Abstract
The aim of the present study is to develop colon-targeted drug delivery systems for diclofenac sodium which release the drug specifically and instantly at target site using amylose as a carrier. Coating formulations were designed based on the full factorial design. The evaluated responses were lag time prior to drug release and T90. Compression-coated tablets of diclofenac sodium containing various proportions of amylose and HPMC were prepared. In vitro drug release studies were done by changing pH method with enzyme. In vivo studies were done to confirm the potential of formulation to release the drug at target site. The dissolution data revealed that the ratio of polymers is very important to achieve optimum formulation. Results showed that the tablet prepared according to the above formulation released drug instantly at pH 6.8 (simulating colonic pH). An in vivo study shows that optimized formulation disintegrated in the target region. The results of this study revealed that factorial design is a suitable tool for optimization of coating formulations to achieve colon delivery. It was shown that coating formulation consisting of amylose 285 mg and HPMC 150 mg coating has the potential for colonic delivery of diclofenac sodium irrespective of change in pH in a patient with IBD.
Introduction
Colonic drug delivery has gained increased importance not just for the delivery of drugs for the treatment of local diseases of colon such as irritable bowel syndrome, inflammatory bowel disease (IBD) including Crohn’s disease and ulcerative colitis, but also for its potential for the delivery of proteins and therapeutic peptides (CitationYang et al., 2002; CitationChourasia & Jain, 2004). Colon as a site offers distinct advantages on account of a near neutral pH, a much longer transit time, reduced digestive enzymatic activity, much greater response to absorption enhancers, and the presence of a number of enzymes acting on polysaccharides viz. β-D-glucosidase, β-D-galactosidase, amylase, pectinase, etc., secreted by a large number and variety of colonic bacteria (CitationSinha & Kumria, 2001; Citation2003). Various systems have been developed for colon-specific drug delivery. These include covalent linkage of a drug with a carrier, coating with pH-sensitive polymers, time-dependent release systems, and enzymatically-controlled delivery systems (CitationLeopold, 1999).
In the treatment of IBD, colon-specific drug delivery systems are designed to deliver anti-inflammatory agents to the sites of inflammation and hence the systemic drug absorption should be minimized as this may lead to unwanted systemic side effects (CitationFriend, 1991; Citation2005; CitationRubinstein, 2005). The pH- and time-dependent approach has the problem of non-specificity for colonic release, due to reasons such as the shallow pH gradient between the small and large intestine and the pH change in disease states. In IBD pH was found to be lower than that seen in healthy volunteers and was ~ 5.3 ± 0.3 in patients with Crohn’s disease (CitationVandamme et al., 2002; CitationFriend, 2005). Inter-subject variations are also observed due to variable gastric residence times depending on the presence or absence of food (CitationDavis et al., 1984; CitationPozzi et al., 1994; CitationUeda et al., 1994; CitationGupta et al., 2001a; b; CitationKatsuma et al., 2002; CitationYang et al., 2003; CitationFriend, 2005) and failure of coating to disintegrate during passage through the ileocecal region (CitationMarvola et al., 1987). Biodegradable polymers provide a more specific approach for targeting the colon, as the enzymes responsible for degradation are found only in the colon. One form of starch, amylose, can be made resistant to pancreatic enzymes through the formation of an amorphous structure, which can be degraded by colonic bacteria (CitationEllis & Ring, 1985). Amylose films can be applied using conventional coating technology to solid oral dosage forms; such films of amylose are fragile and swell in water. The addition of a structuring agent, ethylcellulose, from an aqueous dispersion or organic solution has been found to improve the physical and mechanical properties of the film, without impairing the film’s sensitivity to bacterial degradation (CitationMilojevic et al., 1996; CitationSiew et al., 2000; CitationLeong et al., 2002; CitationTuleu et al., 2002).
The main function of the colon is to absorb water and minerals from food particles and luminal content, due to this, colonic contents have a very low quantity of water, making it difficult for any formulation to dissolve rapidly. This problem can be overcome with the aid of superdisintegrant, which will help to rapidly disintegrate the formulation in the low water content of the colon and provide rapid release once the tablet reaches the target site (CitationLee & Mukherjee, 2002).
