Abstract
Grape seed polyphenols (GPP) are reported to have various biological effects along with strong antioxidant potential. Pharmacokinetic studies of GPP reveal its poor absorption through the intestine. The objective of the present study was to enhance bioavailability of GPP by its complexation with phosphatidyl choline. A complex of GPP was prepared with phosphatidyl choline and characterized on the basis of solubility, melting point, DSC, and IR. Everted intestine sac technique was used to study ex vivo drug absorption of GPP-PC complex and plain GPP. Pharmacokinetic studies were performed in rats and the hepatoprotective activity of GPP-PC complex was also compared with GPP and GPP-PC physical mixture in isolated rat hepatocytes. Analytical reports along with spectroscopic data revealed the formation of the complex. The results of ex vivo study show that the GPP-PC complex has significantly increased absorption compared with GPP, when given in equimolar doses. The complex showed enhanced bioavailability, improved pharmacokinetics, and increased hepatoprotective activity as compared to GPP or GPP-PC physical mixtures. Enhanced bioavailability of GPP-PC complex may be due to the amphiphilic nature of the complex, which greatly enhance the lipid miscibility of GPP. The present study clearly indicates the superiority of complex over GPP, in terms of better absorption, enhanced bioavailability, and improved pharmacokinetics.
Introduction
Phenolic compounds are among the most widely distributed plant secondary products and are found in many plants used as foods and feeds. Grape seeds contain flavonoids (catechin, epicatechin, procyanidins, and anthocyanins), phenolic acids (gallic acid and ellagic acid), and stilbenes (resveratrol and piceid). These grape seeds constituents have been shown to have various health functional activities. Grape seed extract has in vivo antioxidant activity and prevents oxidative damage in tissues by reducing the lipid oxidation and/or inhibit the production of free radicals. Apart from antioxidant action, the grape seed polyphenols (GPP) have been reported to have a variety of biological activities and pharmacological actions, such as hypolipidemic, anti-tumor, anti-inflammatory, hepatoprotective, anti-ulcer, wound healing, and treatment of atherosclerosis (CitationTebib et al., 1997; CitationBagchi et al., 1998; CitationBouhamidi et al., 1998; CitationSato et al., 1999).
The polyphenols obtained from grape seeds are poorly bioavailable when taken by mouth—only a small fraction of a given dose reaches the blood. The poor absorption of polyphenols is likely due to two main factors. First, these are multiple ring molecules not quite small enough to be absorbed from the intestine into the blood by simple diffusion. Nor does the intestinal lining actively absorb them, as occurs with some vitamins and minerals. Second, polyphenols typically have poor miscibility with oils and other lipids. This severely limits their ability to pass across the lipid-rich outer membranes of the enterocytes, the cell that lines the small intestine (CitationScalbert & Williamson, 2000; CitationManach & Scalbert, 2004).
Complexing of the phospholipids, with the standardized botanical extracts, has provided dramatic bioavailability enhancement and faster and improved absorption in the intestinal tract (CitationBombardelli et al., 1989; CitationYanyu et al., 2006; CitationSharma et al., 2010). Earlier in our lab, we prepared and evaluated a complex of boswellic acid and curcumin with phosphatidyl choline and found dramatically enhanced absorption of these plant constituents when administered orally or applied topically in experimental animal models (CitationGupta & Dixit, 2010; CitationSharma et al., 2010). GPP are poorly absorbed and therefore strategies to improve its bioavailability need to be worked. Complexation with phospholipid has been used in the present studies to meet this objective.
Materials and methods
Materials
Grape seeds were obtained from a commercial winery. The soy phosphatidyl choline (Lipoid S 100) was obtained as a gift sample from Lipoid (Ludwigshafen, Germany). Standard gallic acid was purchased from Himedia Labs (Mumbai, India). All other chemicals and solvents were of analytical grade.
