Abstract
This work aims to develop norfloxacin-solid lipid nanoparticles (NFX-SLN) as an oral delivery formulation. Hot homogenization and ultrasonic technique was employed to prepare NFX-SLN using stearic acid as lipid matrix and polyvinyl alcohol as surfactant. The physicochemical characteristics of SLN were investigated by optical microscope scanning electron microscopy and photon correlation spectroscopy. Antibacterial experiments of NFX-SLN were carried out by broth dilution technique. Pharmacokinetics was studied after oral administration in male Sprague-Dawley rats. The results showed that NFX-SLN was spherical and the SLN of the optimized formulation had diameters 301 ± 16.64 nm, polydispersity index 0.15 ± 0.04, zeta potential -30.8 ± 0.69 mv, loading capacity 8.58 ± 0.21% and encapsulation efficiency 92.35 ± 2.24% with good stability at 4 °C. The NFX-SLN had sustained release effect and sustained bactericidal activity. Cytotoxicity studies in cell culture demonstrated that the nanoparticles were not toxic. NFX-SLN resulted in significantly higher plasma drug concentration than native NFX. The SLN increased the relative bioavailability of NFX by 12 folds, prolonged the plasma drug level above the average minimum inhibition concentration from 14 to 168 h. These studies demonstrate that NFX-SLN could be a promising oral formulation for enhanced bioavailability and pharmacological activities.
Introduction
Norfloxacin (NFX) is a widely used third generation quinolone synthetic antibiotic which has the characteristics of broad bacterium contradicting, small side-effects and cross-resistance with other drugs (CitationHan et al., 2005). NFX is used against both gram-positive and gram-negative bacteria as well as trimethoprim/sulfonamide resistant microbes (CitationPreheim et al., 1987). It is also active against Mycoplasma (CitationBrown et al., 1996). This antibiotic shows promise as an antimicrobial agent for a variety of bacterial diseases (CitationWolfson & Hooper 1988; CitationCarratalá et al., 1995). NFX kills bacteria on a concentration-dependent basis (CitationFerrero et al., 1995). The minimum inhibitory concentration for 90% of organisms (MIC90) was below 0.12 μg/ml for E.coli, Salmonella spp, Klebsiella pneumonia, Klebsiellaoxytoca, Proteus vulgaris, Haemophilus influenzae (CitationLavy et al., 1995; CitationAndersson & MacGowan 2003). NFX has been used extensively as both oral and injectable formulations for human and many domestic animal species (CitationHans & Li 1991). Oral administration is a fundamental route for therapy and prevention such as the standard of therapy in the prophylaxis of bacterial infections in cirrhotic patients with gastrointestinal hemorrhage; oral treatment regimen for uncomplicated gonococcal infection or oral prophylaxis suppressing infection caused by aerobic gram-negative bacilli during anti-leukemic therapy (CitationFernández et al., 2006; CitationKaplowitz et al., 1987; CitationVerhoef & Rozenberg 1989).
However, there are drawbacks to limit the usage of NFX. NFX is scarcely dissolved in pure water, ethanol, methanol or aether while freely soluble in hydrochloric acid and natrium hydroxydatum with a low bioavailability mainly attributed to its low aqueous solubility and low permeability (CitationGuyot et al., 1995; CitationBreda et al., 2009). Human pharmacokinetic data for NFX show fraction absorption indicative of a poor permeability (CitationHooper & Wolfson 1985). The absolute bioavailability of NFX in humans and in laboratory animals is reported 40% post oral administration (CitationGips & Soback 1996). Examination of the individual serum concentration/time curves of the volunteers indicated that there may be a “first-pass” effect after oral administration and the site of metabolism is the liver, first-pass hepatic metabolism may be in part responsible for their lesser bioavailability (CitationHooper & Wolfson 1985). Poor bioavailability may provide lower antimicrobial activity and give rise to the development of resistance by the microorganisms (CitationAleem et al., 2008). Furthermore, repeated administrations are required to attain a steady-state level of drug. The common usage of NFX is twice a day for 7 to 10 days or even longer for uncomplicated lower urinary tract infections leading to a frequent dosage (CitationArredondo et al., 2004). Prophylactic NFX to patients with cancer who were neutropenic or likely to develop cytotoxic therapy–induced neutropenia or gastrointestinal hemorrhage are given orally 400 mg twice daily lasting more than 7 days (CitationFernández et al., 2006; CitationCarratala et al., 1998). Repeated dosage may result in higher incidence of adverse effect. Additionally, treatment of some diseases requires high dosage, such as urinary tract infection required high dose NFX for months (CitationBoerema & Saene 1986). Long-term high dose therapy has caused lenticular opacities, decreased spermatogenesis and testicular atrophy in experimental animals nausea and headache in patients(CitationSmith 1987; CitationPittman et al., 1993).
