Abstract
To develop pullulan acetate nanoparticles (PANs) as a drug nanocarrier, pullulan acetate (PA) was synthesized and characterized. Its acetylation degree determined by the proton nuclear magnetic resonance (1H NMR) was 2.6. PANs were prepared by the solvent diffusion method and characterized by transmission electron microscope (TEM), size distribution, and ζ potential techniques. PANs had nearly spherical shape with a size range of 200–450 nm and low ζ potentials both in distilled water and in 10% FBS. The storage stability of PANs was observed in distilled water. PANs were stored for at least 2 months with no significant size and ζ potential changes. The safety of PANs was studied through single dose toxicity test in mice, and the result showed that PANs were well tolerated at the dose of 200 mg/kg in mice. Epirubicin-loaded PANs (PA/EPI) were also prepared and characterized in this study. Moreover, the in vivo pharmacokinetics of PA/EPI was investigated. Compared with the free EPI group, the PA/EPI group exhibited higher plasma drug concentration, longer half-life time (t1/2) and the larger area under the curve (AUC). All results suggested that PANs were stable, safe, and showed a promising potential on improving the bioavailability of the loaded drug of the encapsulated drug.
Introduction
Nanoparticles (NPs) have specific physicochemical properties differing from bulk materials of the same composition, and such properties make them very attractive for commercial and medical development (CitationCurtis et al., 2006; CitationLanone & Boczkowski, 2006; CitationMedina et al., 2007). However, compared with conventional materials, NPs may have differential toxicity profiles due to their different chemical and structural properties (CitationVega-Villa et al., 2008). For example, the interaction between nanomaterials and biological components, e.g. proteins and cells may lead to their unique bio-distribution, clearance, immune response, and metabolism, and therefore it is very necessary to study the in vivo toxicity of nanomaterials (CitationFischer & Chan, 2007).
Pullulan, a very important neutral and linear natural polysaccharide, has been used as a good biomaterial in drug and gene delivery, tissue engineering, and other fields (CitationLeathers, 2003; CitationShingel, 2004; CitationRekha & Sharma, 2007). Many investigations (CitationAkiyoshi et al., 1993; CitationJeong et al., 2006) have reported that some hydrophobized pullulan such as cholesterol-modified pullulan can form self-aggregated NPs to be used as the carrier for drug delivery. As one of the most conventional hydrophobized pullulan derivatives (CitationJung et al., 2003; CitationNa et al., 2003; Citation2004, CitationPark et al., 2007), pullulan acetate (PA) and its modified materials can form self-aggregated NPs in aqueous media. The hydrophobic core of these self-assembled NPs formed by the hydrophobic interactions was considered to act as a reservoir of hydrophobic substances. In our previous report (CitationZhang et al., 2009), PANs prepared by the solvent diffusion method had the potential to be used as a sustained release carrier for Epirubicin (EPI) in vitro. Some investigations (CitationGu et al., 1998; CitationNa et al., 2004; CitationShimizu et al., 2008) showed that NPs of hydrophobized pullulans had good morphology, drug loading, and release properties in vitro, but the biological effects of hydrophobized pullulan NPs in vivo have not been investigated deeply up to date. Herein, based on our previous work, we studied the stability and toxicity of PANs, and further investigated the sustained release behavior in vivo of drug loading in PANs. PA was firstly synthesized and characterized by FT-IR and 1H NMR, and then PANs with moderate size and low potential were prepared by the solvent diffusion method. The storage stability of PANs was studied in the aqueous medium, and the acute toxicity of PANs was evaluated in mice. Morever, EPI was loaded into PANs and its pharmacokinetics was also assessed in rats to compare to the free drug.