In view of this fact, the present study attempts to evaluate the use of compression coating as a novel encapsulation method for coating core tablets containing superdisintegrant for specific and instant drug release in the colon. A colon-specific delivery system was developed by compression-coating drug containing core with amylose and HPMC. The release occurred only upon sufficient erosion of the coat. The polymers degraded rapidly in the presence of simulated enzymes, which are localized in the colonic region of the gastro-intestinal tract, thus rapidly releasing the active ingredient. A similar result was observed in in vitro and in vivo studies on human volunteers and superdisintegrant was found to play an important role in the site-specific and instant drug release.
Materials and methods
Diclofenac sodium was obtained as a gift sample from Arti Pharmaceuticals Ltd. (Mumbai, India) and Hydroxy Propyl Methyl Cellulose (HPMC) was provided by Colorcon India Pvt. Ltd. (Mumbai, India) Amylose (HylonII) was gifted by National Starch Research Center (Hyderabad, India). Other excipients used to prepare tablets and for coating were of standard pharmaceutical grade. All chemical reagents used were of analytical grade and procured locally.
Preparation and evaluation of diclofenac sodium core tablet
Tablet formulations of diclofenac sodium were prepared by wet granulation with and without sodium starch glycolate (SSG) as a superdisintegrant (F1 and F2). Tablets were compressed on a 8 mm standard concave punch on a single rotary tablet compression machine (Rimek, Mumbai) and were subjected to various evaluation tests. For in vivo study, placebo tablets containing radio-opaque agent barium sulfate were prepared with and without SSG and evaluated for disintegration test, weight variation, and breaking force (shown in and ).
Table 1. Formulation of core tablets.
Table 2. Physical properties of Diclofenac core tablet compression coated with Amylose–HPMC K100LV.
Preparation of compression coated tablets
Coating material was prepared by wet granulating amylose and HPMC K100 LV using PVP K30 as a binder. A two-factor three-level factorial design was constructed for optimizing the composition of amylose and HPMC K100LV in coating. About 47% of the coat formulation was placed in the die cavity (diameter 12.5 mm). A diclofenac core tablet (diameter 8 mm) was then carefully positioned in the center of the die cavity and the die cavity was filled with the remainder of the coat formulation. It was then compressed around the core tablets at an applied force of 4000–5000 kg using 12.5 mm round, concave punches. Tablets were evaluated as mentioned above. Placebo tablets were also coated similarly and evaluated.
Optimization of compression coat by full factorial design
A 32 full factorial design was constructed to optimize coat composition to evaluate the effect of content of polymers on targeting efficiency of the drug delivery system in the colon. The amounts of amylose (X1) and HPMC K100LV (X2) were selected as the independent variables; levels of these factors were selected by initial experimentation. All the other formulation aspects and processing variables were kept invariant throughout the study period. and summarize the translation of the coded levels to the experimental units used in the study. All formulations were subjected to evaluation as mentioned above. The cores (F1, F2) prepared earlier were coated with optimized coating composition of amylose and HPMC.
Table 3. Experimental design: Factors and responses.
Table 4. Composition of experimental formulations (runs).
Drug release studies
The dissolution studies of compression-coated tablets were carried out in USP XXIII dissolution type II apparatus (TDT 08L Electrolab) at a rotation speed of 50 rpm, at 37°C. The tablets (n = 3) were transferred to dissolution medium and aliquots were withdrawn at selected time intervals and filtered through Whatman filter paper no. 41. The filtrates were then analyzed by a UV spectrophotometer (V-530 Jasco) at 276 nm. The continuous dissolution method USP XXIII was used for simulating conditions of the GI tract. Initially tablets were added in 700 ml of 0.1 N HCl (pH 1.2) for 2 h (stage I). At the end of 2 h, 233.3 ml of tribasic sodium phosphate solution (Tris HCl) (0.2 M) was added to all the dissolution vessels and the pH was adjusted to 6.8 by using 2 M NaOH or 2 M HCl and dissolution was further continued for 3 h (stage II). The effect of enzyme on the drug release was studied on all formulations by adding 770 mg of α-amylase into buffer, at the beginning of 5th h (stage III) (CitationChebli et al., 2001).
Effect of coating composition
In order to study the influence of HPMC on site specificity of a drug release, the core tablet coated with plain HPMC K100LV (formulation B2) was subjected to dissolution study as mentioned above. It was then compared with the tablet coated with HPMC and amylose (B1).
