Abstract
The aim of this study was to develop a novel nanoparticulate formulation and test its potential for oral peptide drug delivery. Chitosan-6-mercaptonicotinic acid is a novel thiolated chitosan with strong mucoadhesive properties. Nanoparticles were developed by an ionic gellation method. The obtained particles were characterized in terms of mucoadhesion, stability, toxicity, and in vitro release. Human insulin (HI) was chosen as a model peptide drug, incorporated in the particles and orally administered to rats. Human insulin was quantified in the blood by means of ELISA. The size of the obtained particles was in the range of 200–300 nm and the zeta potential was determined to be +8−+23 depending on the amount of thiol groups attached on the polymer. After 3 h of incubation up to 60% of the thiolated chitosan nanoparticles remained attached to the mucosa in contrast to 20% of unmodified chitosan particles. The AUC of HI after oral administration of thiolated chitosan nanoparticles was 4-fold improved compared to unmodified chitosan nanoparticles. Due to these improvements, chitosan-6-mercaptonicotinic acid nanoparticles are promising vehicles for oral delivery of peptide drugs.
Introduction
Peptide drug delivery is extensively studied because of the high therapeutic efficacy of this class of active pharmaceutical ingredients for treatments of many diseases. The improvements in biotechnology made it possible to produce peptides on a commercial scale. The oral administration of peptide drugs is one of the greatest challenges in pharmaceutical technology. When orally administered, peptide drugs are degraded in the low gastric pH as well as by different digestive enzymes in the stomach and small intestine (CitationKhafagy et al., 2007). A promising strategy to tackle this problem is the use of nanoparticulate carriers. In particular, nanoparticles made from biodegradable polymers represent an exciting approach to improve the uptake of orally administered drugs (CitationCui et al., 2006). One polymer of choice is chitosan. Chitosan has the ability to gel spontaneously in contact with multivalent polyanions due to the formation of inter- and intra-molecular cross-linkages. Among poylanions, tripolyphosphate (TPP) is most commonly used because of its safety profile and quick gelling ability. Nanoparticles are formed immediately upon mixing of TPP and chitosan solution as ionic linkages are formed between TPP and chitosan (CitationGan et al., 2005). The advantageous features of chitosan-TPP systems include the formation under mild conditions, a positive surface charge, and a great capacity for the association of peptides, proteins, and oligonucleotides (CitationShu & Zhu, 2000). Indeed, the polymeric component of the particles such as chitosan should assure protection of the drug towards enzymatic degradation and controlled release (CitationSakuma et al., 1997). However, as the stability of these particles is provided by the addition of polyanionic excipients such as TPP, the positive charges of chitosan are neutralized. This results in loss of the mucoadhesive properties of chitosan. Mucoadhesion could alternatively be gained by the choice of specific polymers such as thiomers. Thiomers are thiolated polymers on which a thiol-bearing compound has been covalently attached. They are known to possess mucoadhesive, enzyme inhibition, permeation enhancing, and sustained release properties. Furthermore, thiol groups within these nanoparticles provide stability to such particles via the formation of intra- and inter-molecular disulfide bonds. Recently a novel thiolated chitosan, called chitosan-6-mercaptonicotinamide (chitosan-6-MNA), has been synthesized and characterized (CitationMillotti et al., 2009). This novel thiolated chitosan exhibits a pH-independent reactivity, comparatively high mucoadhesive properties, and biocompatibility. In order to combine these two promising strategies it was the aim of this study to develop a nanoparticulate drug delivery system based on chitosan-6-mercaptonicotinic acid and to evaluate whether these nanoparticles have potential for oral delivery of peptides. Because of already well established in vivo test systems, insulin was chosen as a representative model peptide drug.
Material and methods
Materials
6-Mercaptonicotinic acid (6-MNA), dioxane, N-3(dimethylaminopropyl)-N- ethylcarbodiimide hydrochloride (EDAC), and human insulin were purchased from Sigma-Aldrich (Vienna, Austria). Chitosan (MM 400 kDa; DD 70–80%) and tris(2-carboxyethyl)phosphine hydrochloride (TCEP) were obtained from Fluka. CytoTox-ONE(TM) homogeneous Membrane Integrity Assay was purchased from Promega. Surfactant-Free Carboxyl White Polystyrene Latex and Carboxyl White Polystyrene Latex were purchased from Interfacial Dynamics. Pyrogen Ultra (sensitivity = 0.06 EU/ml) was obtained from Lonza. ELISA kit for human insulin was purchased from Mercodia (Uppsala, Sweden).