Methods
Materials
Pullulan (Mw = 200,000) was purchased from Hayashibara (Tokyo, Japan). Epirubicin·HCl (EPI·HCl) was purchased from Hisun Pharmaceutical Co. (Zhejiang, China). Poly (vinyl alcohol) (PVA) with an average molecular weight of 30,000–70,000 was obtained from Sigma-Aldrich (St. Louis, MO). All reagents for high performance liquid chromatography (HPLC) analysis, including acetonitrile and methanol, were HPLC grade. Other chemical reagents were of analytical grade and obtained from commercial sources. ICR mice and Wistar rats were purchased from the Institute of Radiology, Chinese Academy of Medical Science. All animal experiments were performed in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
Synthesis and characterization of PA
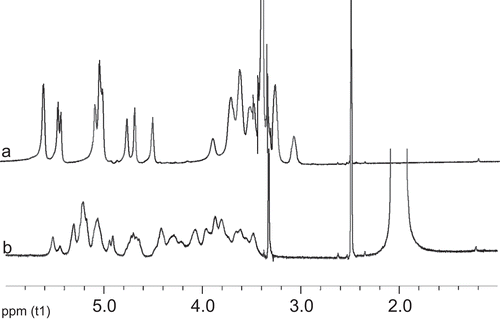
PA was synthesized according to the method described in previous literature (CitationJung et al., 2003; CitationZhang et al., 2009). Briefly, pullulan (2 g) was suspended in 20 ml of formamide and dissolved by vigorous stirring at 54°C. Pyridine (6 ml) and acetic anhydride (5.5 ml) were added to the above solution, and the mixture was subsequently stirred at 54°C for 48 h. The reactant was precipitated with distilled water, and then washed with distilled water and methanol. The solid material was vacuum-dried at 50°C for 48 h. The final product was identified by Fourier transform infrared (FT-IR) (Thermo, Nicolet is10, Los Angeles, CA ) and 1H NMR (Varian, Varian INOVA. 400 M NMR, Palo Alto, CA) spectrometry. The degree of substitution (DS) was defined as the number of acetyl groups per glucose unit of pullulan. It was determined by 1H NMR (CitationZhang et al., 2009). The DS values are expressed by the following equations: DS = 10A/(3B + A), where A is the integration value of acetyl protons at 1.8–2.2 ppm and B is that of OH protons and H-1 to H-6 protons of pullulan moiety observed at more than 3.5 ppm.
Preparation and characterization of PANs and PA/EPI
Nanoparticles were prepared according to a solvent diffusion method (CitationFessi et al., 1989; CitationGovender et al., 1999; CitationBilati et al., 2005; CitationZhang et al., 2009). Briefly, PA (100 mg) was dissolved in 10 ml of N, N-Dimethylformamide (DMF). The solution was then added to 0.5% PVA aqueous solution through a syringe under moderate magnetic stirring. The produced PANs were collected with centrifugation (Beckman Coulter, Inc. Avanti J-25, Fullerton, CA) at 18,000 rpm for 15 min at 4°C. Subsequently, PANs were dispersed in the distilled water and 10% fetal bovine serum (FBS), respectively, with PA concentration of 1 mg/ml to carry out the later experiments.
EPI-loaded PANs were prepared as follows: EPI·HCl (10 mg) was dissolved in DMF (2 ml) and then triethylamine (TEA) was added to this solution to remove hydrochloride. The mixed solution was stirred in the dark for 12 h. PA (100 mg) dissolved in 8 ml of DMF was added into this mixed solution. The PA/EPI were collected with centrifugation (Beckman Coulter, Inc. Avanti J-25, Fullerton, CA) at 18,000 rpm for 15 min at 4°C, then the supernatant removed and washed twice with distilled water, at last, dispersed in distilled water by sonication for several minutes with a probe-type sonifier (Automatic Ultrasonic Processor UH-500A, China) at 100 W.
The particle size and ζ potential were determined by dynamic light scattering (Malvern Instruments Ltd., Zeta sizer 2000, Worcestershire, UK). The morphology of NPs was observed using TEM (FEI, TECNAI G2F-20, Eindhoven, Holland).
The stability of PANs in water
In order to study the stability of PANs, the above dispersions were stored for 2 months at 4°C. Macroscopic characteristics of NPs dispersions such as opalescence and precipitation were observed from time to time. Moreover, the size and ζ potential of PANs were also determined by dynamic light scattering method once a month, and all measurements were performed in triplicate.