Solid lipid nanoparticles (SLN) are valuable as an oral delivery carrier to increase the absorption of a poor water soluble drug and enhance the permeability and retention effect (CitationLi et al., 2009; CitationMaeda 2001; CitationZhang et al., 2008). It can improve the bioavailability of poorly hydrophilic and lipophilic drugs when these drugs are encapsulated in SLN (CitationLuo et al., 2006). The mechanisms of bioavailability enhancement by SLN is that they possess adhesive properties that make them adhere to the gut wall and release the drug exactly where it should be absorbed (CitationMüller & Keck 2004). In addition, the lipids are known to have properties that promote the oral absorption of lipophilic drugs and drugs in general (CitationPorter & Charman 2001; CitationCharman 2000). SLN can protect labile macromolecules from stomach acid and from the “first-pass” metabolism in the gastrointestinal tract (CitationManjunath & Venkateswarlu 2005). Better absorption and bioavailability enhancement enables dosage reduction and minimizes side effects (CitationMunoz et al., 1994; CitationKalaria et al., 2008). The use of biodegradable materials for nanoparticles preparation allows for sustained drug release within the target site over a period of days or even weeks, lowering the frequency of administration (CitationPanyam & Labhasetwar 2003; CitationVasir et al., 2003).
Stearic acid is a saturated 18-carbon chain fatty acid and usually synthesized from acetyl-CoA, metabolized by β-oxidation and expected to have good biocompatibility and low toxicity in body. The fatty acid is solid at room or body temperature, thus it is chosen for commonly pharmaceutical use as lipid matrix to prepare SLN (CitationZhang et al., 2000). Polyvinyl alcohol (PVA) is the most commonly used surfactant in the formulation of nanoparticles due to its excellent mechanical strength, biocompatibility, and non-toxicity and has been approved by the US Food and Drug Administration for medical and food applications (CitationDavda & Labhasetwar 2002). In this work, NFX-SLN was prepared using stearic acid as lipid matrix and PVA as surfactant. The performances and the pharmacological activities of NFX-SLN were studied in vitro and in vivo.
Materials and methods
Materials
Stearic acid was obtained from Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China). PVA and MTT (3-[4, 5-dimehyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide) were purchased from Sigma (St. Louis, MO, USA). NFX was bought from Shandong Lukang Pharmaceutical Co., Ltd. (Jining, China). NFX reference standard and Staphylococcus aureus (CCVCC2248) were purchased from China Institute of Veterinary Drug Control (Beijing, China). The solvents used for HPLC were of liquid chromatography (LC) grade. Methanol and acetonitrile were purchased from Fisher Scientific (Fair Lawn, NJ, USA). The water for HPLC was prepared with a Milli-Q system (Millipore, Bedford, MA). The citric acid was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) and the ammonium acetate was obtained from Beijing Chemical Reagents Company (Beijing, China). Other chemicals and reagents not specified in the text were of analytical grade or equivalent. Baby hamster kidney cell line (BHK-21) was from American Type Culture Collection (ATCC) (Manassas, VA, USA). Sprague-Dawley (SD) rats were from Academy of Military Medical Sciences (Beijing, China). They were acclimatized for 1 week before use with free access to food and water. All animal procedures were conducted with the approval of the China Agricultural University Institutional Animal Care and Use Committee. Humane care of all animals was stringently followed throughout all animal-involved studies.