Synthesis of chitosan-6-mercaptoicotinic acid
First, chitosan middle molecular mass was depolymerized in order to obtain chitosan with molecular mass of 20 kDa (Bravo-Osuna). Briefly, 200 ml of a 2% (m/v) chitosan solution (400 kDa) in 6% (v/v) acetic acid solution was depolymerized by the addition of 20 ml of a 7% (m/v) NaNO2 solution. After 1 h reaction, chitosan was precipitated by the addition of 5 M NaOH upon reaching a pH of 9. The precipitate was filtered and washed with acetone. Afterwards the precipitate was resuspended in 100 ml 0.1 M acetic acid solution dialyzed against water and freeze-dried. Chitosan-6-mercaptonicotinic acid was synthesized following a method previously described (CitationMillotti et al., 2009). Briefly, 1 g of the depolymerized chitosan was mixed with 2.5 g of 6-mercaptonicotinic acid dissolved in 100 ml of a dioxane–water mixture (80 ml + 20 ml). The pH was adjusted to 5 and EDAC was added in final concentrations of 5 mM, 10 mM, and 25 mM. The pH was adjusted to 6 and the reaction was left to proceed for 7 h. At the end of the reaction, disulfide bonds formed during the reaction were reduced by the addition of TCEP. The final product was then dialyzed and freeze-dried.
Nanoparticles formation
Nanoparticles were formed following the ionic gelation method. Thiolated chitosan and unmodified chitosan were dissolved in 0.05% (v/v) acetic acid solution in a final concentration of 0.2 and 0.25% (m/v) solution, respectively, and the pH of the polymer solution was adjusted to 4.5. Tripolyphosphate was dissolved in distilled water at concentrations of 0.1% (m/v) and slowly added to the polymer solution of 0.2% (m/v), and in a concentration of 0.2% (m/v) and slowly added to the polymer solutions of 0.25% (m/v). The solution was stirred for 40 min. Thiolated chitosan nanoparticles were stirred for an additional 4 h to favor the formation of disulfide bonds within the particle. Due to the diversity of concentrations proposed by the literature, the formation of the particles was followed by measuring the opalescence at 400 nm and the size along the preparation process. Particles were collected by centrifugation for 45 min at 4500 rpm and resuspended in water. This process was repeated three times. Thereafter, trehalose in a final concentration of 3% (m/v) was added and the particles were lyophilized. For mucoadhesion studies, the marker fluorescein diacetate (FDA) was incorporated in the obtained nanoparticles. First, 1 ml of the particles suspension after centrifugation was transferred to 1 ml of a 0.1% (m/v) FDA solution in acetonitrile. The suspension was then incubated in a thermomixer at 25°C for 1 h. Afterwards the particles were centrifuged for 10 min at 13,400 rpm and lyophilized. The volume of the TPP solution necessary to reach 0.55 absorbance was identified for each polymer individually. Afterwards, insulin was dissolved in the TPP solution and used in insulin-to-polymer (m/m) ratio of 1:5. Although the volume of TPP added to the polymers was different, the amount of insulin added was the same for all the formulations. The TPP solutions containing the not incorporated insulin were removed by centrifugation. The unbound insulin was quantified by HPLC. The purification step was repeated three times. The association efficiency was calculated as association efficiency = (total amount of insulin-free insulin)/(total amount of insulin)*100 (CitationFernandez-Urrusuno et al., 1999). Insulin was thereafter incorporated in the formulation showing the most promising results.
Determination of the degree of modification
The total amount of thiol group present was measured as follows. 6-MNA exhibits an absorption maximum in the range of 260–280 nm in the UV-VIS range. The freeze-dried conjugates were dissolved in a 0.1 M acetate buffer pH 3.3-dioxane mixture (1 + 1) in a final concentration of 0.2 mg/ml. The amount of ligand bound to chitosan was calculated from the corresponding calibration curve made with 6-mercaptonicotinic acid (0–0.16 µmol/ml) dissolved in 0.1 M acetate buffer pH 3.3-dioxane mixture (1 + 1) with absorbance values at 268 nm (Beckman DU 650 spectrophotometer) from 0–0.9 giving origin to the following equation: y = 7.3574x – 0.003, R2 = 0.9987.
Determination of reduced thiol groups
The amount of thiol groups in their reduced form was measured via iodometric titration (CitationBravo-Osuna et al., 2007). Briefly, different concentrations of the conjugate have been prepared (0.5–0.05 mg/ml) in 0.5 M acetate buffer pH 2.7. Then 1 ml starch aqueous solution (1% m/v) was added to 2 ml of each sample concentration. Afterwards, 0.1 M iodine was added until the majority of the samples of each conjugate displayed a blue-violet color. The oxidation reaction was allowed to proceed for 24 h, protected from light. A calibration curve was elaborated under the same conditions with cysteine-HCl solution in a concentration of 10–40 µmol/l. The excess iodine that reacts with starch was measured at 560 nm. The following equation was obtained: y = −54.648x + 2.0563; R2 = 0.9974.