In vivo toxicity
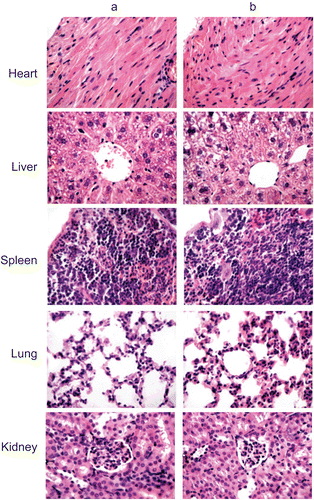
The toxicity of PANs was evaluated in vivo according to the previous reported method (CitationYoksan & Chirachanchai, 2008; CitationSonaje et al., 2009). Adult male and female ICR mice (18–22 g) were randomly divided into two groups, each with 10 mice. The experimental group received a single intravenous (i.v.) dose of blank PANs (200 mg/kg); the other group was treated with a single i.v. dose of normal saline. All animals were fed with normal diet, and water was provided ad libitum. Animals were observed carefully for the onset of any signs of toxicity and monitored for changes in food intake and body weight at 1, 8, and 15 days. After being sacrificed at 15 days, internal organs of each animal were harvested and observed grossly. For histological examinations, specimens of major organs such as heart, liver, spleen, lung, and kidney were fixed in 10% phosphate buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
In vivo pharmacokinetics and bioavailability
In vivo pharmacokinetic study was conducted by the routine method (CitationMross et al., 1988; CitationBibby et al., 2005; CitationCao & Feng, 2008; CitationDevalapally et al., 2008). The drug content of the PA/EPI was determined according to our previous study (CitationZhang et al., 2009) and PA/EPI with 3.34% was selected to carry out the pharmacokinetics studies. Female Wistar rats of 180~250 g and 4~6 weeks old were held in an air-conditioned facility, provided with standard food and filtered water. Animals were randomly assigned to two groups, each with six rats, which received an i.v. injection via the tail vein of free EPI and the PA/EPI solution in saline at 10 mg/kg equivalent dose, respectively. All animals were observed for mortality, general condition, and potential clinical signs.
The blood samples were collected with heparinized tube at 0 (pre-dose), 0.0167, 0.0833, 0.167, 0.5, 1, 2, 4, 8, 12, 24, and 48 h post-treatment. Plasma samples were harvested by centrifugation at 3000 rpm for 15 min and stored at −20°C until analysis. Liquid–liquid extraction was performed prior to the HPLC analysis. Briefly, the plasma (100 µl) was mixed with dichloromethane-methanol (4:1, v/v) on a vortex-mixer for 3 min to extract the drug. Upon centrifugation at 10,000 rpm (15,000 g) for 15 min, the upper aqueous layer was removed by aspiration and the organic layer was transferred to a tube and evaporated under nitrogen at 50°C. The residue was dissolved in 100 µl of anhydrous methanol by vortex. For the HPLC analysis, the C-18 column was used and the mobile phase (0.02 M KH2PO4/CH3CN/CH3OH = 49:17:34 v/v/v) was delivered at a rate of 1 ml/min. Sample (20 μl) was injected and the column effluent was detected with a UV detector at 232 nm. The main pharmacokinetic parameters were calculated by DAS 1.0 (Anhui, China) program.
Bioavailability (BA) is a measurement of the rate and extent of a therapeutically active drug that reaches the systemic circulation and is available at the site of action. When a medication is administered intravenously, its bioavailability is 100%. When the standard consists of intravenously administered drug, this is known as relative bioavailability (BAR). The BAR of PA/EPI after administration was calculated using the following formula (CitationSonaje et al., 2009):
where AUC is the area under the curve.
Statistical analysis
All data are presented as a mean value with its standard deviation indicated (mean ± SD). Statistical analysis was conducted using the Student’s t-test. Differences were considered to be statistically significant when the p-values were less than 0.05.