Preparation of norfloxacin-loaded nanoparticles
Norfloxacin-solid lipid nanoparticles were formulated by hot homogenization and ultrasonic technique (CitationXie et al., 2010). Briefly, NFX was mixed with 3 g stearic acid in a 50 ml tube and heated in a boiling water bath. After the lipid was melted and the drug was dissolved in the melted lipid, 30 ml PVA solution preheated on a boiling water bath was poured into the lipid phase under magnetic stirring to form an o/w emulsion. The emulsion was sonicated for 5 min (VC X 750 Vibra-CellTM, Sonics & Materials, Inc., Newtown, CT, USA, using the 13 mm microprobe with amplitude 35%) to form a nanoemulsion. The nanoemulsion was quickly poured into 200 ml cold water to obtain a nanoparticle suspension. The nanoparticles were collected by centrifugation at 9000 rpm (Centrifuge 5810 R; Eppendorf, Germany) for 30 min and washed 3 times with distilled water. The SLN were re-suspended in 100 ml distilled water and lyophilized for 72 h (LGJ-12 Freeze Dryer; Beijing Songyuanhuaxing Science Technology Development Co., Ltd., China). The control SLN was prepared similarly without adding NFX.
Determination of mean diameter (MD), polydispersity index (PDI) and zeta potential (ZP)
The mean diameter, PDI and zeta potential of the SLN were determined by photon correlation spectroscopy (PCS) using Zetasizer Nano ZS90 (Malvern Instruments, UK). The samples of SLN were suspended in distilled water at a concentration of 2.7 mg/ml for particle size and PDI analysis, and a concentration of 0.3 mg/ml for zeta potential determination.
Determination of loading capacity (LC) and encapsulation efficiency (EE)
The wavelength scan using a UV spectrophotometer (U-1800, Hitachi Tech Co., Japan.) showed that NFX had a specific absorption at 272.4 nm. To determine the drug content in SLN, a weighed amount of freeze-dried SLN was suspended in 1% NaOH solution in a 15 ml tube and heated on a boiling water bath for 5 min. After cooling down, the solution was centrifuged at 9000 rpm (Centrifuge 5810 R; Eppendorf, Germany) at 4 °C for 15 min. The supernatant was analyzed at 272.4 nm. The control nanoparticles without NFX were treated similarly as blanks for measurements. Loading capacity (LC) was calculated directly. NFX in total SLN was calculated by LC multiply the recovered freeze-dried SLN. Loading capacity (LC) and encapsulation efficiency (EE) are defined as follows:
Microscopic analysis
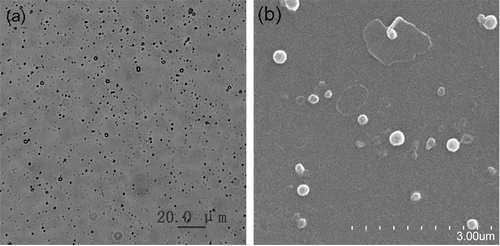
One milligram of NFX-SLN prepared with 3g strearic acid, 300mg NFX and 30ml 1% PVA was suspended in 1 ml distilled water. Two microliters of the suspension were placed on a microscope slide and the nanoparticles were dried at room temperature for 5 min. Photomicrographs of the SLN were taken using an inverted optical microscope (Olympus 1X71, Olympus, Japan).
Morphology analysis
The morphology of NFX-SLN prepared with 3g strearic acid, 300mg NFX and 30ml 1% PVA was studied by SEM (SE S-3400N; Hitachi, Japan). Briefly, 1 mg samples were suspended in 1 ml distilled water and 2 µl of the suspension were placed on a cover glass and dried at room temperature over 30 min. The samples were coated with gold and examined at an accelerating voltage of 20 kV.
In vitro release study
Norfloxacin-solid lipid nanoparticles (3 mg) prepared with 3g strearic acid, 300mg NFX and 30ml 1% PVA were suspended in 2 ml 0.1 mol/ml hydrochloric acid (HCl) to mimic the in vivo stomach decomposition process (CitationLuo et al., 2006) in a dialysis bag (MW: 8000–14400) and dialyzed against 38 ml 0.1 mol/ml HCl in a 50 ml tube at 37 °C under magnetic stirring at 60 rpm. Since NFX was freely soluble in hydrochloric acid, the volume of receiver solution was large enough to completely dissolve the released drug. Native NFX suspension with the same drug concentration was used as the control. To determinate the NFX amount diffused through the dialysis bag, at fixed time intervals samples (2 ml) were withdrawn from the receiver solution and the same amount of 0.1 mol/ml HCl was added to maintain a constant volume. NFX in the release medium was measured spectrophotometrically at 272.4 nm (U-1800, Hitachi Tech Co., Japan). The control nanoparticles without NFX were treated similarly and used as blanks for measurement. To determine the drug remained in SLN within dialysis bags after release, the SLN was collected by centrifugation of the suspension and the measurement was carried out by the method in determination of LC. All the experiments were carried out in triplicate.