Particles characterization
Thiol groups present on the particles were quantified by iodometric titration. Briefly, 250 µl of particles suspension (immediately after purification step) were incubated with 250 µl of an acetate buffer solution at pH 2.7. Afterwards, 500 µl of starch aqueous solution (4% m/v) were added. The solution was then incubated for 24 h with 300 ml of 0.1 M iodine solution. After this step the same procedure was adopted as described above. Size distribution and zeta potential were determined in distilled water with a particle sizer (Z potential/Particle sized PSS Nicomp, Santa Barbara, CA). In order to check the influence of lyophilization, the particles size and Z potential were also measured after the particles have been lyophilized.
Mucoadhesion studies
Freshly excised porcine intestinal mucosa was glued on a half pipe and placed at an angle of 45°C in an incubator at 37°C and 100% humidity. Afterwards the mucosa was continuously rinsed with 100 mM phosphate buffer pH 6.5. A constant flow rate was provided by a peristaltic pump. The flow rate was 5 ml/min. Afterwards, 30 mg of particles were applied to the mucosa and continuously rinsed with the buffer. After 1, 2, and 3 h the mucosa with the remaining marker was incubated in 20 mL of 5 M NaOH for 40 min at 37°C under gentle shaking. In this way, fluoresceine diacetate (FDA) is hydrolyzed to sodium fluoresceine (NaFlu). After centrifugation at 13,400 rpm for 5 min, fluorescence of each sample was measured with a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 520 nm. The calibration curve was done by incubating the mucosa in the incubator and adding a known amount of nanoparticles in a decreasing concentration and proceeding with the hydrolysis of FDA.
Stability studies
Stability of the particles in acid media was evaluated by a method described by CitationDhawan et al. (2004) Briefly, the particles were incubated in 0.1 M HCl in a final concentration of 1% (m/v). At pre-determined time points the transmission of the samples was measured at 400 nm. The stability of the particles at physiological pH was determined following a method described by CitationGrabovac and Bernkop-Schnürch (2007) Particles were incubated in 0.1 M phosphate buffer pH 6.8 in a final concentration of 1% (m/v). At pre-determined time points the size of particles was measured.
Toxicity studies
Cell culture conditions
For the Caco-2 cell line, the medium was composed of 80% minimum essential medium (MEM) (with Earle’s salts), 20% 10 mM phosphate buffered saline (PBS) pH 7.2, 1× non-essential amino acids, and 1% penicillin-streptomycin liquid. The media used for the EAhy926 cells consisted of 90% Dulbecco’s modified Eagle’s medium, 10% 10 mM phosphate buffered saline (PBS), 2 mM L-glutamate, and 1% penicillin-streptomycin liquid.
LDH release assay
A standardized number of cells was seeded in a 96 wells plate (100 µl per well) and grown for 24 h in an incubator (37°C, 5% CO2, 95% relative humidity) prior to stimulation. Medium was removed and 100 µl of the polymer (0–100 µg/ml in medium) were added. Experiments were performed in triplicate. The plate was incubated at 37°C, 5% CO2, and 95% relative humidity for 24 h. The standard assay set-up included: blank (culture medium alone), growth control (culture medium with the cells), lysis control (untreated cells in medium where 100% lysis was performed), particulate positive and negative controls (26 nm carboxyl White Polystyrene latex as positive control and 160 nm Surfactant-Free Carboxyl white polystyrene Latex as negative control). The CytoTox-ONE assay kit was performed as indicated by the producer. The average value of the blank was subtracted from every fluorescence value.
In vitro release studies from nanoparticles suspension
At first, 8 mg of the lyophilized nanoparticulate material were suspended in 2 ml of the release medium consisting of 0.1 M phosphate buffer pH 7.8. Aliquots of 100 µl were withdrawn after 0, 15, 30, 45, 60, 90, 120, 180, 240, and 360 min. The withdrawn volume was replaced by release medium. Samples were analyzed by HPLC.
HPLC analysis
The mobile phase consisted of eluent A:acetonitrile (ACN) and eluent B:0.1% (v/v) trifluoroacetic acid (TFA). A gradient elution was performed with the flow ratio of 1 ml/min over 0–22 min (linear gradient from 91% A/9%B to 39% A/61% B). Insulin was analyzed quantitatively at a wavelength of 254 nm. For determining the linearity of the system, eight concentrations in the range from 0.03–2 mg/ml of insulin were prepared and analyzed.