Isolation of grape seed polyphenols (GPP)
A solvent extraction technique utilizing 50% ethanol as solvent was used for the extraction of polyphenols from grape seeds with slight modifications (CitationNawaz,H., 2006). Briefly, 100 g of milled grape seeds were extracted with 500 ml of 50% ethanol solution for 12 h at room temperature in the dark. The solvent layer was filtered through Whatman filter paper and the residue was further extracted with 400 ml 95% ethanol solution for 12 h at room temperature. The solvent layer was again filtered and the combined filtrate was further ultrafiltered using Millipore filtration cell with 0.22 µm pore size filter. The final concentrate was collected and dried under reduced pressure in a rotary evaporator.
Estimation of total polyphenols using gallic acid as standard
The level of total polyphenols was determined with Folin-Ciocalteau (F&C) reagent according to the method of CitationSingleton et al. (1999) using gallic acid as a standard phenolic compound. A range of gallic acid concentrations from 100–1000 µg/ml was used to prepare the calibration curve; 0.5 ml of the standard solution/isolated GPP was added to 10 ml water and 0.5 ml F&C reagent. After 5 min, 8 ml of 7.5% sodium carbonate solution was added. The solution was allowed to stand for 2 h in the dark. Absorbance was taken at 765 nm using a Shimadzu UV–Vis spectrophotometer. The concentration of total polyphenols in grape seeds extract was determined as micrograms of gallic acid equivalent using an equation obtained from the calibration curve.
Preparation of GPP-PC complex
The grape seed polyphenols (GPP) and Soy-phosphatidyl choline (PC) in a molar ratio of 1:2 were taken in a round bottom flask containing 20 ml dichloromethane. This mixture was stirred for 2 h at 40°C on a magnetic stirrer. The resultant clear solution was concentrated by evaporation using a rotary evaporator (Model RE-52, SONAR, New Delhi, India) and 10 ml of n-hexane was added to it with continuous stirring. The GPP-PC complex was precipitated and the precipitate was filtered, washed repeatedly with n-hexane to remove traces of PC, and then dried under vacuum (). The GPP-PC complex was kept in amber colored glass bottle and stored at room temperature (CitationBombardelli et al., 1989; CitationGupta & Dixit, 2010; CitationSharma et al., 2010).
Characterization of complex
Solubility study
Solubility studies were performed by adding a small amount of the GPP, PC, and GPP-PC complex to 10 ml of different solvents and shaking the contents in volumetric flasks.
Differential scanning calorimetry (DSC)
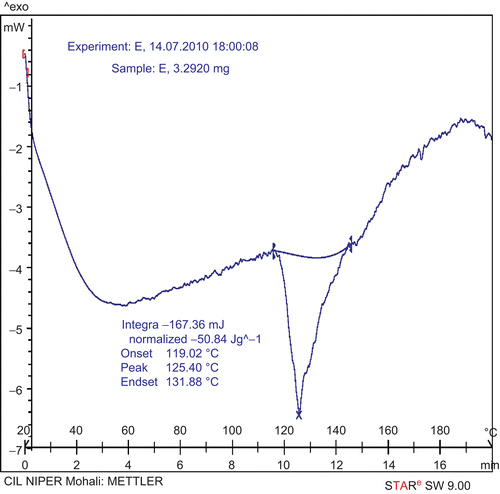
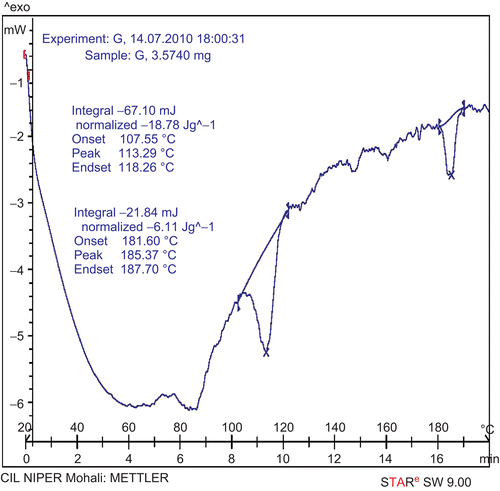
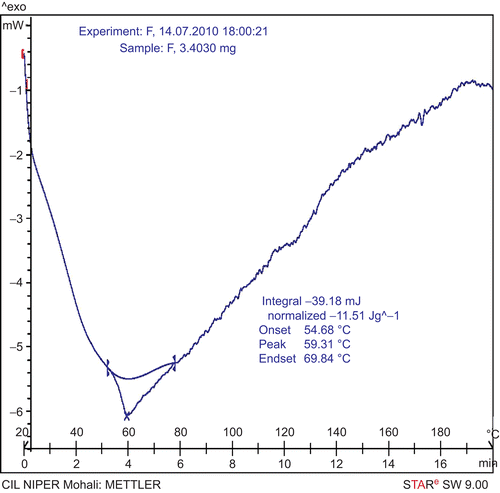
DSC thermograms were recorded with a METTLER STAR SW 9.00 DSC instrument. The samples were sealed in the aluminum crimp cell and heated from 20 to 200°C at 10°C/min. The onset, peak, and end-set temperatures of GPP, PC, and GPP-PC complex were determined and compared.
