered as an optimization formulation ().
A stable SLN formulation was achieved after stirring ∼ 3000 rpm for 20 min and sonicating the formulation for 4 min with a minimum average particle size of 206.39 ± 9.37 nm and a maximum percentage drug entrapment of 90.86 ± 5.20% ().
Characterization of SLN
Shape and surface morphology of the SLN prepared with optimized parameters was observed by transmission and scanning electron microscopy. The study revealed that most of the SLN were fairly spherical in shape, the surface of the particle showed a characteristic smoothness, and that the particle size was in the nanometric range ( and ).
Formulation of MN-loaded SLN-bearing hydrogel and characterization
Carbopol 934 was selected for the preparation of MN-loaded SLN-bearing hydrogel. The optimized SLN dispersion was then incorporated into hydrogel. The prepared formulation was first studied to establish the release kinetics of the drug from the carrier systems. The pH was found to be 6.8 ± 0.1 which is acceptable for topical applications.
Spreadability is an important property for topical formulations. Application of the formulation on the desired part would be more comfortable if the base spreads easily, exhibiting maximum ‘slip’ and ‘drag’. The diameter was found to be 5.8 ± 0.2 cm, which is indicative of good spreadability of the MN-loaded SLN-bearing hydrogel.
In vitro drug release studies
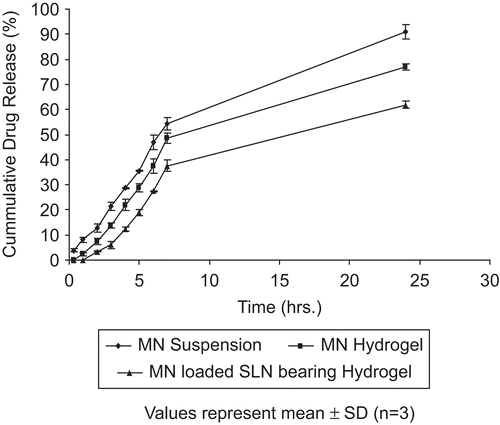
To evaluate the drug release pattern, the dialysis bag method was used where a mixture of methanol:PBS (pH 6.4) (30:70) was used as the diffusion medium. Different release patterns were observed from the MN suspension, MN hydrogel, and MN-loaded SLN-bearing hydrogel. An initial rapid release was observed in the case of MN suspension, whereas MN hydrogel and MN-loaded SLN-bearing hydrogel depicted a slow initial release with a lag time of 0.5 h and 1 h, respectively (). The direct exposure of MN hydrogel and MN suspension to diffusion media may account for rapid initial release in hydrogel and suspension. MN-loaded SLN-bearing hydrogel formulations depicted better controlled drug release profile for a prolonged period, suggesting their applicability in topical drug delivery. An earlier similar release profile was obtained in the case of flurbiprofen by CitationJain et al. (2005).
In vitro skin permeation, stripping, and retentivity studies
A negligible amount of MN was obtained in the receptor compartment from any of the applied formulations after 24 h in a rat skin permeation experiment (). On conducting the tape stripping 10 times after 24 h, it was observed that MN recovered in tape stripping was highest (145.5 ± 6.84 µg/cm2) with MN-loaded SLN-bearing hydrogel (). In the case of MN suspension and MN hydrogel it was 11.4 ± 2.95 µg/cm2 and 13.2 ± 3.52 µg/cm2, respectively. The results confirm the presence of drug in the upper layers of the skin which could further provide a better treatment profile for pathological conditions like superficial mycoses.
Table 2. Amount of MN permeated and retained in hairless rat skin from different formulations (tape stripping experiments).
Further, a skin retentivity study conducted after tape stripping also suggested nearly 10-fold greater skin retention in the case of MN-loaded SLN-bearing hydrogel as compared to the other two formulations (). The retentivity profile could be attributed to occlusive and bioadhesive properties of SLN, where occlusion decreases the transepidermal water loss, which further increases skin hydration and permeability. Further, it may also lead to accumulation of bioactives into the upper skin layers, reducing drug flux and creating a reservoir able to prolong skin residence time (CitationPuglia et al., 2006), and, hence, could provide better treatment to upper skin infections and disorders. Similar results for MN skin permeation and deposition were obtained by CitationPeira et al. 2008) using a microemulsion-based vehicle for topical delivery.