In vivo evaluation of the delivery system
The protocol for the studies was approved by the Animal Ethical Committee of Vienna, Austria, and adhered to the principles of Laboratory Animal Care. For the studies, non-diabetic male Spargue-Dawley rats with an average body weight of 250 g were used. Rats were fasted 3 h prior to the experiment. Before the gavage of formulations, 150 µl of blood samples were withdrawn from the tail vein and collected in 500 µl Eppendorf tubes containing 30 µl of a 3.8% (m/v) citrate solution. Thereafter the samples were centrifuged for 10 min at 13,400 rpm. The serum was separated and stored at −20°C. Groups of five rats were dosed with the thiolated chitosan particulate formulation or with chitosan particulate formulation as controls. An aqueous suspension of nanoparticles was administrated via a gavage syringe. Afterwards blood samples were collected from the tail vein at intervals of 30, 60, 120, 180, 240, and 360 min, and treated as described above. The concentration of insulin within the serum was determined by an ELISA test kit for human insulin.
Pharmakokinetic and statistical data analysis
Calculation has been done by the use of the computer software OriginPro. The areas under the concentration time curves (AUC) were calculated according to the linear trapezoidal rule. Statistical data analyses were performed using the Student’s t-test with p < 0.05 as the minimum level of significance.
Results and discussion
Nanoparticles formation and insulin incorporation
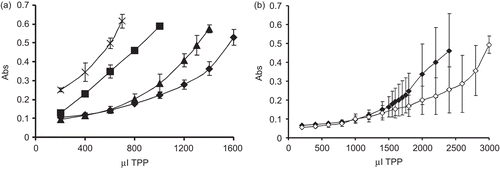
The characterization of the polymers used to develop the particles is presented in and , respectively. The nanoparticles present less thiol groups compared to the polymers of which they have been prepared as during the formation of nanoparticles disulfide bonds are formed. These disulfide bonds are essential in order to stabilize the particles. The lyophilization process does not have an important influence on particles size and Z potential (data not shown). The zeta potential was positive for all particles, but the value decreases as the degree of substitution of the polymer increases. This observation can be explained by the covalent linkage of 6-MNA to chitosan transforming the primary amino group in an amide substructure. Two chitosan and TPP concentrations were analyzed. The formation of nanoparticles was followed by measuring the opalescence to detect the optimum volume of TPP that has to be added. Indeed formation of nanoparticles is only possible for some specific concentrations of chitosan and TPP (CitationXu & Yumin, 2003). At various TPP and chitosan concentrations, three different systems can be visually identified: solution, suspension, and aggregates (CitationPan et al., 2002). The formation of the particles can be followed by measuring turbidity (CitationNurkeeva et al., 2003). Indeed, the appearance of the solution changes when a certain amount of TPP ions are added to the chitosan solution, from a clear to opalescent solution that indicates a change of the physical state of chitosan to form particles, or eventually aggregates (CitationKatas & Alpar, 2006). The formation of nanoparticles is presented in . By using a concentration of 0.25% chitosan and 0.2% TPP particles formation is very reproducible and the standard deviation is comparatively low. Therefore, this concentration combination was selected for future studies. Furthermore, it was noticed that the optimal absorbance to reach is 0.55. Above an absorbance value of 0.6 particles form agglomerates and precipitate. Generally, the disadvantage of nanoparticles and colloidal carriers is their tendency to agglomerate during storage due to the immense surface area of the system and the resulting thermodynamic instability that favors aggregation of the colloidal particles. Freeze-drying is a well established method for the preservation of unstable molecules over long periods of time (CitationSameti et al., 2003). By freeze-drying the herein developed nanoparticles, it is easily possible to resuspend them in water and the size has been proven to remain constant. As NP3 showed the best results, insulin was encapsulated in NP3. Insulin was dissolved in the TPP solution which presents a pH of 7.5. At this pH insulin is soluble and negatively charged, so this will prevent the interaction between TPP and insulin. Once the insulin is added to the positively-charged chitosan, insulin, as well as TPP will form a complex. The amount of encapsulated insulin was quantified to be 32.42% (± 3.42) for unmodified chitosan nanoparticles and 36.26% (± 4.58) for NP3 thiolated chitosan nanoparticles. The encapsulation efficiency was therefore in the same order for unmodified and thiolated chitosan.
Table 1. Characterization of the polymer used to develop nanoparticles.
Table 2. Particles characterization.