Melting point
Melting point of GPP, PC, and GPP-PC complex was determined by melting point apparatus (Scientific International, New Delhi, India).
Infrared spectroscopic analysis
FTIR spectra were obtained on the 8400 Shimadzu FTIR spectrometer with the wave number 500~4000 cm−1 using KBr pellets. Briefly, 500 mg potassium bromide and 5 mg sample were taken into a mortar, mixed, and grinded to a fine powder. A homogeneous disc was prepared by compressing the powder and inserting it into an IR sample holder carefully. The spectrum was recorded between 500~4000 cm−1.
Ex vivo absorption study using everted small intestine sac method
Intestine of goat (2–2.5 inch piece) was taken, washed, and freed of intestinal contents and everted using a glass rod. One end of the intestine was fastened using thread while a thread tied cannula was fitted at the other end, and kept in PBS solution (pH 7.4).
Three flasks were taken and filled with 50 ml phosphate buffer saline in each. GPP was added into the first flask, whereas GPP-PC physical mixture and GPP-PC complex were added into the second and third flasks, respectively; 2.0 ml of mammalian ringer’s solution was injected in each of the three intestine pieces and immersed in the separate flasks containing GPP, GPP-PC physical mixture, and GPP-PC complex. Temperature of the flasks was maintained at 37 ± 1°C and aeration was provided to the intestine pieces throughout the study. After the specified time interval the serosal fluid of each intestine fragment was assayed for polyphenol content using a UV spectrophotometer taking gallic acid as a standard. The study was repeated three times with fresh pieces of intestine each time and results are presented as mean ± standard error of mean (SEM).
Pharmacokinetic study
Estimation of total polyphenols in serum after oral administration in rats
Eighteen albino rats (wistar strain) of either sex (120–160 g) were divided into three groups consisting of six rats in each group. To the animals of one group, GPP was administered at a dose of 1 g/kg orally. GPP-PC physical mixture was administered to the animals of the second group at a dose equivalent to 1 g/kg of GPP. To the third group of animals GPP-PC complex was given at a dose equivalent to 1 g/kg of GPP. Blood was collected from retro orbital plexus at different time interval. Blood was allowed to clot at room temperature for ~ 1 h, centrifuged at 3000 rpm for 15 min. The clear serum was separated and kept at −20°C prior to analysis.
The concentration of polyphenols was estimated by taking 0.5 ml serum using a spectrophotometer as described previously.
Pharmacokinetic parameters
The pharmacokinetic parameters of GPP, GPP-PC physical mixture, and GPP-PC complex were obtained with the help of a software program (KINETICA 5.0) and the parameters were compared to that of free GPP.
Screening of hepatoprotective activity
The hepatoprotective activity of GPP is well known and the mechanism underlying the hepatoprotective action is already established in animal models. In the present work, we compared the hepatoprotective action of GPP with GPP-PC complex and GPP-PC physical mixture by in vitro testing on isolated hepatocytes rather than in vivo animal study, which requires a number of animals and their sacrificing.
