Abstract
In the present investigation, pulsatile release beads were prepared by ionic gelation technique. Theophylline dual-cross-linked beads were prepared by dropping dispersed phase of theophylline, Delonix regia gum (DRG), and sodium alginate into the dispersion phase of different concentration of calcium chloride solution followed by aluminium chloride solution. The formulated beads were further coated by Eudragit L & S 100 in the ratio 1:2 w/w in order to achieve desired lag time. In vitro release study showed lag time of 5–7 h before release of theophylline from the formulated beads, which were found to be intact for 6 h. Thus, formulated dual cross-linked beads when administered at bed time may release theophylline when needed most for chronotherapeutics of early morning asthmatic attacks in chronic patients. In vivo radio imaging study carried out in New Zealand white strain rabbit confirms the findings of in vitro results.
Introduction
Multiparticulate systems provide a more reproducible transit to the colon than single-unit dosage forms and release drug more rapidly because of a higher surface area. Conversely, they are more prone to premature drug release in the upper gastrointestinal tract. In this study an attempt has been made to formulate dual cross-linked pulsatile beads of theophylline by ionic gelation technique using calcium chloride, aluminium trichloride, Delonix regia gum, and sodium alginate.
Sodium alginate (SA) is an alginic acid sodium salt, a naturally occurring non-toxic polysaccharide found in brown algae. Alginate has been widely used as a food and pharmaceutical excepient such as tablet binder, disintegrant, diluent in capsule formulation, and gelling agent to delay the drug dissolution from the tablet. It is made up of two molecules of uronic acid, one α-L-glucoronic, one β-D-mannuronic acid, and homopolymeric blocks with an alternating sequence. In the ionic gelation process the uronic acids cross-links with divalent cations, such as Ca++. Three-dimensional structure of calcium alginate gel is formed when each Ca++ ion takes part in nine co-ordination links with an oxygen atom. This principle has been applied for preparing alginate-based beads as a drug delivery system. Calcium–alginate beads by ionotropic gelation have been prepared by dropping the drug-containing SA dispersion into a calcium chloride solution (CitationOstberg et al., 1994). The inert environment within the polymer network of alginates allows for the entrapment of a wide range of bioactive substances, cells, and drug molecules with minor interactions between them and the biopolymer. For macromolecular drugs an alginate-based system is useful for formulation of pulsatile drug delivery system (CitationGriffith, 2000). The release of drug from the gel matrix of calcium–alginate beads depends upon the diffusion and the swelling of the beads (CitationPasparakis & Bouropoulos, 2006). The release pattern of loaded drug substance could be modified by incorporating some water-soluble polymers along with sodium alginate, such as chondroitin sulfate (CitationWang & He, 2002), konjac glucomannan (CitationKikuchi et al., 1997), gelatin (CitationMurata et al., 1996), and chitosan (CitationMurata et al., 1993; CitationAlmeida & Almeida, 2004). The strength of beads depends on the manuronic/glucuronic ratio in the alginate and on the type of divalent ion. Ca-alginate beads are sensitive to phosphate (PO4−−) ions. The presence of PO4−− leads to early erosion of the beads. This is one of the major shortcomings of Ca++ alginate beads. Thus, there is need to use trivalent metal ions such as Al+++ and Fe+++ which were found to be superior to divalent metal ions such as Ca++, Ba++, Cu++, and Cd++.
Delonix regia gum (DRG) is a non-toxic, non-ionic polysaccharide consisting of a (1-4) D-mannose (i.e. 2, 3, 6-tri-O-methyl-D-mannose) main backbone with branch points from their 6-positions linked to D-galactose (i.e. 2,3,4,6-tetra-O-methyl-D-galactose). It consists of (1-4)-D-mannose units with –D- galactose units attached by (1–6) linkages. It is derived from the seeds of Delonix regia (Bojer) Raf. (Flame tree, Gul mohar), family Fabaceae. There are two mannose residues for every galactose residue (CitationKapoor, 1972). It has been reported that DRG can be used as a tablet binder and disintegrant in pharmaceutical formulations (CitationKale et al., 2009) and hydrogel plug material in pulsatile drug delivery (CitationTekade & Gattani, 2009).
The objective of the present investigation was to prepare dual cross-linked pulsatile release beads containing theophylline (TPH) as a model drug and employing a simple technique (i.e. ionotropic gelation) using sodium alginate and DRG. The purpose of combining sodium alginate and DRG for formulation of dual cross-linked bead is to get the advantage of increased swelling of both these natural polymers in higher pH followed by burst release after a pre-determined lag time. The prepared beads were further coated with eudragit polymers having pH-dependent solubility in order to increase the lag time prior to rapid and complete drug release. It has been reported that Eudragit L 100 & S 100 in a ratio of 1:2 w/w showed maximum solubility in pH 6.8 buffer solution. Thus, this combination of these two polymers was selected for coating of dual cross-linked beads.