Effect of superdisintegrant
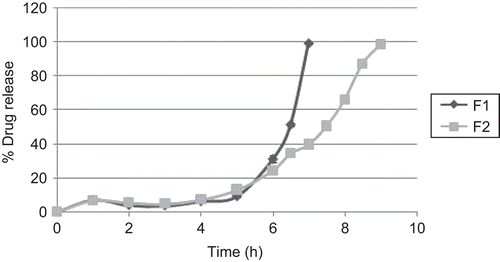
The core tablets with (F1) and without (F2) superdisintegrant were coated with optimized coating formula of amylose and HPMC K100 LV (285:150 ratios). The tablets were further subjected to dissolution studies as above.
Effect of pH of media
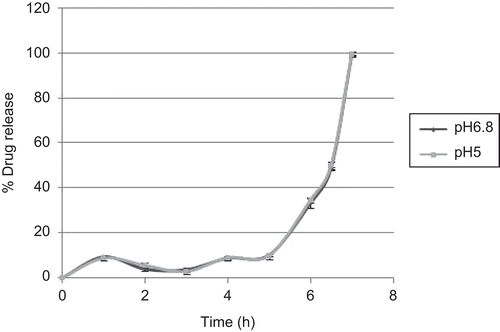
The effect of pH on drug release from compression-coated tablet with amylose and HPMC K 100 LV (285:150 ratio) in different colonic pH (5.0 and 6.8) was studied using modified dissolution medium at the beginning of stage III, i.e. acetate buffer and Tris-HCl buffer separately.
In vivo studies
X-ray imaging was used to monitor the passage of tablets through the gastrointestinal system. Healthy male volunteers, with a mean age of 24 years (range 22–40) and 50–80 kg body weight, participated in in vivo studies. They were non-alcoholics, non-smokers, and had not taken any drugs. The purpose of the study was fully explained, and written consent was received from all volunteers. Subjects were fasted overnight and administered barium sulfate containing core tablet coated with amylose and HPMC K100LV polymer. Two volunteers received core tablets with superdisintegrant and two volunteers received core tablets without superdisintegrant with 200 ml of water. Abdominal radiographs were taken at fixed time intervals, and the tablets were visualized using digital X-ray imaging (CR30X AGFA, MSXML 4.0). Tablets were visualized to determine whether they reached and remained in the terminal ileum or ceacum for 4.30 h. Studies were conducted with the approved protocol from Medical Ethical Committee of Nagpur, (Ref. no. 55721052009) in accordance with internationally accepted principles. Each volunteer received ~ 0.1 rem of radiation during the gastrointestinal X-ray radiograph. Normally, in a routine abdominal investigation with barium sulfate, a patient receives 0.7 rem of radiation (Office for Official Publications of the European Commission Directorate 2000). Therefore, the total radiation dose (~ < 0.7 rem) received by each volunteer was found to be lower than that of standard abdominal radiography.
Results
It is reported that amylose can be used as a carrier for colon-specific drug delivery as a matrix former or as coating (CitationMilojevic et al., 1996; CitationSiew et al., 2000). In order to achieve effective treatment of colonic disease, the drug should be released specifically and instantly in the colon; hence, an attempt was made to minimize the drug release in the physiological environment of the stomach and small intestine to ensure instant drug release in the specific physiological environment of the colon. This was achieved by applying amylose (as a compression coat) over the diclofenac sodium core tablets to enable effective treatment of IBD. The core tablets of diclofenac sodium were made with and without superdisintegrant. Further, the optimum quantity of superdisintegrant (SSG) was decided to be 5% on the basis that further increase did not increase DT significantly. The tablet containing SSG disintegrated within 1 min, while tablet without SSG disintegrated in 10 min.
Evaluation of diclofenac compression coated tablets
The compressed tablets were analyzed for thickness, hardness, and friability. All formulations were within specifications for weight variation, drug content, hardness, and friability, as shown in .
Full factorial design
The values of dependent variables of each of the formulations in the trial run are given in . In order to determine the levels of factors which yield optimum dissolution responses, a statistical model, Y = b0 + b1X1 + b2X2 + b12X1X2 + b11X1X1 + b22X2X2, incorporating interactive and polynomial terms, was used to evaluate the responses. Here Y is the dependent variable, b0 is the arithmetic mean response of the nine runs and bi is the estimated coefficient for the factor Xi. The main effects (X1 and X2) represent the average result of changing one factor at a time from its low to high value. The interaction terms (X1X2) represent the changes in response when two factors are simultaneously changed. The polynomial terms (X1X1 and X2X2) are included to investigate non-linearity; coefficients with second order terms (X12, X22) indicate the quadratic nature of the phenomenon. The data clearly indicates that the release after 5 h and t90% are strongly dependent on selected variables. The fitted full equations were generated using design expert software relating the responses to the transformed factors are as:
Table 5. Experimental runs and observed results of Release after 5h (Y 1) and T90% (Y 2).