Figure 1. (a) Monitoring of particles formation due to addition of indicated amounts of a 0.2% (m/v) solution of TPP to 10 ml of a 0.25% (m/v) solution of polymers. Opalescence was measured at 400 nm. Legend: (×) NP3, (▪) NP2, (▴) NP1, (♦) unmodified chitosan. The results are mean of at least five experimental values ± SD. (b) Monitoring of particles formation due to the addition of indicated amounts of a 0.1% (m/v) solution of TPP to 10 ml of a 0.1% (m/v) solution of polymers. Opalescence was measured at 400 nm. Legend: (♦) NP1, unmodified chitosan (◊). The results are mean of at least five experimental values ± SD.
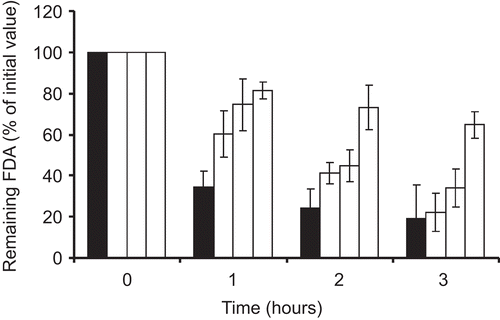
Mucoadhesion studies
The results of mucoadhesion studies showed that chitosan-6-mercaptonicotionc acid nanoparticles were more mucoadhesive compared to unmodified chitosan nanoparticles. shows the comparison of FDA incorporated in nanoparticles that remained attached on the small intestinal mucosa after a pre-determined time period. The coating of nanoparticles with mucoadhesive polymers is known to be an advantageous method to improve mucoadhesive properties of particles (CitationTakeuchi et al., 2001). However, as shown by this study, by introducing a thiol-bearing group on the mucoadhesive polymer, the mucoadhesiveness can be further improved. The mucoadhesive properties of thiomers are the result of disulfide exchange reactions or disulfide bonds formation between the thiols present on the molecule and the thiols present on the mucus. Furthermore, chitosan-6-mercaptonicotinic acid in particular can additionally contribute to increase mucoadhesiveness due to its increased hydrophobic nature. It has been proposed that mucoadhesion can be enhanced by exploiting the hydrophobic interactions between a hydrophobic entity and the hydrophobic sub-structures of mucins (CitationShi & Caldwell, 2000). Indeed mucins have hydrophobic groups such as the naked blocks of protein backbone chains, so that the hydrophobic interactions can be exploited to find new mucoadhesive polymers (CitationPeppas & Huang, 2004). The increase in mucoadhesive character should provide a delivery system with the possibility to keep the carried drug for a prolonged period of time close to the site of absorption.
Figure 2. Amount of FDA remaining on the porcine small intestinal mucosa; FDA incorporated in unmodified chitosan nanoparticles (black bars), chitosan-6-MNA NP1 (dark grey bars), chitosan-6-MNA NP 2 (light grey bars), and chitosan-6-MNA NP 3 (white bars). Indicated values are means of at least three experiments ± SD.
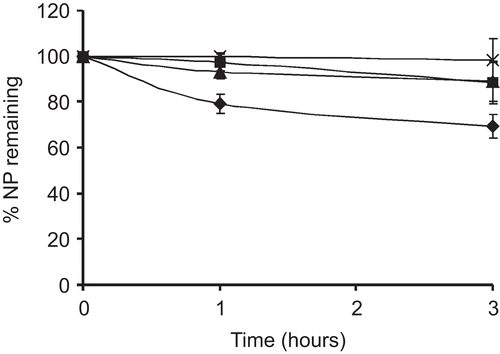
Stability studies
The stability of the nanoparticles in acidic environment was determined by measuring the transmittance. As the decrease in turbidity is directly dependent on the disintegration of the particles, transmission is used to measure the concentration of non-disintegrated particles (CitationDhawan et al., 2004). As shown in , the acidic suspension of particles became more transparent over time. In contrast, unmodified chitosan nanoparticles dissolve very rapidly at acidic pH. Indeed, ∼ 20% of the particles are dissolved within 2 h. Thiolated chitosan nanoparticles, on the other hand, remained more stable for a prolonged period of time. The stability increased with increasing amount of thiol groups present. Indeed, thiol groups present on the particles can form disulfide bridges and can therefore highly stabilize the particles. NP3, which presents the highest amount of thiol groups, remained stable for almost the whole time of the experiment. Apart from disulfide bonds formation, chitosan-6-mercaptonicotinic acid can stabilize the particles also by hydrophobic interactions of the hydrophobic groups present on the polymer. Thus, chitosan-6-MNA could offer protection of the incorporated drug also in the acidic environment. The thiolated chitosan particles were more stable compared to unmodified chitosan particles also at physiological pH (). Unmodified chitosan particles precipitated while thiolated chitosan nanoparticles, although aggregated to some extent, remained in the nano-size range. CitationLee et al. (2004) reported a similar behavior. According to CitationLee at al. (2004) unmodified chitosan nanoparticles aggregate due to the strong intra- and inter-molecular hydrogen bonding, while long acyl groups lessen these interactions. They also suggested that aliphatic and aromatic acyl groups should eliminate the need of stabilizers which are otherwise necessary to stabilize the suspension of chitosan particles.