Materials and methods
Materials
DRG was isolated and characterized in our laboratory. Sodium alginate (Viscosity 3500 cps) was obtained from Sigma Aldrich Chemie GmbH (Steinheim, Germany). Theophylline was supplied as a gift sample from Mehta Pharmaceutical Industries (Thane, India). All other chemicals used were of analytical grade. Double distilled water was used throughout the experiments.
Methods
Preparation of Ca++/Al+++ single cross-linked beads
Pre-weighed amounts of sodium alginate and DRG were completely dissolved in distilled water (). TPH was added into the above solution and stirred for a few minutes using a mechanical stirrer to achieve uniform mixing. Then, whole dispersion was dropped into the CaCl2/AlCl3 solution using an insulin syringe and then cured for 15–25 min for further gelation of alginate:DRG beads. Following cross-linking for pre-determined times; the cross-linked beads were separated by filtration and washed thrice with 50 ml double distilled water.
Table 1. Composition and conditions for formulation of dual cross-linked beads.
Preparation of dual cross-linked beads
The Ca++ cross-linked beads (B6, B7, and B8) were directly dropped into 50 ml solution of various concentrations of aluminum chloride (% w/v) for 15–25 min and then the Ca++ and Al+++ dual cross-linked beads were separated by filtration, washed trice with 50 ml double distilled water, air dried, and finally vacuum-dried at 40°C till constant weight.
Coating of formulated dual cross-linked beads
Solution (10% w/w) of Eudragit L 100 and S 100 in 1:2 w/w ratios was prepared in methanol. Triethyl citrate (10% w/w with respect to polymer weight) was added to the solution as a plasticizer. The resulting suspension was sprayed on TPH containing dual cross-linked beads using a fluidized bed coater (Mini Glatt Model, Glatt GmbH, Binzen, Germany) till weight gain was 15% w/w. Operating parameters are summarized in . At each stage, the beads were fluidized for an extra 5 min and samples were dried in an oven for 2 h at 35–40°C.
Table 2. The operating parameters used for coating of TPH beads.
In vitro characterization of fabricated beads
Size analysis of dual cross linked beads.
Size analysis of dual cross-linked beads was carried out using Motic microscopy. In motic microscopy randomly selected 50 beads of these formulations were observed under a Motic DMWB2-223 digital microscope fitted with 1/3 CCD camera imaging accessory and using Motic Images 2000 (1.3 Version) image analysis software.
The images of beads were analyzed for their average diameter and different shape factors such as roundness and circularity factor. The roundness values (CitationNeau et al., 2000) and circularity (CitationPodczeck & Newton, 1994) of the formulated dual cross-linked beads were determined by the following equations:
where P is the perimeter of the pellet image and A is the area determined by the total number of pixels within the feature. The factor 0.9399 corrects the perimeter for the effect of the corners produced by digitization of the image. A roundness value of 1 corresponds to the image of a perfect sphere.
Determination of yield.
The yield of formulated beads were calculated using the weight of final product after drying with respect to the initial total weight of the drug and polymer used for preparation of beads and percent yields were calculated as per the following formula,
where Pm and Tm are practical and theoretical weight of the beads, respectively.
Drug content
Drug content of beads was determined by a previously reported method (CitationTekade & Gattani, 2009). Accurately weighed 50 mg of each formulation of drug loaded dual cross-linked alginate: DRG beads were individually placed in 100 ml phosphate buffer of pH 6.8. It was then mechanically stirred to promote swelling and breaking of cross-linked structure which causes liberation and dissolution of TPH in phosphate buffer. These solutions were then filtered through Whatmann’s filter paper (diameter 0.45 µm) and subjected for analysis by a UV spectrophotometer (1700, Shimadzu, Japan) at 272 nm. Entrapment efficiency of the beads was calculated by the following formula,
where AQ and TQ are the actual quantity and theoretical quantity of drug present in the beads, respectively.