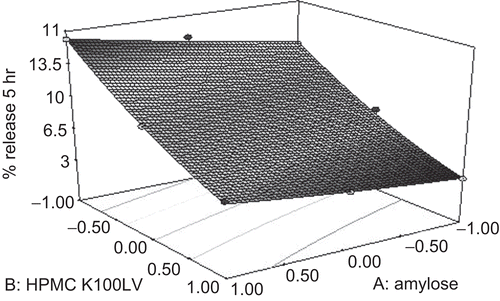
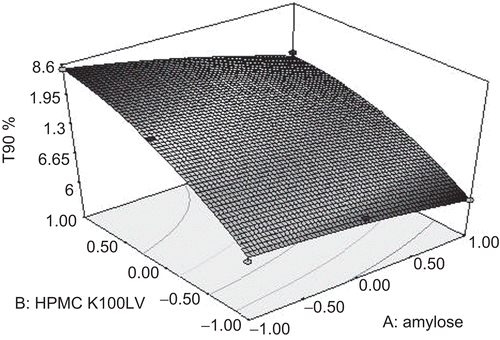
The equations. represent the quantitative effect of independent variables (X1 and X2) on the responses (Y1 and Y2). The optimum formula was decided from intensive grid search (formulation A3). The surface response plot was drawn to estimate the effect of independent variables on each response ( and ). Increase in concentration of amylose did not contribute significantly to drug release in 5 h (required lag time), whereas increase in HPMC K100LV decreased the drug release which may be due to swelling of polymer that caused gel formation and preventing the drug release (). HPMC K100LV appreciably decreased t90%, whereas amylose marginally decreased t90% (). Both the parameters were reduced as a result of interaction of factors. To justify the validity of the equations, three optimum formulations were selected by grid search performed over the entire experimental domain. The criteria for selection of optimum were primarily based on the lag time (5 h) and T90% (minimum time). Formulations corresponding to this optimum were prepared and evaluated for various response properties. The predicted and observed values were found to be in good agreement (r2= 0.9954). shows the values of observed and predicted responses using factorial design along with the residual errors for these three optimum formulations. The predicted error for the response variables ranged between −0.057 to 0.097, thus low magnitude of error designates a high prognostic ability of response surface methodology. Analysis of variance (ANOVA) () indicates that the assumed regression models were significant and valid for each considered response (Akhgari et al., 2005).
Table 6. Composition of observed and predicted response parameters.
Table 7. Analysis of variance (ANOVA) of dependent variables.
In vitro release study
Effect of coating polymer ratios
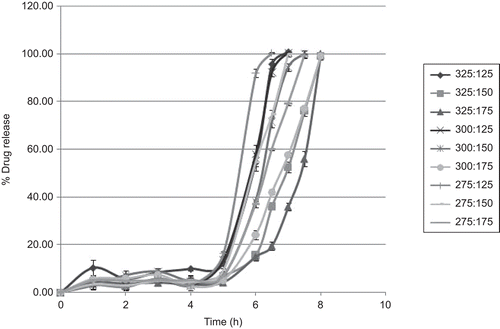
The release profiles for compression-coated diclofenac tablets prepared as per trial runs of factorial design by changing pH method are shown in . At pH 1.2 (simulating stomach) and 6.8 (simulating intestine) none of the formulations released their drug content up to 5 h and were intact. The high rate of drug release was observed for formulations R4 and R7 (containing low level of amylose and HPMC). Slow drug release was observed for formulations R2, R3 and R6 (higher HPMC concentration).
Effect of coating composition
The tablets were coated with a composition containing HPMC and amylase, where HPMC was included to improve coating properties and degradation of α-1-4-glucosidic bonds of amylose by α-amylase was supposed to cause a breach in the coat, allowing drug release. This was validated by making tablets coated only with HPMC K100LV (without amylose) (B2) which did not allow the fast drug release ().