In vitro studies involved isolation of hepatocytes and examination of the effect of toxicants along with the test samples. The rat hepatocytes were isolated according to CitationSeglen (1975) with slight modifications by recirculating enzymatic perfusion technique. The viability of the cells was determined by trypan blue exclusion method (CitationVisen et al., 1991). The hepatocytes thus isolated were kept in the medium in petri dishes for 15 min at 37°C. The petri dishes were divided into five groups of three petri dishes each. Group A was kept as normal, group B was given CCl4 treatment (10 µl), group C CCl4 + GPP solution (10 µl), group D CCl4 + GPP-PC physical mixture (equivalent to 10 µl of GPP), and group E CCl4 + GPP-PC complex. After 24 h incubation, contents of the petri dishes were centrifuged. The supernatant was removed to determine the levels of transaminases (GOT and GPT) and LDH. The whole set of experiments was done twice and the results expressed indicate mean of six readings of each group.
Assessment of anti-hepatotoxic activity
Assessment of anti-hepatotoxic activity was done by determining the glutamyl oxalacetic acid transaminase (GOT), glutamyl pyruvate transaminase (GPT), and lactate dehydrogenase (LDH) activity. The enzyme assay was carried out by Reagent Kits maintained by Bayer India Ltd. and the procedures were essentially those described in the literature available with kits (CitationReitman & Frankel, 1957; CitationKing & Wootton, 1964).
Statistical analysis
Results are presented as mean ± standard error of mean (SEM) and percentage degree of reversal against hepatotoxin by test. The percentage was calculated by considering enzyme level difference between CCl4 and normal group as 100% degree of reversal using the following equation:
Total variation present in a set of data was analyzed through one-way analysis of variance (ANOVA) followed by Dunnett’s test using GraphPad Prism 3 (GraphPad Software, Inc., La Jolla, CA).
Results
Estimation of total polyphenols
A calibration curve of gallic acid was prepared at 765 nm using a spectrophotometer. The plot of concentration vs absorbance exhibited a linear relationship. The equation of the straight line for gallic acid was y = 0.0007x + 0.0115 and R2 value 0.9974. The amount of polyphenols was found to be 92.34% in the isolated ultrafiltered concentrate.
Characterization of complex
Solubility study
As shown in , the GPP-PC complex has a different solubility profile than plain GPP, which indicates the formation of complex.
Table 1. Solubility of GPP, PC, and GPP-PC complex.
Differential scanning calorimetry (DSC)
Interaction of drug with excipient can be examined by differential scanning calorimetry (DSC), which also gives information about compatibility of drug with excipient. In DSC, an interaction is concluded by elimination of endothermic peaks, appearance of new peaks, change in peak shape and its onset, peak temperature/melting point, and relative peak area. DSC thermograms of GPP, PC, and GPP-PC complex are shown in . The thermogram of GPP showed a single peak with an onset of 119.02°C and maximum occurrence at 125.40°C. Thermogram of PC exhibit two different peaks at 113.29 and 185.37°C, which may be due to the hot movement of phospholipids polar head group and due to phase transition from gel to liquid crystalline state, respectively. In the thermogram of the complex a single peak with an onset at 54.68°C and maximum occurrence at 59.31°C appeared, which is different from the peaks of the individual components (GPP and/or PC) of the complex.
Melting point
Melting point of GPP, PC, and GPP-PC complex was found to be 124–126, 110–112, and 60–62°C, respectively.
Infrared spectroscopic analysis
The IR spectrum of GPP-PC complex was found to be significantly different from the IR spectrum of GPP and the PC alone which supports the reaction of the−OH group of GPP at choline part of PC and hence complex formation.
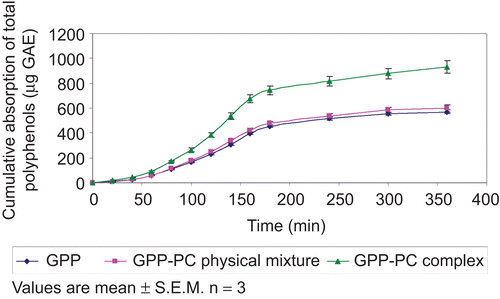
Ex vivo absorption studies
The absorption of GPP-PC complex was found to be greater than plain GPP or GPP-PC physical mixture at different time intervals. The curve between cumulative absorption and time for GPP, GPP-PC physical mixture, and GPP-PC complex is shown in , which clearly indicates the enhanced absorption of the GPP-PC complex.