Results and discussion
Characterization of PA
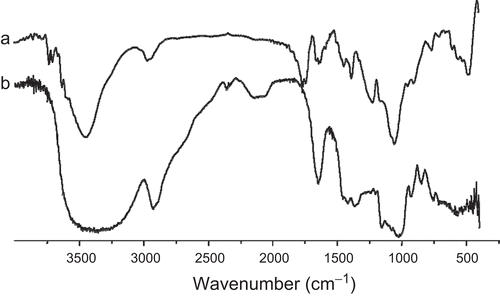
Pullulan has three free hydroxyl groups on each glucose unit. It is easy to synthesize hydrophobic pullulan derivative, PA, by means of replacing the hydroxyl groups of the glucose unit with acetate groups. shows FT-IR spectra of pullulan and PA, which were similar to our previous literature (CitationZhang et al., 2009). shows 1H NMR spectra of pullulan and PA in DMSO-d6. The acetylation degree of PA calculated by 1H NMR method was 2.6, which is smaller than 2.7 reported by CitationZhang et al. (2009). Based on our previous report, PA with lower DS may form smaller NPs in size. Moreover, CitationLi and Huang (2008) also reported that the smaller size of NPs may be in favor of longer circulation time in vivo. Therefore, PA with acetylation degree of 2.6 was used to prepare PANs and PA/EPI in our study.
Characterization of PANs and PA/EPI
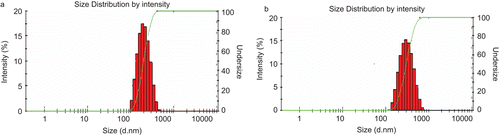
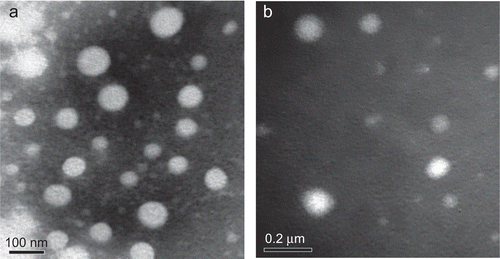
The self-assembled NPs were prepared by solvent diffusion method. This method had several advantages such as a rapid and simple preparation procedure, great potential for large industrial scale production, and easy control of the particle size (CitationZhang et al., 2009). The size and the size distributions of PANs and PA/EPI in distilled water were measured by DLS. The morphological characteristics were observed by TEM at the same time. As shown in , the mean diameters of PANs and PA/EPI were 247.6 ± 34.8 nm and 343.4 ± 87.7 nm, with the narrow size distributions (the polydispersity indexes (PDI) < 0.3, ). Under TEM observations (), PANs and PA/EPI were nearly spherical in shape and uniform sized.
Table 1. Size and PDI of PANs and PA/EPI (![]() ± s, n = 6).
± s, n = 6).
ζ potential of NPs in different media
The ζ potentials of PANs and PA/EPI in distilled water were −3.353 ± 1.296 mV and −3.297 ± 1.025 mV. To model the circulation in vivo, we observed the ζ potentials of NPs in 10% FBS, which had a composition very similar to the body liquids. The ζ potentials of PANs and PA/EPI in 10% FBS were −1.460 ± 0.297 and −1.902 ± 1.112 mV. According to the previous report (CitationLevchenko et al., 2002; CitationAlexis et al., 2008), neutral NPs would exhibit a decreased rate of macrophage phagocytosis system (MPS) uptake. However, MPS is the major contributor for the clearance of NPs, thus the reducing rate of MPS uptake could be considered as the best strategy for prolonging the circulation of NPs (CitationLi & Huang, 2008). Therefore, PA/EPI prepared in this study would exhibit the longer blood circulation time than free EPI in vivo.
The storage stability of PANs
Stability is an important facet of preparation and a necessary step in the development process, it will indicate the potentiality of industry production. The possibilities of modulating the loaded drug’s pharmacokinetic parameters are dependent on physicochemical properties such as stability, size, and surface characteristics (CitationLourenco et al., 1996). PANs dispersions maintained slight opalescence within 2 months. As shown in , the size, size distribution, and ζ potential of PANs showed no significant changes during 2 months. Therefore, it could be concluded that PANs in aqueous media were stable for at least 2 month at 4°C. Stabilization of colloidal systems is traditionally viewed as arising from either electrostatic or steric effects (CitationLourenco et al., 1996). A ζ potential of at least −30 mV for electrostatic stabilized systems is desired to obtain a physically stable suspension according to the literature (CitationMüller & Jacobs., 2002). The higher zeta potential value indicates the better stability (CitationDai et al., 2010). The absolute value of ζ potential was lower than 5 mV in our study. The result showed that electrostatic stabilization was not provided efficiently in the suspension; therefore, a steric stabilization may occur and provide the stability of the PANs. It may also be important information for developing a new nanosuspension liquid formulation about pullulan acetate.