Swelling study
Swelling study was carried out according to previously reported methods (CitationRemunan-Lopez & Bodmeier 1997; CitationHalder et al., 2005; CitationSharma & Pawar, 2006; CitationMuangsin et al., 2007; CitationPongjanyakul & Puttipipatkhachorn, 2007). The diameter of 20 completely dried beads were measured using a digital photomicroscope (Motic 2000, Version 2.0, China) and then placed separately in a petridish containing 20 ml of 0.1 N HCl (pH 1.2), phosphate buffer (pH 6.8), or distilled water at room temperature (37 ± 0.5°C). At pre-determined time intervals, the diameter of each bead was determined at two different positions and swelling (%) of the beads was calculated according to the following equation,
where D0 and Dt are the initial diameter of the beads and the diameter of the beads at a given time, respectively.
Scanning electron microscopy (SEM)
The surface topography was studied using a scanning electron microscope. The formulated dual cross-linked beads were coated with palladium for 5 min. Morphological examination of beads surface was performed at 10 KV at appropriate magnification using a scanning electron microscope (JEOl JSM-5800LV, Tokyo, Japan).
In vitro drug release studies
In vitro drug release studies were performed in a USP XXIII dissolution apparatus (TDT-08L, Electrolab, India) with paddle speed at 50 rpm. Studies were conducted in 900 mL of simulated gastric fluid (0.1 N HCl, pH 1.2) for 2 h and simulated colonic fluid (pH 6.8) for remaining hours of the study at 37 ± 0.5°C. At the end of 2-h simulated gastric fluid was replaced with simulated colonic fluid and the study was continued for the next 10 h. At regular intervals of time, an aliquot was removed and replenished with fresh pre-warmed medium. The aliquot was assayed for theophylline content using a UV spectrophotometer (1700, Shimadzu, Japan) at 272 nm.
In vivo radio imaging study in rabbits
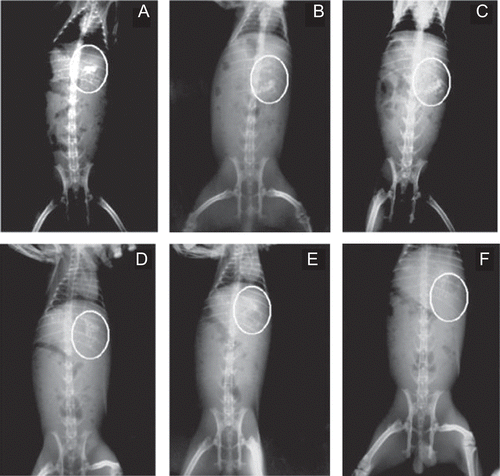
In vivo radio imaging study was performed according to a previously reported method (CitationTekade & Gattani, 2010). The study protocol for in vivo study was approved by the institutional animal ethics committee of R. C. Patel Institute of Pharmaceutical Education & Research (Shirpur, India). Three adult male New Zealand white strain rabbits (n = 3) weighing ∼ 2.0–2.5 kg were used for this study. Rabbits were fasted overnight before the start of the study. For in vivo radio imaging study, dual cross-linked beads of barium sulfate equivalent to TPH quantity in optimized batch B8 were prepared to aid in proper visualization during gastro-intestinal transit. The formulated beads of barium sulfate were administered through plastic tubing followed by flushing with 25–30 ml of water. During the entire study rabbits had free access to water only. Photographs were taken at 1, 2, 4, 5, 6, and 7 h.
Results and discussion
In vitro characterization of formulated beads
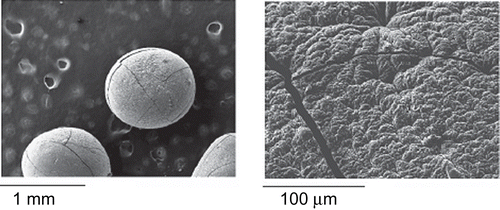
Eight formulations of TPH-loaded dual cross-linked beads were prepared by ionic gelation technique. The important characteristics of prepared beads are summarized in . All the formulated beads prepared with different drug-to-polymer ratio were found to be uniform size and nearly spherical in shape (). The roundness and circularity values for the formulated beads indicate spherical shape.
Table 3. Physical characterization of formulated dual cross–linked beads.
Photo microscopic studies showed that the shape of the single as well as dual cross-linked beads was near spherical. Also, no difference in the shape of the beads after dual cross-linking with trivalent ion was observed.