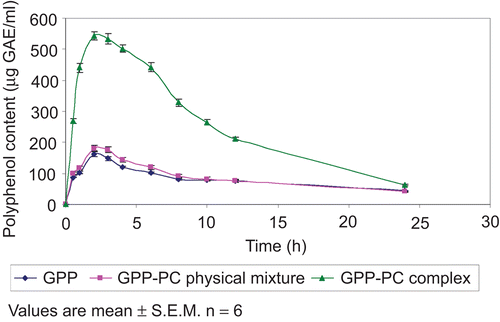
Pharmacokinetic study
The main pharmacokinetic parameters of GPP, GPP-PC physical mixture, and GPP-PC complex in the rat are shown in , and the plasma concentration–time profile is shown in . The polyphenol content is reported in terms of µg gallic acid equivalents per ml (µg GAE/ml). GPP-PC complex showed very high plasma concentrations, low clearance, and long half-life in rats as compared to GPP or GPP-PC physical mixture. Our study has shown that maximum plasma drug concentration (Cmax) of rats treated with GPP and GPP-PC physical mixture was 166.47 ± 5.27 and 184.29 ± 4.73 µg GAE/ml with Tmax 2.38 ± 0.06 and 2.39 ± 0.04 h, respectively. In contrast, the Cmax for GPP-PC complex was 554.39 ± 8.46 µg GAE/ml with Tmax 2.49 ± 0.03 h. The elimination half-life was found to be significantly higher in the rats treated with GPP-PC complex (2.35 ± 0.03 h) as compared to rats treated with plain GPP (2.13 ± 0.04 h) or GPP-PC physical mixture (2.15 ± 0.02 h). Furthermore, the clearance was very low in the rats treated with GPP-PC complex (21.64 ± 1.12 l/h), when compared with the rats treated with plain GPP (92.18 ± 3.17 l/h) or GPP-PC physical mixture (81.43 ± 2.13 l/h).
Table 2. Comparison of pharmacokinetic parameters.
Hepatoprotective activity
The results obtained are reported in . Incubation of rat hepatocytes with 10 µl CCl4 resulted in an induction of hepatotoxicity, which was indicated by a significant increase in the GOT, GPT, and LDH levels in the toxin control group (CCl4-treated group) in comparison to the normal group. The GPP, GPP-PC physical mixture, and GPP-PC complex significantly (p < 0.001) decreased the GOT level by 44.13, 58.89, and 94.64% and GPT level by 43.39, 62.48, and 98.82%, respectively. In the case of LDH level GPP, GPP-PC physical mixture, and GPP-PC complex decreased the same by 44.98, 56.15, and 92.29%, respectively.
Table 3. Hepatoprotective activity of GPP, GPP-PC physical mixture, and GPP-PC complex on cultured rat hepatocytes.
Discussion
Phytomedicines, complex chemical mixtures prepared from plants, have been used for health maintenance since ancient times. However, many phytomedicines are limited in their effectiveness because they are poorly absorbed when taken by mouth. The recent trend is to improve the therapeutic performance of the conventional drug by formulating them as a new drug delivery system rather than going for cumbersome and costly research for a new entity (CitationBombardelli et al., 1989; CitationYanyu et al., 2006; CitationSharma et al., 2010).
The effectiveness of any herbal product is dependent upon delivering an effective level of the active compounds. GPP has its widespread use in treatment of a number of disorders including antioxidant action. Poor absorption of GPP through the intestine as reported in pharmacokinetic studies warrants high doses to reach therapeutic levels of GPP, which is sometimes inconvenient. There are usually several factors responsible for this, but a particularly widespread problem is poor miscibility of polyphenols with oils and other lipids (CitationD’Archivio et al., 2010). In this case, improved bioavailability can be achieved by enhancing the rate and extent of drug miscibility with lipid rich membrane of enterocytes. Phospholipids play a major role in drug delivery technology. There are numerous advantages of phospholipids in addition to solubilizing property while considering them for a carrier system. Improved absorption by complexation with phospholipids has been reported in a number of cases and potential formulations with silymarin etc. are marketed worldwide (CitationBarzaghi et al., 1990; CitationBuzzelli et al., 1993).