Effect of pH of media on drug targeting
The effect of pH on drug release from optimized formulations (A3) in different colonic pH mimicking patients with IBD (pH 5.0, acetate buffer) and healthy volunteers (pH 6.8, Tris–HCl buffer) is shown in . The results suggested that pH has no effect on dissolution behavior of the optimized formulations.
Effect of superdisintegrant on drug targeting
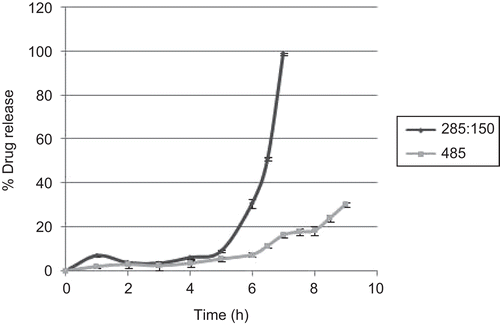
The release of diclofenac from core tablets coated with optimized blends of Amylose:HPMC K100LV (A3) is shown in . Fastest drug release was obtained for tablets containing sodium starch glycolate (F1), as compared to the tablet containing no superdisintegrant (F2).
In vivo X-ray results
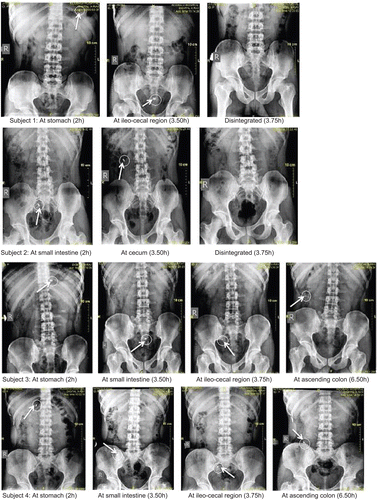
For this study, an optimized formulation coated with amylose and HPMC K100LV (A3) was used. Two volunteers received core tablets with SSG and two volunteers received core tablets without SSG. The details of the results are shown in and . From the abdominal radiographs, taken at different points of time, it was seen that there is variation in time required for the tablet to enter the colonic region, still a tablet with superdisintegrant disintegrated in the colonic region.
Table 8. The position of the tablets throughout the gastrointestinal tract in the subjects at certain points in time. Subjects 1 and 2 ingested tablets with superdisintegrant and subjects 3 and 4 ingested tablets without superdisintegrant, both coated with optimized amylose and HPMC compression coat.
Discussion
Thus, inclusion of SSG was done to enable fast release of diclofenac sodium soon after the degradation of amylose coat by the colonic bacterial enzymes. Further, a full factorial design was constructed to optimize the concentration of amylase and HPMC K 100LV in the compression coat.
Results of the full factorial formulation design indicate that an increase in concentration of amylose did not contribute significantly to drug release in 5 h (required lag time), whereas an increase in HPMC K100LV decreased the drug release, which may be due to swelling of the polymer that caused gel formation and prevented the drug release (). HPMC K100LV appreciably decreased t90%, whereas amylose marginally decreased t90% (). Both the parameters were reduced as a result of interaction of factors. To justify the validity of the equations, three optimum formulations were selected by grid search performed over the entire experimental domain. The criteria for selection of optimum were primarily based on the lag time (5 h) and T90% (minimum time). Formulations corresponding to this optimum were prepared and evaluated for various response properties. The predicted and observed values were found to be in good agreement (r2 = 0.9954). shows the values of observed and predicted responses using a factorial design along with the reidual errors for these three optimum formulations. The predicted error for the response variables ranged between −0.057 and 0.097, thus low magnitude of error designates a high prognostic ability of response surface methodology. Analysis of variance (ANOVA) () indicates that the assumed regression models were significant and valid for each considered response (CitationAkhgari et al., 2005).