Production yield and entrapment efficiency
The production yield and drug entrapment efficiency were found to be high for beads of dual cross-linked formulation batches B6, B7 and B8. Moreover, amongst these three batches entrapment efficiency was highest in the case of batch B7, suggesting 20%w/v concentration of AlCl3 is the optimum and the entrapment efficiency decreases with further increase in concentration of trivalent cross-linking agent. The entrapment efficiency in the case of single cross-linked beads of batches B1, B2, and B3 prepared using CaCl2 as a cross-linking agent were found to be less due to less cross-linking time and dissolution of some amount of TPH in the solvent used for cross-linking, whereas Batch B4, which is also single cross-linked using AlCl3 showed greater entrapment efficiency, suggesting trivalent Al+++ is a better cross-linking agent than divalent Ca++. This fact also suggests the need for dual cross-linking ().
Swelling study
Swelling capacity of the beads was mostly determined by Sodium alginate as well as DRG content and cross-linking time. The maximum degree of swelling was observed in batches B2 and B4. Beads with lower sodium alginate content that is formulation B1 has lower swelling ability than B2. In the case of beads with higher cross-linking time, formulations B3 and B8 were characterized by lower swelling ability than the B1, B4, and B6 batches of beads with lower cross-linking time. This shows that an increase in polymer concentration increases the swelling capacity, whereas increase in cross-linking time lower the swelling capacity ().
Scanning electron microscopy
SEM photographs at different magnifications revealed the spherical nature of the formulated dual cross-linked beads. They observed smooth surface of the beads with small cracks ().
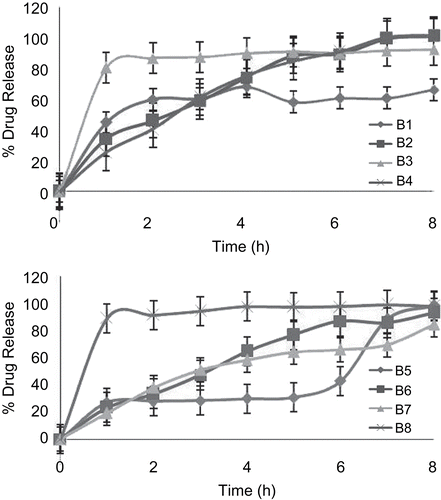
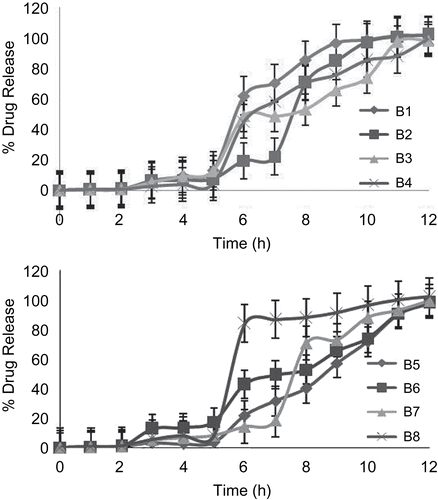
In vitro drug release studies
In vitro release studies were carried out using USP dissolution apparatus 2 (Paddle type) at 50 rpm. During in vitro release study, it was observed that release of drug from uncoated as well as coated beads takes place after complete swelling. Swelling of uncoated beads starts immediately, whereas swelling of coated beads is pH-dependent, and thus increased lag time was observed before rapid and complete drug release. In the case of uncoated formulations B3 and B8, initial burst release is 80.50% and 92.63%, respectively. This might be due to formation of brittle beads which may break and lead to rapid and complete drug release. The reason for formation of brittle beads may be attributed to higher concentration of cross-linking agent in the case of batch B3 and greater cross-linking time in the case of batch B8. While in the remaining all the uncoated formulations observed sustained release of drug (). Amongst B6, B7, and B8, which were dual cross-linked, B7 showed a lag time of 7 h. Both the batches B6 and B8 give lag times of 5 h. A sigmoidal release pattern was observed in the case of B8, which may be attributed to its brittleness due to high cross-linking time. In the case of batch B6, where curing time was less, sustained release was shown after a lag time. Formulation B7 showed a lag time of 7 h, while in the remaining formulations a 5 h lag time was observed. Thus, batch B7 has been optimized for in vivo radio imaging study ().
In vivo radio imaging study
The results of the in vivo radio imaging study in rabbits demonstrated the intactness of the dual cross-linked beads for 6 h, establishing the results of the in vitro study (). The observed 5–7 h lag time during in vitro study was supported by in vivo radio imaging study, which showed intactness of the beads for 6 h in rabbits.
In vitro–in vivo correlation
In vitro release data have been correlated with in vivo radio imaging study conducted in rabbits. We observed a 5–7 h lag time during in vitro release, whereas radio imaging technique showed intactness of beads till 6 h, suggesting IVIVC for product performance in rabbits.