Stability studies
Stability studies of SLN were performed after the samples were stored at 4 °C for 6 months. The values of MD, PDI, ZP and LC were measured for the evaluation of the physical stability of the nanoparticles. In vitro release was also carried out and compared with that of newly prepared SLN.
Antibacterial activity studies
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by the broth dilution technique of National Committee for Clinical Laboratory Standards (NCCLS; now renamed as Clinical and Laboratory Standards Institute, CLSI, 2000). Briefly, 11 mg NFX-SLN (containing 944 μg NFX) was suspended in 4 ml distilled water and serial dilutions were made with distilled water (NFX concentration: 1.2, 0.9, 0.6, 0.45 0.3 and 0.225 µg/ml). The dilutions (2.7 ml) were mixed with 10 µl Staphylococcus aureus (CCVCC2248) containing 0.5 McFarland (1.5 × 108 CFU/ml) to a final bacterial concentration of 5.5 × 105 CFU/ml. The mixture was incubated at 37 °C for 12 h and 24 h in an incubator (Jiangsu Taicang Experimental Equipment Company, China) with shaking at 130 rpm. The MIC of NFX was defined as the lowest concentration inhibiting visible growth of bacteria. The MBC is measured by subculturing the broths used for MIC determination onto fresh agar plates. The MBC is the lowest concentration of the drug that results in killing 99.9% of the bacteria being tested. The native NFX and the released NFX were also made serial dilutions to the final concentrations as above and the MIC and MBC were measured by the same way.
Sustained antibacterial studies were conducted by broth dilution technique. Ten microliters bacterial culture containing 0.5 McFarland (1.5 × 108 CFU/ml) of organisms were added to 2 ml Mueller-Hinton broth medium in a 4 ml tube containing NFX or NFX-SLN with a drug concentration of 0.34 µg/ml. Control nanoparticles (CNP) and Mueller-Hinton broth (MHB) were used as controls. The mixtures were incubated at 37 °C in an incubator with shaking (130 rpm). At fixed time points (6, 12, 24, 36, 48, 72 h), and ten serial dilutions of the mixtures were cultured on Luria broth agar plates at 37 °C. The colonies were counted when they could be observed by naked eyes. Growth graphs were plotted by calculating the number of colonies in 1 ml of the mixture versus the incubating time (hour). All experiments were carried out in triplicates.
Cytotoxicity evaluation
Cytotoxicity evaluation of NFX-SLN was conducted by MTT assay in BHK-21 cell line. The cells were seeded into a 96-well microplate at a density of 1 × 104 cells per well in 0.1 ml DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics and cultured in a humidified 5% CO2 incubator at 37 °C. After 24 h, the medium was replaced by 100 µl DMEM complete medium containing 58.3 µg NFX-SLN (drug concentration: 50 µg/ml). Native NFX or control nanoparticles (CNP) at same concentrations were also used. After 24 h co-incubation, cell viability was assessed by MTT assay. Typically, 5 mg/ml of MTT in PBS were added to each well reaching a final MTT concentration of 0.5 mg/ml and incubated for 4 h. Then the supernatants were removed and the formazan crystals were dissolved in 150 µl DMSO. Aliquots were drawn from each well and the absorbance at 490 nm was determined by a microplate reader (Bio-Rad 680). Cells without adding SLN or NFX were taken as control and set to 100% viability. The cell viability (%) compared to control cells were calculated by (OD490sample/OD490control) × 100.
Pharmacokinetics
A group of 20 male SD rats weighing on an average of 360 ± 10 g were used for the pharmacokinetic studies. They were randomly divided into 4 groups with 5 animals in each group. NFX was administrated to them orally (20 mg/kg of body weight) in the form of either NFX or NFX-SLN suspended in 2 ml sterilized double-distilled water. Each treatment contained 2 groups. At different times (0, 0.17, 0.5, 1, 1.5, 2, 3, 4, 8, 14, 24, 36, 48, 72, 96, 120, 144, 168 h) post dosage, blood samples were collected from tail vein and at one time point only one group of each treatment were collected. The blood samples were collected by turns from 2 groups over the observation period. The experimental protocols concerning the handling of rats were in accordance with the requirements of the Institutional Animal Care and Use Committee at China Agricultural University.