In the present experiment, the complex of GPP was prepared with soy phosphatidyl choline and characterized by solubility, DSC, melting point, and IR spectroscopic analysis.
The GPP-PC complex was studied for absortion through the intestine and compared with plain GPP and GPP-PC physical mixtures. An everted intestine sac technique was used to study the ex vivo drug absorption; because it is advantageous as large changes can be observed in a comparatively small amount of serosal fluid. Moreover, eversion permits the epithelial cells of the mucosal surface to be exposed directly to the oxygenated mucosal fluid (CitationBarthe et al., 1998). The amount of polyphenols absorbed from the mucosal fluid to serosal fluid through the intestine at various time intervals was observed. The results of ex vivo study show that the GPP-PC complex has significantly increased absorption compared with GPP or GPP-PC physical mixture, when given in equimolar doses. This increase may be due to the amphiphilic nature of the GPP-PC complex, which greatly enhances the water and lipid solubility of the GPP. The overall pharmacokinetics of GPP has been improved after complexation with phosphatidyl choline.
The results of pharmacokinetic study of GPP, GPP-PC complex, and GPP-PC physical mixture have shown that the bioavailability has been improved after oral administration with phospholipid complex of GPP. The higher concentration was also maintained for a longer period of time. Thus, in complexed form the phosphatidyl choline enhanced the plasma concentration of GPP in a significant manner and the effect persisted for a longer period of time. The improved bioavailability of complex may be due to increased lipid miscibility of GPP in complex form. The elimination half-life of GPP was increased when it was in the form of complex, also the clearance of GPP was low in the complex form. The complex persisted for a longer time in the body with higher bioavailability.
GPP are reported to have hepatoprotective properties when given orally. In the present study the GPP, GPP-PC physical mixture, and GPP-PC complex were studied for liver protective action in isolated rat hepatocytes. The hepatotoxicity induced by CCl4 is due to its metabolite CCl3•, a free radical that binds to lipoprotein and leads to peroxidation of lipids of endoplasmic reticulum (CitationRecknagael, 1967). In this study also GPP in complexed form showed better hepatoprotective effect in terms of lowering the GOT, GPT, and LDH, as compared with GPP and GPP-PC physical mixture. This increased hepatoprotection may be due to fast and increased uptake of GPP by the hepatocytes. Moreover, the GPP-PC physical mixture was found to be a little bit better than plain GPP in terms of providing increased hepatoprotection. It may be explained if hepatoprotection offered by PC is taken into account. Use of PC as a hepatoprotective agent is well documented in the literature (CitationDas & Vasudevan, 2006; CitationRaj et al., 2010).
The data from pharmacokinetic studies clearly indicate that the complex persisted with higher plasma concentration and for a longer time in the body. The improved pharmacokinetic profile of GPP will also help in reducing the dose size and frequency of doses. Thus, the formulations containing GPP-PC complex instead of plain GPP can be helpful in reducing the amount and frequency of administered dosage.
The GPP-PC complex can be given orally in the form of suspension or in capsule form. Further, the GPP-PC complex can be utilized to develop novel delivery systems such as vesicles. In vesicular form the complex may be used for the localized effect of GPP on skin to combat photoaging and for the treatment of skin cancer.
Conclusion
In the present work a complex of GPP was prepared with phosphatidyl choline to enhance the bioavailability of GPP. The prepared complex was characterized and evaluated in terms of absorption through intestine, pharmacokinetic studies, and hepatoprotective potential. The results of present study clearly indicates the superiority of GPP-PC complex over plain GPP or GPP-PC physical mixture, in terms of better absorption, enhanced bioavailability, and improved pharmacokinetics.
Acknowledgements
The authors thank LIPOID GmbH (Germany) for providing the gift sample of phosphatidyl choline.
Declaration of Interest
The authors also acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, India for providing financial assistance (Grant no. 09/150/(0100)/2009/EMR-I) in the form of Senior Research Fellowship to one of the author, Nishant Kumar Gupta.