Conclusion
In conclusion, it is possible to achieve pulsatile release of the drug from the beads by dual cross-linking and coating by Eudragit L & S 100. In vitro as well as in vivo study showed a lag time of 5–7 h, which is desired for chronotherapeutics of early morning attacks in chronic asthmatic patients. Thus, when we administered formulated dual cross-linked beads at bed time, they may release the drug early morning. Although the in vitro study showed a lag time of 5–7 h and the radio imaging study showed intactness of the beads for 6 h in rabbits, it is difficult to correlate the lag time on the basis of intactness of beads. Thus, plasma study needs to be performed in order to confirm the findings of in vitro as well as in vivo radio imaging study. Moreover, formulated dual cross-linked beads may not perform in a similar manner in humans due to some physiologic differences between species and therefore needs clinical investigation.
Declaration of interest
The financial support of the All India Council for Technical Education, New Delhi, under research promotion scheme (Grant No. 8023/BOR/RID/RPS-166/2007-08) is gratefully acknowledged. The authors are thankful to the sophisticated test and instrumentation centre, Kochi for help in SEM and DSC studies. The Management and Principal (R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur) are gratefully acknowledged for providing facilities to carry out this research work.
References
- Almeida, P.F., Almeida, A.J. (2004). Cross-linked alginate–gelatine beads: a new matrix for controlled release of pindolol. J Contr Rel. 97:431–9.
- Griffith, L.G. (2000). Polymeric biomaterials. Acta Mater. 48:263–77.
- Halder, A., Maitim, S., Sa, B. (2005). Entrapment efficiency and release characteristics of polyethyleneimine-treated or untreated calcium alginate beads loaded with propranolol–resin complex. Int J Pharm. 302:84–94.
- Kale, R.H., Joshi, U.M., Ambhore, D.P., Sitaphale, G.R. (2009). Evaluation of Delonix regia Raf. endospermic mucilage as tablet binder. Int J Chem Tech Res. 1:11–15.
- Kapoor, P.V. (1972). A galactomannan from the Delonix regia. Phytochemistry. 11:1129–32.
- Kikuchi, A., Kawabuchi, M., Sugihara, M., Sakurai, Y., Okano, T. (1997). Pulsed dextran release from calcium – alginate gel beads. J Contr Rel. 47:21–9.
- Muangsin, N., Piyakulawat, P., Praphairaksit, N., Chantarasiri, N. (2007). Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS Pharm SciTech. 8:E1–E11.
- Murata, Y., Miyamoto, E., Kawashima, S. (1996). Preparation of chitosan-reinforced alginate gel beads - effects of chitosan on gel matrix erosion. J Contr Rel. 38:101–8.
- Murata, Y., Miyamoto, E., Kawashima, S., Maeda, T. (1993). Preparation of chitosan-reinforced alginate gel beads – effects of chitosan on gel matrix erosion. Int J Pharm. 96:139–45.
- Neau, S.H., Chow, M.Y., Hileman, G.A., Durrani, M.J., Gheyas, F., Evans, B.A. (2000). Formulation and process considerations for beads containing Carbopol® 974P, NF resin made by extrusion-spheronization. Int J Pharm. 199:129–40.
- Ostberg, T., Lund, E.M., Graffner, C. (1994). Calcium alginate matrices for oral multiple unit administration: IV. Release characteristics in different media. Int J Pharm 112:241–8.
- Pasparakis, G., Bouropoulos, N. (2006). Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int J Pharm. 323:34–42.
- Podczeck, F., Newton, J.M. (1994). A shape factor to characterize the shape of spheroids. J Pharm Pharmacol. 46;82–5.
- Pongjanyakul, T., Puttipipatkhachorn, S. (2007). Xanthan–alginate composite gel beads: molecular interaction and in vitro characterization. Int J Pharm. 331:61–71.
- Remunan-Lopez, C., Bodmeier, R. (1997). Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J Contr Rel. 44:215–25.
- Sharma, S., Pawar, A. (2006). Low density multiparticulate system for pulsatile release of meloxicam. Int J Pharm. 313:150–8.
- Tekade, A.R., Gattani, S.G. (2009). Development and evaluation of pulsatile drug delivery system using novel polymer. Pharm Dev Tech. 14:380–7.
- Tekade, A.R., Gattani, S.G. (2010). Development and evaluation of pulsatile drug delivery system using novel polymer. Part II: in vivo radio imaging study. Pharm Develop Tech. doi: 10.3109/10837450903397628.
- Wang, K., He, Z. (2002). Alginate-konjac glucomannan-chitosan beads as controlled release matrix. Int J Pharm. 244:117–26.