HPLC assay
Sample extracts were prepared by mixing 150 µl plasma with 300 µl mixture of methyl alcohol and acetonitrile (1/1 v/v) and vortexed for 3 min, and then centrifuged at 14000 rpm (Sigma 1-14; Sartorius, Germany) for 25 min. Two hundred microliters supernatant were taken for high performance liquid chromatography (HPLC) analysis using VP-ODS: 250 mm × 4.6 mm (Shimadiu Coperation, Kyoto, Japan). The HPLC condition was: mobile phase: (0.05 mol/l citric acid and 0.01 mol/l ammonium acetate, pH of the aqueous phase was adjusted by triethylamine to 4.5)/methyl alcohol (65/35, v/v); flow rate: 1 ml/min; UV detector wavelength: 278 nm; the injection Volume: 50 µl. The plasma concentration of NFX was found to be linear over the range 0.125–10 µg/ml. The correlation coefficient was 0.9991. The limit of quantification (LOQ) of UV detection was 0.1µg/ml. The relative standard deviations (R.S.D.) of accuracy and precision for three different plasma concentrations of NFX (0.1, 1 and 4 µg/ml) were 12.33%, 5.57% and 0.78% for intra-day analysis, and 7.10%, 6.58% and 6.03% for inter-day analysis.
Statistical analysis
The values of maximum serum concentration (Cmax) and time to peak serum concentration (Tmax) were obtained directly from the concentration–time plotting. The area under the concentration–time curve (AUC0-inf), mean residence time (MRT) and elimination half-life (T½) was analyzed based on noncompartmental pharmacokinetics statistical moment. The relative bioavailability at infinity was calculated as: Fr = AUCNFX-SLN, 0-inf /AUCNFX, 0-inf. Results were expressed as mean ± SD. Statistical analysis was performed using the one-way ANOVA. The differences were considered significant at the level of P < 0.05. Microsoft Excel (2003) (Microsoft Inc., USA) was used for all statistical analysis.
Results and discussion
Characteristics of NFX-loaded nanoparticles
The characteristics of SLN were listed in . The values of MD, PDI, ZP, LC and EE were significantly affected by the amount of NFX added in the preparation. With the increase of NFX from 150 to 600 mg, the values of MD, PDI, ZP and LC increased significantly. Although SLN with 600 mg NFX had higher LC value, its EE value was lower than those with both 150 and 300mg NFX, because of more drugs diffusing into the continuous phase. SLN with 300 mg NFX had higher drug loading capacity than SLN with 150 mg NFX and lower values of MD and PDI than SLN with 600 mg NFX, thus, 300 mg of NFX was selected for the preparation of SLN for further studies. NFX was usually orally given a single dose of 400 mg for patients (CitationWella M et al., 1998; CitationEandi M et al., 1983), which equaled to 4.662 g of the SLN prepared with 300 mg NFX. Considering the improvement of the drug bioavailability and the enhanced therapeutic efficacy, the dose could be reduced (CitationPandey R & Khuller GK 2005; CitationPandey R et al., 2005).
Table 1. Characteristics of SLN (mean ± SD, n = 3).
PVA concentration had significant effects on the values of MD, PDI and EE. When PVA concentration increased from 0.5% to 1%, EE increased significantly and MD and PDI decreased. But further increase of PVA concentration from 1% to 2% resulted in no significant changes of MD, PDI and EE. Therefore, 1% PVA was chosen for preparation of SLN.
Optical and SEM micrographs showed that NFX-SLN were well dispersed with good particle size distributions (), and were spherical with smooth surfaces (). NFX-SLN had higher values of MD, PDI and ZP than that of control (), indicating that the drug played an important role in these characteristics. Lipid matrix encapsulated drug leading to a larger volume. NFX with negative charge enhanced the total surface charge. A high ZP (>30 mV) could provide an electric repulsion to avoid the aggregation of particles (CitationLevy et al., 1994). The nanoparticle system with a high LC and EE could reduce the quantity of carrier required for the administration of a sufficient amount of pharmacologically active agent to the target site.