Table 3. Stability of nanoparticles at intestinal pH.
Toxicity studies
Toxicity of the newly developed nanoparticles was investigated by the LDH assay and compared to that of unmodified chitosan. LDH is an enzyme normally found in the cytosol. Upon the cell lysis this enzyme is released into the culture medium. It can consequently be measured by a coupled enzymatic assay that results in the conversion of resazurin into a fluorescent resofurin product. As the amount of fluorescence is proportional to cell lysis, the lysed cells can therefore be quantified. The cell line used for the study was the human colorectal carcinoma cell line (Caco-2). After 24 h of incubation, the toxicity of the chitosan-6-mercaptonicotinic acid nanoparticles was in the same range as that for unmodified chitosan nanoparticles (data not shown). The percent of dead cells was ∼ 1%. As this value is very low, the newly developed nanoparticles could be regarded as non-toxic.
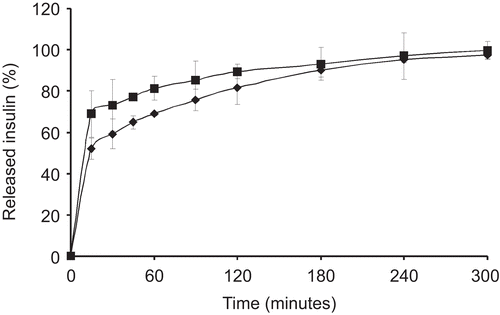
In vitro release
Insulin is readily degraded by the intestinal enzymes and it would therefore be an advantageous feature to slow down the release rate out of the formulation to provide a concentration gradient for the absorption. Chitosan is used in oral formulations due to its safety; however, it presents a very fast drug release profile (CitationKhoo et al., 2003). Thiolated chitosans instead, due to the inter- and intra- disulfide bonds formation, are able to slow down the release of the incorporated compound. In this case, 70% of the insulin was released out of unmodified chitosan nanoparticles within the first 30 min, while 50% of the insulin was released in the same time period from chitosan-6-mercaptonicotinic acid particles (). This means that unmodified chitosan nanoparticles release insulin 1.4-fold faster compared to thiolated chitosan nanoparticles. Both formulations released 100% of the incorporated insulin within 300 min, but the profile for chitosan-6-mercaptonicotinic acid particles was more retarded within the first 120 min.
In vivo studies
The in vivo evaluation was done by measuring the HI in the plasma after the oral administration of the HI-containing nanoparticles. The particles were administered in form of a suspension by the use of a gavage syringe. The amount of human insulin absorbed was measured by means of an ELISA kit for human insulin with no cross-reactivity to rat insulin. The results are presented in .
Figure 5. Serum concentration of human insulin after oral administration of formulations containing unmodified chitosan (♦) and chitosan-6-mercaptonicotinic acid (▪). Indicated values are means ± SD of at least five rats.
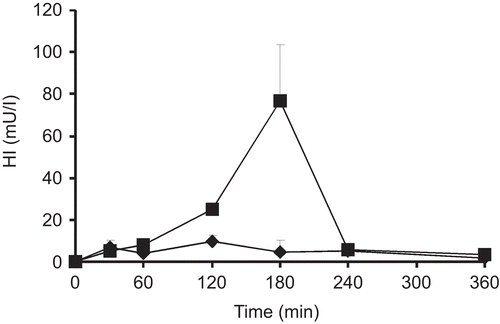
Tmax for unmodified chitosan formulation was reached after 120 min, whereas for thiolated chitosan nanoparticles Tmax was reached 180 min after oral administration. The Cmax of human insulin detected in the plasma after oral administration of unmodified chitosan particles was determined to be 9.75 mU/l. In contrast, Cmax after oral administration of chitosan-6-MNA was 76.6 mU/l. The AUC for unmodified chitosan nanoparticles was 1810 mU/lmin. Consequently, the AUC of the thiolated formulation was 4-fold higher than that of unmodified chitosan nanoparticles.