Primary skin irritation studies
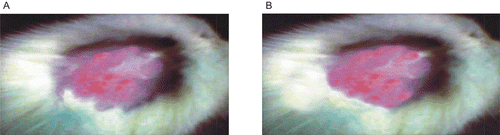
One of the major disadvantages associated with the MN therapy is skin irritation (erythema), which strongly limits its utility and acceptability by the patients. Ideally, the delivery system of MN should be able to diminish or abolish these erythematic episodes. It was hypothesized that encapsulation of MN in SLN would reduce the direct contact of the MN (the triggering factor for the erythematic events) with the stratum corneum, thus resulting in reduced erythematic episodes. The results obtained from the primary skin irritation studies are listed in . The skin-irritation studies indicated that MN-loaded SLN-bearing hydrogel resulted in considerably less irritation as compared to MN hydrogel and MN suspension after 24 h of application. The erythema continued to increase even after 24 h in the case of MN hydrogel and MN suspension, whereas no erythema was observed in the case of MN-loaded SLN-bearing hydrogel. Thus, SLN-based gel demonstrated a remarkable advantage over simple gel in improving the skin tolerability of MN, indicating their potential in improving patient acceptance and topical delivery of MN.
Table 3. Mean erythemal scores observed for various formulations.
In vivo studies
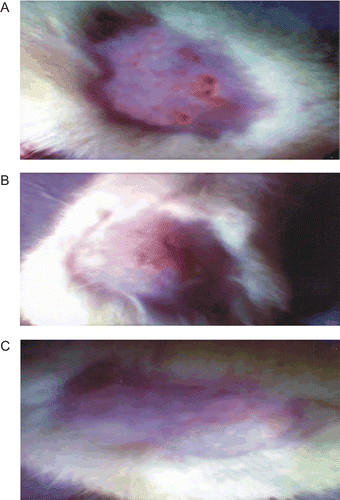
The in vivo efficacy of MN-loaded SLN-bearing hydrogel was assessed in a male albino rat model (Sprague Dawley; 100–150 g). Isolate of candida albicans was used for production of cutaneous candidiasis in albino rats. depicts the efficacy of MN-loaded SLN-bearing hydrogel formulation against cutaneous candidiasis in rats as compared to that of MN hydrogel and MN suspension. The isolates of viable candida albicans were removed from the lesions of all the treated animals. It was observed that MN-loaded SLN-bearing hydrogel depicts greater efficiency in the treatment of candidiasis, as only one animal out of six exhibited a positive culture test while in the case of MN hydrogel and MN suspension treatment it was in three and four animals out of six, respectively. Fast recovery from fungal infection was found in the case of MN-loaded SLN-bearing hydrogel. Such good performance of the SLN was possibly due to better occlusive and bioadhesive properties ( and ).
Table 4. Colony forming unit of Candidia albicans in skin of rats after treatment with different formulations.
Conclusion
The MN-loaded SLN could be fabricated with the help of a modified solvent injection method and successfully incorporated into hydrogel for topical application. The in vitro and in vivo data indicate that MN-loaded SLN-bearing hydrogel provides sustained release of MN. The obtained results reflect the potential of SLN as a carrier for topical administration of MN which is demonstrating greater drug deposition into skin and better in vivo anti-fungal efficacy in a rat model with less erythemal score. The study provides evidences that SLN could be a better module for prolonged release profile with wide applications in topical drug delivery.
Acknowledgements
The authors are grateful to All India Institute of Medical Sciences, New Delhi for providing Scanning and Electron Microscopy facility, and M/s Medley Pharmaceuticals Mumbai, India for providing the gift sample of Miconazole nitrate.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Almeida, A.J., Runge, S., Muller, R.H. (1997). Peptide-loaded solid lipid nanoparticles (SLN): influence of production parameters. Int J Pharm. 149:255–65.
- Boltri, L., Canal, T., Esposito, P.A., Carli, F. (1993). Lipid nanoparticles: evaluation of some critical formulation parameters. Proc Int Symp Contr Rel Bioact Mater. 20:346–7.
- Chen, H.B., Chang, X.L., Yang, X.L., Du, D.R., Liu, W., Liu, J., Weng, T., Yang, Y.J., Xu, H.B. (2006). Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Contr Rel. 110:296–306.
- Draize, J., Woodard, G., Calvery, H. (1944). Methods for the study of irritation and toxicity of substances topically applied to skin and mucous membranes. J Pharmacol Exp Ther. 82:377–90.