Table 2. Diameter and ζ potential of PANs (![]() ± s, n = 3).
± s, n = 3).
In vivo toxicity
To evaluate whether i.v. administration (at dose of 200 mg/kg) of PANs was associated with any toxicity in vivo, animals were treated with a single dose of empty NPs. No significant differences between the PANs group and the control group in clinical signs, e.g. diarrhea, fever, and other systemic symptoms, and no mortality occurred throughout the entire study course. Additionally, there were no significant differences in body weight and food intake for both male and female mice between the two studied groups ( and ). shows the microscopic examination of major organs including heart, liver, spleen, lung, and kidney sections stained with H&E. Pathological changes in the major organs including heart, liver, spleen, lung, kidney, stomach, and intestinal segments were scarcely observed at 15 days. Moreover, no evidence of inflammatory reactions was observed in the experimental group. All the above results indicated that no apparent toxicity of the PANs was found in the experimental animals after i.v. at a dose of 200 mg/kg.
Table 3. Body weight of PANs i.v. injected in mice at 200 mg/kg dose (![]() ± s, g).
± s, g).
Table 4. Food intake of PANs i.v. injected in mice at 200 mg/kg dose (![]() , g).
, g).
In vivo pharmacokinetics
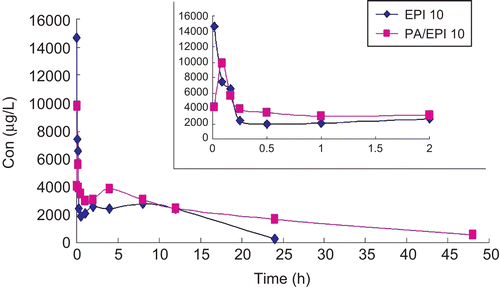
The plasma levels of EPI were determined following a single i.v. injection of EPI or PA/EPI (10 mg/kg EPI equiv.) in female Wistar rats. The plasma levels over 48 h are shown in and the PK parameters are summarized in . The peak concentration of total EPI in plasma was 14.65 mg/L at 1 min after injection and then decreased to nearly undetectable levels after 24 h. The maximum concentration (Cmax) of PA/EPI at 5 min was 9.80 mg/L, which was obviously lower than that of EPI because of sustained release from NPs. According to the previous report (CitationMross et al., 1988; CitationJakobsen et al., 1991; Citation1994), free EPI rapidly disappeared from the circulation due to its short half-life. In this study, the terminal elimination half-life of free EPI was 8.129 h (), which was consistent with that of 7.3 h of doxorubucin (CitationGustafson et al., 2002; CitationBibby et al., 2005). In contrast, PA/EPI showed a much longer circulation time and its elimination half-life time was 17.231 h, 2.12-times that of free EPI. The clearance of EPI loaded in NPs was 0.104 L/h, which is 3.16-times smaller than free EPI. Moreover, the mean residence time (MRT) of PA/EPI in the plasma was 15.854 h, 1.9-times that of free EPI. Altogether, PA/EPI had the longer t1/2 and MRT, lower Vd and CL than the free drug in rats. At the same time, the AUC of EPI and PA/EPI from 0–24 h were 48,128.269 and 91,006.508 µg h/L, respectively. The bioavailability of free EPI was 100%, the BAR of PA/EPI calculated through the equation above by the AUC0–24h was 189%, much higher than the free drug. According to the previous report (CitationLi & Huang, 2008), we believed that the longer t1/2 of PA/EPI in plasma may be due to its low ζ potential and moderate size. As prolonged plasma circulation is the driving force for increased tumor targeting (CitationSeymour et al., 1995), CitationTsuchihashi et al. (1999) prepared long circulating liposomes to improve therapeutic efficacy of doxorubicin. In this study, the slow release of EPI from PANs in the blood suggested that the bioavailability of EPI improved when it was loaded into PANs. However, whether it could enhance the uptake of passive permeability in targeting tumor tissues or not needs to be further proved by the experiments such as bio-distribution study and pharmacodynamic test.