Chitosan nanoparticles are held together by ionic interactions and cannot be maintained if certain pH is reached. The isoelectric point of insulin is 5.3 (CitationFernandez-Urrusuno et al., 1999). At a pH below 5.3, both insulin and chitosan are positively charged, causing the complexes to dissociate due to electrostatic repulsion and, therefore, complete insulin release (CitationMao et al., 2005). As the nanoparticles were non-coated by their passage through the stomach, it is likely that unmodified chitosan formulation dissociated. A big part of the incorporated insulin was therefore lost due to enzymatic and pH degradation. On the other hand, strong cohesion of chitosan-6-MNA nanoparticles due to the formation of inter- as well as intra-molecular disulfide bonds within the polymeric network keep the particles un-dissociated for a longer time, as demonstrated by the stability studies. Consequently, due to the cohesiveness of the thiolated formulation, insulin should be at least to some extent protected from enzymatic degradation as the enzymes have to penetrate the carrier matrix in order to degrade the incorporated therapeutic peptide. Moreover, chitosan-6-MNA exhibits a very high mucoadhesion that provides an intimate contact of the formulation with the mucosa. It has been demonstrated that they are superior compared to unmodified chitosan. Indeed, permeation enhancing properties of thiolated polymers are the result of inhibition of protein thyrosine phosphatase (CitationClausen et al., 2002; CitationBernkop-Schnurch et al., 2003) and not only charged induced transient loosening of thigh junctions (Citationvan der Merwe et al., 2004) like in the case of unmodified chitosan. Insulin can therefore be absorbed to a greater extent from thiolated formulation by exploiting the paracellular transport.
The results are in good accordance with previous studies showing the superiority of thiolated polymers over non-thiolated polymers. Chitosan-4-thio-buthylamidine (chitosan-TBA) has shown great potential as an oral insulin tablet formulation administered in rats. Indeed the AUC of insulin for chitoasan-TBA was 2.95-fold higher compared to unmodified chitosan (CitationKrauland et al., 2004). Poly(vinyl pyrrolidone) combined with poly(acrylic acid)-cysteine particles showed a 2.3-fold improvement of the AUC of insulin compared to the non-thiolated formulation (CitationDeutel et al., 2008). Furthermore, the pharmacological efficacy of trimethyl chitosan-cysteine in decreasing the blood glucose level was 1.1-fold higher compared to unmofidied chitosan and its increased insulin transport through rat intestine compared to unmodified chitosan was 1.7–2.6-fold improved (CitationYin et al., 2009).
Generally, thiolated polymers showed a 2–3-fold improvement over non-thiolated polymers. On the other hand chitosan-6-MNA showed a 4-fold improvement over the non-thiolated polymer. This difference between chitosan-6-MNA to other thiolated polymers might be explained by the aromatic nature of the thiolated ligand 6-MNA exhibiting it’s full reactivity already at physiological pH values. However, no direct comparison was done between aromatic and aliphatic thiols. Furthermore, study variabilities have to be taken into account and direct comparison should be carried out to prove the superiority of aromatic thiomers compared to aliphatic ones.
Conclusion
Within this study a novel nanoparticulate insulin delivery system has been evaluated in vitro and in vivo after oral administration. Mucoadhesion and stability of the thiolated particles was significantly higher compared to unmodified chitosan particles. Furthermore, thiolated particles were more stable both in acidic as well as intestinal pH when compared to unmodified chitosan particles. These enhanced properties allowed to reach a significant plasma insulin concentration after oral administration. The advantages of this novel delivery system for the oral application are likely to be useful also for other peptides and could be a promising novel approach for their oral administration.
Acknowledgements
This work has been supported by the EC. NanoBioPharmaceutics is an Integrated Project funded within the 6th Framework Programme of the European Commission.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bernkop-Schnurch, A., Kast, C.E., Guggi, D. (2003). Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J Contr Rel. 93:95–103.
- Bernkop-Schnürch, A., Pinter, Y., Guggi, D., Kahlbacher, H., Schöffmann, G., Schuh, M., Schmerold, I., Del Curto, M.D., D’Antonio, M., Esposito, P., Huck, C. (2005). The use of thiolated polymers as carrier matrix in oral peptide delivery - Proof of concept. J Contr Rel. 106:26–33.
- Bravo-Osuna, I., Teutonico, D., Arpicco, S., Vauthier, C., Ponchel, G. (2007). Characterization of chitosan thiolation and application to thiol quantification onto nanoparticle surface. Int J Pharm. 340:173–81.
- Clausen, A.E., Kast, C.E., Bernkop-Schnurch, A. (2002). The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm Res. 19:602–8.
- Cui, F., Shi, K., Zhang, L., Tao, A., Kawashima, Y. (2006). Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: preparation, in vitro charachterization and in vivo evaluation. J Contr Rel. 114:242–50.