- Evren, H.G., Giuseppina, S.M., Cristina, B., Silvia, R., Franca, F., Tamer, G., Carla, C. (2008). Cyclosporine A loaded SLNs: evaluation of cellular uptake and corneal cytotoxicity. Int J Pharm. 364:76–86.
- Goymann, M.C., Schubert, M.A. (2003). Solvent injection as a new approach for manufacturing lipid nanoparticles—evaluation of the method and process parameters. Eur J Pharm Biopharm. 55:125–31.
- Jain, S.K., Chourasia, M.K., Masuriha, R., Soni, V., Jain, A., Jain, N.K., Gupta, Y. (2005). Solid lipid nanoparticles bearing flurbiprofen for transdermal delivery. Drug Deliv. 12:207–15.
- Jenning, V., Gysler, A., Schafer-Korting, M., Gohla, S.H. (2000). Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 49:211–8.
- Joshi, M., Patravale, V. (2006). Formulation and evaluation of nanostructured lipid carrier (NLC)-based hydrogel of valdecoxib. Drug Dev Ind Pharm. 32:911–8.
- Maebashi, K., Itoyama, T., Uchida, K., Yamaguchi, H. (1995). A novel model of cutaneous candidiasis produced in prednisolone-treated guinea pigs. J Med Vet Mycol. 19:390–2.
- Maia, C.S., Mehnert, W., Schafer-Korting, M. (2000). Solid lipid nanoparticles as drug carriers for topical glucocorticoids. Int J Pharm. 196:165–7.
- Muller, R.H., Mader, K., Gohla, S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery—review of the state of the art. Eur J Pharm Biopharm. 50:161–77.
- Pedersen, M., Edelsten, M., Nielsen, V.F., Scarpelline, A., Skytte, S., Slot, C. (1993). Formation and antimycotic effect of cyclodextrin inclusion complexes of econazole and miconazole. Int J Pharm. 90:247–54.
- Peira, E., Eugenia, M., Trotta, C.C., Cavalli, R., Trotta, M. (2008). Positively charged microemulsions for topical application. Int J Pharm. 346:119–23.
- Pershing, L.K., Corlett, L.J., Jorgensen, C. (1994). In vivo pharmacokinetics and pharmacodynamics of topical ketoconazole and miconazole in human stratum corneum, Antimicrob. Agents Chemother. 38:90–5.
- Puglia, C., Filosa, R., Peduto, A., Caprariis, P., Rizza, L., Bonina, F., Blasi, P. (2006). Evaluation of alternative strategies to optimize ketorolac transdermal delivery. AAPS Pharm Sci Tech. 7:E1–9.
- Shah, K.A., Patravale, V.B., Date, A., Joshi, M.D. (2007). Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 345:163–71.
- Siekmann, B., Westesen, K. (1992). Submicron-sized parental carrier system based on solid lipids. Pharm Pharmacol Lett. 1:123–6.
- Sivaramakrishnan, R., Nakamura, C., Mehnert, W., Korting, H.C., Kramer, K.D., Schafer-Korting, M. (2004). Glucocorticoids entrapment into lipid carriers—characterization by parelectic spectroscopy and influence on dermal uptake. J Contr Rel. 97:493–502.
- Song, C., Liu, S. (2005). A new healthy sunscreen system for human: solid lipid nanoparticles as carrier for 3, 4, 5-trimethoxybenzoylchitin and the improvement by adding Vitamin E. Int J Biol Macromol. 36:116–9.
- Sylvia, A., Muller, R.H., Wissing, S.A. (2003). Cosmetic applications for solid lipid nanoparticles (SLN). Int J Pharm. 254:65–8.
- Wissing, S.A., Muller, R.H. (2001). A novel sunscreen system based on tocopherol acetate incorporated into solid lipid nanoparticles. Int J Cos Sci. 23:233–43.
- Wissing, S.A., Muller, R.H. (2002a). Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Contr Rel. 81:225–33.
- Wissing, S.A., Muller, R.H. (2002b). The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int J Pharm. 242:377–9.
- Wissing, S.A., Muller, R.H. (2003). The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—in vivo study. Eur J Pharm Biopharm. 56:67–72.
- Zhinan, M., Qunrong, W., Sheng, H., Xiaokuan, L., Xiangliang, Y. (2005). Triptolide loaded solid lipid nanoparticles hydrogel for topical application. Drug Dev Ind Pharm. 31:161–8.
- Zur Muhlen, A., Schwarz, C., Mehnert, W. (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery—drug release and release mechanism. Eur J Pharm Biopharm. 45: 149–55.