Table 5. Pharmacokinetic parameters of EPI and PA/EPI i.v. injected in rats at the equivalent 10 mg/kg dose.
Conclusions
In this study, PA with the DS of 2.6 was synthesized and characterized. PANs and PA/EPI with moderate size and low potential were prepared by the solvent diffusion method. The nanoparticles were stable in aqueous media for at least 2 months in vitro, furthermore, PANs were safe in mice at 200 mg/kg and showed the potential to improve the bioavailability of the loaded drug in vivo.
Declaration of interest
This work was supported by the Major State Basic Research Program of China (No. 2006 CB933300).
References
- Akiyoshi, K., Moriguchi, N., Yamaguchi, S., Sunamoto, J. (1993). Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules. 26:3062–8.
- Alexis, F., Pridgen, E., Molnar, L.K., Farokhzad, O.C. (2008). Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 5:505–15.
- Bibby, D.C., Talmadge, J.E., Dalal, M.K., Kurz, S.G., Chytil, K.M., Barry, S.E., Shand, D.G., Steiert, M. (2005). Pharmacokinetics and biodistribution of RGD-targeted doxorubicin-loaded nanoparticles in tumor-bearing mice. Int J Pharm. 293:281–90.
- Bilati, U., Allemann, E., Doelker, E. (2005). Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 24:67–75.
- Cao, N., Feng, S.S. (2008). Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS): conjugation chemistry, characterization, in vitro and in vivo evaluation. Biomaterials. 29:3856–65.
- Curtis, J., Greenberg, M., Kester, J., Phillips, S., Krieger, G. (2006). Nanotechnology and nanotoxicology: a primer for clinicians. Toxicol Rev. 25:245–60.
- Dai, W., Zhang, D., Duan, C., Jia, L., Wang, Y., Feng, F., Zhang, Q. (2010). Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul. 27:234–41.
- Devalapally, H., Rajan, K.S., Akkinepally, R.R., Devarakonda, R.K. (2008). Safety, pharmacokinetics and biodistribution studies of a beta-galactoside prodrug of doxorubicin for improvement of tumor selective chemotherapy. Drug Dev Ind Pharm. 34:789–95.
- Fessi, H., Puisieux, F., Devissaguet, J.P., Ammoury, N., Benita, S. (1989). Nanocapsule formation by interfacial deposition following solvent displacement. Int J Pharm. 55:R1–R4.
- Fischer, H.C., Chan, W.C. (2007). Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol. 18:565–71.
- Govender, T., Stolnik, S., Garnett, M.C., Illum, L., Davis, S.S. (1999). PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Contr Rel. 57:171–85.
- Gu, X.G., Schmitt, M., Hiasa, A., Nagata, Y., Ikeda, H., Sasaki, Y., Akiyoshi, K., Sunamoto, J., Nakamura, H., Kuribayashi, K., Shiku, H. (1998). A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res. 58:3385–90.
- Gustafson, D.L., Rastatter, J.C., Colombo, T., Long, M.E. (2002). Doxorubicin pharmacokinetics: macromolecule binding, metabolism, and excretion in the context of a physiologic model. J Pharm Sci. 91:1488–501.
- Jakobsen, P., Sorensen, B., Bastholt, L., Mirza, M.R., Gjedde, S.B., Mouridsen, H.T., Rose, C. (1994). The pharmacokinetics of high-dose epirubicin and of the cardioprotector ADR-529 given together with cyclophosphamide, 5-fluorouracil, and tamoxifen in metastatic breast-cancer patients. Cancer Chemoth Pharm. 35:45–52.
- Jakobsen, P., Steiness, E., Bastholt, L., Dalmark, M., Lorenzen, A., Petersen, D., Gjedde, S.B., Sandberg, E., Rose, C., Nielsen, O.S., et al. (1991). Multiple-dose pharmacokinetics of epirubicin at four different dose levels: studies in patients with metastatic breast cancer. Cancer Chemoth Pharma. 28:63–8.