References
- Bagchi, D., Garg, A., Krohn, R.L., Bagchi, M., Bagchi, D.J., Balmoori, J., Stohs, S.J. (1998). Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 30:771–6.
- Barthe, L., Woodley, J., Kenworthy, S., Houin, G. (1998). An improved everted gut sac as a simple and accurate technique to measure para-cellular transport across the small intestine. Eur J Drug Metab Pharmacokinet. 23:313–23.
- Barzaghi, N., Crema, F., Gatti, G. (1990). Pharmacokinetic studies on IdB 1016, a silybinphosphatidyl choline complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet. 15:333–8.
- Bombardelli, E., Curri, S.B., Della, L.R. (1989). Complexes between phospholipids and vegetal derivatives of biological interest. Fitoterapia. 90(Suppl 1):1–9.
- Bouhamidi, R., Prevost, V., Nouvelot, A. (1998). High protection by grape seed proanthocyanidins (GSPC) of polyunsaturated fatty acids against UV-C induced peroxidation. Life Sci. 321:31–8.
- Buzzelli, G., Moscarella, S., Giusti, A. (1993). A pilot study on the liver protective effect of silybinphosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 31:456–60.
- D’Archivio, M., Filesi, C., Varì, R., Scazzocchio, B., Masella, R. (2010). Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 11:1321–42.
- Das, S.K., Vasudevan, D.M. (2006). Effect of lecithin in the treatment of ethanol mediated free radical induced hepatotoxicity. Indian J Clin Biochem. 21:62–9.
- Gupta, N.K., Dixit, V.K. (2010). Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J Pharm Sci. In press. DOI: 10.1002/jps.22393.
- King, E.J., Wootton, I.D.P. (1964). Micro-analysis in medical biochemistry. London: Churchill.
- Manach, C., Scalbert, A. (2004). Polyphenols: food sources and bioavailability. Am J Clin Nutr. 79:727–47.
- Nawaz, H., Shi, J., Mittal, G.S., Kakuda, Y. (2006). Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep Purif Technol. 48:176–81.
- Raj, P.V., Nitesh, K., Chandrashekhar, H.R., Rao, C.M., Rao, J.V., Udupa, N. (2010). Effect of lecithin and silymarin on d-galactosamine induced toxicity in isolated hepatocytes and rats. Indian J Clin Biochem. 25:169–74.
- Recknagael, R. (1967). Carbontetrachloride hepatotoxicity. Pharmacol Rev. 19:145–96.
- Reitman, S., Frankel, A.S. (1957). A colorimetric method for the determination of serum glutamyl oxalacetic acid glutamyl pyruvate transaminase. Am J Clin Pathol. 28:56–8.
- Sato, M., Maulik, G., Ray, P.S., Bagchi, D., Das, D.K. (1999). Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J Mol Cell Cardiol. 31:1289–97.
- Scalbert, A., Williamson, G. (2000). Dietary intake and bioavailability of polyphenols. J Nutr. 130:2073S–85S
- Seglen, P.O. (1975). Preparation of isolated rat liver cells. Methods Cell Biol. 13:29–83.
- Sharma, A., Gupta, N.K., Dixit, V.K. (2010). Complexation with phosphatidyl choline as a strategy for absorption enhancement of boswellic acid. Drug Deliv. 17:587-95. DOI: 10.3109/10717544.2010.501461.
- Singleton, V.L., Orthofer, R., Lamuela, R.M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 299:152–3.
- Tebib, K., Rouanet, J.M., Besancon, P. (1997). Antioxidant effects of dietary polymeric grape seed tannins in tissues of rats fed a high cholesterol-vitamin E-deficient diet. Food Chem. 59:135–41.
- Visen, P.K.S., Shukla, B., Patnaik, G.K., Chandra, R., Singh, V., Kapoor, N.K., Dhawan, B.N. (1991). Hepatoprotective activity of picroliv isolated from Picrorrhiza kurrooa against thioacetamide toxicity on rat hepatocytes. Phytother Res. 5:224–7.
- Yanyu, X., Yunmei, S., Zhipeng, C., Qineng, P. (2006). The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm. 307:77–82.