- Deutel, B., Greindl, M., Thaurer, M., Bernkop-Schnürch, A. (2008). Novel insulin thiomer nanoparticles: in vivo evaluation of an oral drug delievry system. Biomacromolecules. 9:278–85.
- Dhawan, S., Singla, A.K., Sihna, V.R. (2004). Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech 5:1–7.
- Fernandez-Urrusuno, R., Calvo, P., Remunan-Lopez, C., Vila-Jato, J.H., M.J., A. (1999). Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res. 16:1576–81.
- Gan, Q., Wang, T., Cochrane, C., Mccarron, P. (2005). Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf B. 44:65–73.
- Grabovac, V., Bernkop-Schnürch, A. (2007). Development and in vitro evaluation of surface modified poly(lactide-co-glycolide) nanoparticles with chitosan-4-thiobuthylamidine. Drug Dev Ind Pharm. 33:767–74.
- Guggi, D., Krauland, A.H., Bernkop-Schnürch, A. (2003). Systemic peptide delivery via the stomach: in vivo evaluation of an oral dosage form for salmon calcitonin. J Contr Rel. 92:125–35.
- Katas, H., Alpar, H.O. (2006). Development and charachterization of chitosan nanoparticles for siRNA delivery. J Contr Rel. 115:216–25.
- Khafagy, E.S., Morishita, M., Onuki, Y., Takayama, K. (2007). Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Delivery Rev. 59:1521–46.
- Khoo, C.G., Frantzich, S., Rosinski, A., Sjostrom, M., Hoogstraate, J. (2003). Oral gingival delivery systems from chitosan blends with hydrophilic polymers. Eur J Pharm Biopharm. 55:47–56.
- Krauland, A.H., Guggi, D., Bernkop-Schnürch, A. (2004). Oral insulin delivery: the potential of thiolated chitosan-insulin tablets on non-diabetic rats. J Contr Rel. 95:547–55.
- Lee, D.W., Powers, K., Baney, R. (2004). Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr Polym. 58:371–7.
- Mao, S., Germershaus, O., Fischer, D., Linn, T., Schnepf, R., Kissel, T. (2005). Uptake and transport of PEG-graft-trimethyl-chitosan copolymer-insulin nanocomplexes by epithelial cells. Pharm Res. 22:2058–68.
- Millotti, G., Samberger, C., Fröhlich, E., Bernkop-Schnürch, A. (2009). Chitosan-graft-6-mercaptonicotinic acid: synthesis, characterization, and biocompatibility. Biomacromolecules. 10:3023–7.
- Nurkeeva, Z.S., Mun, G.A., Khutoryanskiy, V.V., Bitekenova, A.B., Dubolazov, A.V., Esirkegenova, S.Z. (2003). pH effects in the formation of interpolymer complexes between poly(N-vinylpyrrolidone) and poly(acrylic acid) in aqueous solutions. Eur Phys J E. 10;65–8.
- Pan, Y., Li, Y., H., Z., Zheng, J., Xu, H., Wei, G., Hao, J., Cui, F. (2002). Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 249:139–47.
- Peppas, N.A., Huang, Y. (2004). Nanoscale technology of mucoadhesive interactions. Adv Drug Deliv Rev. 56:1675–87.
- Sakuma, S., Suzuki, N., Kikuchi, H., Hiwatari, K., Arikawa, K., Kishida, A., Akashi, M. (1997). Oral peptide delivery using administered salmon calcitonin by polystyrene nanoparticles having poly(N-isopropylacrylamide) branches on their surfaces. Int J Pharm. 158:69–78.
- Sameti, M., Bohr, G., Ravi Kumar, M.N.V., Kneuer, C., Bakowski, U., Nacken, M., Schmidt, H., Lehr, C.M. (2003). Stabilization by freeze-drying of a cationically modified silica nanoparticles for gene delivery. Int J Pharm. 226:51–60.
- Shi, L., Caldwell, K.D. (2000). Mucin adsorption to hydrophobic surfaces. J Colloid Interface Sci. 224:372–81.
- Shu, X.S., Zhu, K.J. (2000). A new approach to prepare trypolyphosphate/chitosan complexbeads for controlled drug delivery. Int J Pharm. 201:51–8.
- Takeuchi, H., Yamamoto, H., Kawashima, Y. (2001). Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv Drug Delivery Rev. 47:39–54.
- Van Der Merwe, S.M., Verhoef, J.C., J.H.M., V., Kotze, A.F., Junginger, H.E. (2004). Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur J Pharm Bioparm. 58:225–35.
- Xu, Y., Yumin, D. (2003). Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 250:215–26.
- Yin, L., Ding, J., He, C., Cui, L., Tang, C., Yin, C. (2009). Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 30:5691–700.