- Jeong, Y.I., Na, H.S., Oh, J.S., Choi, K.C., Song, C.E., Lee, H.C. (2006). Adriamycin release from self-assembling nanospheres of poly(DL-lactide-co-glycolide)-grafted pullulan. Int J Pharm. 322:154–60.
- Jung, S.W., Jeong, Y.I., Kim, S.H. (2003). Characterization of hydrophobized pullulan with various hydrophobicities. Int J Pharm. 254:109–21.
- Lanone, S., Boczkowski, J. (2006). Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr Mol Med. 6:651–63.
- Leathers, T.D. (2003). Biotechnological production and applications of pullulan. Appl Microbiol Biotechnol. 62:468–73.
- Levchenko, T.S., Rammohan, R., Lukyanov, A.N., Whiteman, K.R., Torchilin, V.P. (2002). Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int J Pharm. 240:95–102.
- Li, S.D., Huang, L. (2008). Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 5:496–504.
- Lourenco, C., Teixeira, M., Simoes, S., Gaspar, R. (1996). Steric stabilization of nanoparticles: size and surface properties. Int J Pharm. 138:1–12.
- Medina, C., Santos-Martinez, M.J., Radomski, A., Corrigan, O.I., Radomski, M.W. (2007). Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 150:552–8.
- Mross, K., Maessen, P., van der Vijgh, W.J., Gall, H., Boven, E., Pinedo, H.M. (1988). Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J Clin Oncol. 6:517–26.
- Müller, R.H., Jacobs, C. (2002). Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 237:151–61.
- Na, K., Bum Lee T., Park, K.H., Shin, E.K., Lee, Y.B., Choi, H.K. (2003). Self-assembled nanoparticles of hydrophobically-modified polysaccharide bearing vitamin H as a targeted anti-cancer drug delivery system. Eur J Pharm Sci. 18:165–73.
- Na, K., Lee, K.H., Bae, Y.H. (2004). pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J Cont Rel. 97:513–25.
- Park, K.H., Song, H.C., Na, K., Bom, H.S., Lee, K.H., Kim, S., Kang, D., Lee, D.H. (2007). Ionic strength-sensitive pullulan acetate nanoparticles (PAN) for intratumoral administration of radioisotope: ionic strength-dependent aggregation behavior and (99m)Technetium retention property. Colloids Surf B Biointerfaces. 59:16–23.
- Rekha, M.R., Sharma, C.P. (2007). Pullulan as a promising biomaterial for biomedical applications: a perspective. Trends Biomater Artif Organs. 20:116–21.
- Seymour, L.W., Miyamoto, Y., Maeda, H., Brereton, M., Strohalm, J., Ulbrich, K., Duncan, R. (1995). Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. Eur J Cancer. 31:766–70.
- Shimizu, T., Kishida, T., Hasegawa, U., Ueda, Y., Imanishi, J., Yamagishi, H., Akiyoshi, K., Otsuji, E., Mazda, O. (2008). Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 367:330–5.
- Shingel, K.I. (2004). Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr Res. 339:447–60.
- Sonaje, K., Lin, Y.H., Juang, J.H., Wey, S.P., Chen, C.T., Sung, H.W. (2009). In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials. 30:2329–39.
- Tsuchihashi, M., Harashima, H., Kiwada, H. (1999). Development of a pharmacokinetic/pharmacodynamic (PK/PD)-simulation system for doxorubicin in long circulating liposomes in mice using peritoneal P388. J Contr Rel. 61:9–19.
- Vega-Villa, K.R., Takemoto, J.K., Yanez, J.A., Remsberg, C.M., Forrest, M.L., Davies, N.M. (2008). Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 60:929–38.
- Yoksan, R., Chirachanchai, S. (2008). Amphiphilic chitosan nanosphere: studies on formation, toxicity, and guest molecule incorporation. Bioorg Med Chem. 16:2687–96.
- Zhang, H.Z., Gao, F.P., Liu, L.R., Li, X.M., Zhou, Z.M., Yang, X.D., Zhang, Q.Q. (2009). Pullulan acetate nanoparticles prepared by solvent diffusion method for epirubicin chemotherapy. Colloids Surf B Biointerfaces. 71:19–26.