In vitro drug release studies
Effect of coating polymer ratios
The release profiles for compression-coated diclofenac tablets prepared shows no drug release up to 5 h and tablets were intact. This is attributed to the coat which formed a swollen gel which does not allow diffusion of drug through the core tablet till 5 h and lag time was found to increase with increase in HPMC and amylose polymer concentration. The drug was released from the formulation after 5 h in the presence of enzyme at pH 6.8 (stage II). Due to enzymatic degradation of amylase, the polymer back bone underwent breakdown, leading to a subsequent reduction in their molecular strength, and the formulation was unable to hold the drug entity any longer. The high rate of drug release for formulations R4 and R7, containing low levels of amylose and HPMC in this stage, was attributed to the more rapid dissolution of swollen amylose by α-amylase enzymes which create perforations for fluid diffusion through the gel layer and subsequent swelling of sodium starch glycolate to disintegrate the tablet. Slow drug release was observed for formulations R2, R3, and R6, which was due to higher HPMC concentration, leading to the formation of a thick gel layer, resisting the tablet to disintegrate and release the drug.
Effect of coating composition
Slow drug release from tablets coated only with HPMC K100LV (without amylose) (B2) is due to gel formed around the tablet due to HPMC K100LV, which does not allow enough pore formation and penetration of fluid to cause tablet disintegration. This shows that the site specificity of release is based on biodegradation of amylase, and HPMC helps in film formation along with amylose.
Effect of pH of media on drug targeting
The results suggested that pH has no effect on dissolution behavior of the optimized formulations; since HPMC is a pH-independent polymer and amylose also remains unaffected by pH, the formulation is able to release the drug specifically in the colon, irrespective of change in pH. This ability is very useful, as the colon pH is found to be lowered in conditions such as IBD (CitationKatsuma et al., 2002; CitationFriend, 2005).
Effect of superdisintegrant on drug targeting
Fastest drug release for tablets containing sodium starch glycolate (F1), as compared to the tablet containing no superdisintegrant (F2), is attributed to sodium starch glycolate, which is a superdisintegrant with rapid swelling action that causes a bursting effect after penetration of water into the tablets. However, core tablets without superdisintegrant did not expand enough to burst the tablet, and the gel formed due to HPMC delayed the drug release. This effect was further confirmed by in vivo study in a human volunteer ().
In vivo X-ray results
From the abdominal radiographs of human volunteers taken at regular time intervals, there was seen a variation in gastric transit time of formulation. Despite these variations, the formulation with superdisintegrant coated with amylose and HPMC (A3) disintegrated at the cecum or ileocecal region, which is the initial segment of colon. This disintegration is due to degradation of amylose coat by amylase enzyme present in the colonic region, secreted by bifid bacteria, and the subsequent bursting effect of the superdisintegrant. The tablet without superdisintegrant with the same coating composition of 285:150 of amylose and HPMC, respectively, remained intact in the ascending colon after 6 h. These results are in agreement with in vitro release studies, which show drug release after the addition of amylase enzyme and underline the importance of superdisintegrant in achieving the targeted drug delivery to the colon.
Conclusion
It was shown that an appropriate factorial design and optimization technique can be successfully used in the development of compression coating formulations to achieve colon delivery. Response surface plots and optimization enabled the formulation of diclofenac sodium tablets coated with a combination of amylose and HPMC. It was shown that in the presence of inter-subject variation and changed pH the optimized formulation showed release specifically and instantly in the colon, which may be very effective for the treatment of colonic disease.
Acknowledgements
This work was supported by the BCUD, University of Pune. The authors are thankful for the kind support provided by Dr A. R. Madgulkar (Principal, AISSMS College of Pharmacy, Pune, India). JNTU Hyderabad. Evonik-Degussa for Eudragit polymers as a gift, Portable X ray services.
Declaration of Interest
This work was supported by the BCUD, University of Pune.
References
- Akhgari, A.H., Afrasiabi, G., Sadeghi, F., Azimaie, M. (2005). Statistical optimization of indomethacin pellets coated with pH-dependent methacrylic polymers for possible colonic drug delivery. Eur J Pharm Biopharm. 305:22–30.
- Chebli, C., Cartilier, L., Hartman, N.G. (2001). Substituted amylose as a matrix for sustained-drug release: a biodegradation study. Int J Pharm. 222:183–9.
- Chourasia, M.K., Jain, S.K. (2004). Pharmaceutical approaches to colon targeted drug delivery systems. Eur J Pharm Sci. 6:32–66.
- Davis, S.S., Hardy, J.G., Taylor, M.A., Stockwell, A., Whalley, D.R., Wilson, C.G. (1984). The in vivo evalution of an osmotic device using gamma scintography. J Pharm Pharmacol. 36:740–2.
- Ellis, H.S., Ring, S.G. (1985). A study of some factors influencing amylose gelation. Carbohydr Polym. 5:201–3.
- Friend, D.R. (1991). Colon-specific drug delivery. Adv Drug Deliv Rev. 7:49–199.
- Friend, D.R. (2005). New oral delivery systems for treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 57:247–65.
- Gupta, V.S., Assmus, M.W., Beckert, T.E., Price, J.C. (2001a). A novel pH- and time-based mulit-unit potential colonic drug delivery system. II. Optimization of multiple response variables. Int J Pharm. 213:93–102.
- Gupta, V.S., Beckert, T.E., Price, J.C. (2001b). A novel pH- and time-based multi-unit potential colonic drug delivery system. I. Development. Int J Pharm. 213:83–91.
- Katsuma, M., Watanabe, S., Kawai, H., Takemura, S., Masuda, Y., Fukui, M. (2002). Studies on lactulose formulations for colon-specific drug delivery. Int J Pharm. 249:33–43.
- Lee, H., Mukherjee, S. (2002). Drug delivery-oral colon-specific. In: Swarbrick, J., Boylan, J., eds. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker, 871–85.
- Leong, C.W., Newton, J.M., Basit, A.W., Podczeck, F., Cummings, S.G. (2002). The formation of colonic digestible films of amylase and ethylcellulose from aqueous dispersions at temperature below 370c. Eur J Pharm Biopharm. 54:291–7.
- Leopold, C.S. (1999). Coated dosage forms for colon-specific drug delivery. PSTT. 2:197–204.
- Marvola, M., Aito, H., Ponto, P., Kanniksoki, A., Nykanen, S., Kokkonen, P. (1987). Gastrointestinal transit and concomitant absorption of verapamil from single unit sustained release tablet. Drug Dev Ind Pharm. 13:1593–609.
- Milojevic, S., Newton, J.M., Cummings, J.H., Gibson, G.R., Botham, R.L., Ring, S.G., Stockham, M., Allwood, M.C. (1996). Amylase as a coating for drug delivery to the colon: prepation and in vitro evalution using 5- aminosalicylic acid pellets. J Contr Rel. 38:75–84.
- Pozzi, F., Furlani, P., Gazzangia, A., Davis, S.S., Wilding, I.R. (1994). The time-clock system: a new dosage form for fast and complete release of drug after a predetermined period of time. J Contr Rel. 31:99–108.
- Radiation Protecting 118 Referral Guidelines for Imaging. Office for Official Publications of the European Commission Directorate-General for the Environment, Luxembourg. Available online at: http://europaeuint/comm/energy/nuclear/radioprotection/publication/doc/118_enpdf. 2000, accessed 20th July 2009.
- Rubinstein, A. (2005). Colonic drug delivery. Drug Discov Today: Techno. 2:33–7.
- Siew, L.F., Basit, A.W., Newton, J.M. (2000). The properties of amylase-ethylcellulose films cast from organic-based solvents as potential for colonic drug delivery. Eur J Pharm Sci. 11:133–9.
- Sinha, V.R., Kumria, R. (2001). Polysaccharides in colon-specific drug delivery. Int J Pharm. 224:19–38.
- Sinha, V.R., Kumria, R. (2003). Microbially triggered drug delivery to the colon. Eur J Pharm Sci. 18:3–18.
- Tuleu, C., Basit, A.W., Waddington, W.A., Eill, P.J., Newton, J.M. (2002). Colonic delivery of 4-aminosalicylic acid using amylose-ethylcellulose-coated hydroxypropylmethylcellulose capsules. Aliment Pharmacol Ther. 16:1771–9.
- Ueda, S., Ibuki, R., Kawamuza, A., Murata, S. (1994). Development of a novel drug delivery system, time-controlled explosion system (TES). J Drug Target. 2:133–40.
- Vandamme, T.F., Lenourry, A., Charrueau, C., Chaumeil, J.C. (2002). The use of polysaccharides to target drugs to the colon. Carbohydrate Polym. 48:219–31.
- Yang, L., Chu, J.S., Fix, J.A. (2002). Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 235:1–15.
- Yang, L., Watanabe, S., Li, J., Chu, J.S., Katsuma, M., Yokohama, S., Fix, J.A. (2003). Effect of colonic lactulose availability on the timing of drug release onset in vivo from a unique colon-specific delivery system. Pharm Res. 20:33–43.