In vitro release of NFX from SLN
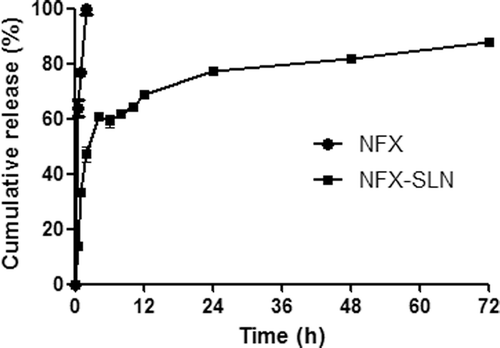
This 0.1 mol/ml HCl was used to mimic stomach acidity (CitationLi et al., 2000). The in vitro release behavior of NFX-SLN and native NFX is summarized as the cumulative percentage release of NFX-SLN, as shown in . The release of native NFX was almost complete by 2h. For the NFX-SLN, an initial fast release was observed during the first 4 h, the release reached 47 ± 2.86% by 2h and 59.09 ± 2.02% by 4h. The fast release phase could be attributed to the large surface area of nanoparticles with drug enrichment or precipitation in outer shell of the particles, leading to a relatively short distance of diffusion and hence fast release of the drug (CitationMühlen et al., 1998; CitationHu et al., 2002; CitationÜner 2006). From a therapeutic standpoint, a fast release profile could be considered as an advantage as a sufficient amount of a drug and improvement of the penetration of the drug (CitationJenninga et al., 2000). After the fast release, it was a sustained release and the total release reached 77.21 ± 3.63% by 24 h. Then a prolonged, relatively steady and slow release was observed. The release reached 88.36 ± 1.27% by 72 h. After 72 h releasing, the remaining drug in SLN within dialysis bags was 8.62 ± 1.39%. This release trend is similar to our previous studies on other drug-loaded SLN (CitationXie et al., 2008; CitationHan et al., 2009; CitationXie et al., 2009).
Stability studies
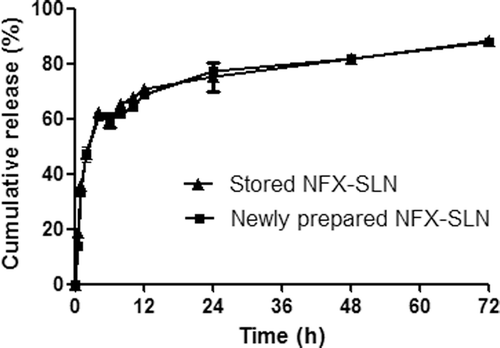
After 6 months of storage at 4°C, ZP and LC displayed no significant changes, but the values of MD and PDI increased significantly (). In vitro release studies showed that stored SLN had the same release ability as that of newly prepared SLN. At different time points (), the cumulative release values of the stored SLN were nearly the same as those of the newly prepared SLN, indicating the SLN maintained their physical characteristics as a controlled release formulation. These results demonstrate that the SLN had good stability at 4°C.
Table 2. Comparison of characteristics of stored and newly prepared SLN (mean ± SD, n = 3).
Antibacterial activity
In vitro antibacterial activity studies showed that the released NFX had the same MIC and MBC as that of the native NFX (), indicating that the bioactivity of NFX was not changed during the preparation and release procedures. This preparation method avoided the using of organic solvent, at the same time, maintained drug stability since the temperature in the preparation did not exceed 100 °C while the melting point of NFX is 228 °C (CitationGao 1995). Moreover, the MIC and MBC of NFX-SLN were the same as that of native NFX at 12 h, and lower than that of native NFX at 24 h, suggesting that SLN increased the antibacterial activity of NFX. These results are similar to those obtained by Ranjita Misra et al and Esmaeili et al (CitationMisra et al., 2009; CitationEsmaeili et al., 2007). Fusion of the lipid composition with the bacterial membranes might account for the enhanced antibacterial effect of NFX-SLN (CitationSachetelli et al., 2000). Due to membrane merging, the antibiotic could easily penetrate inside the bacteria allowing the increased bactericidal efficacy observed with the drug, thus circumventing the normal pathway of penetration (CitationBeaulac et al., 1996; CitationBeaulac et al., 1998).
Table 3. MIC and MBC of NFX (µg/ml, n = 3).
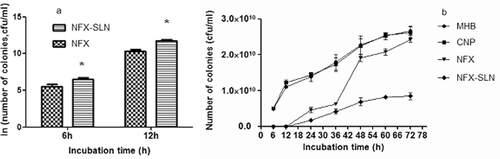
Sustained antibacterial activity studies were carried out based on the growth kinetics of Staphylococcus aureus and a viable colony count method was conducted. At 6 h and 12 h NFX-SLN was less effective (), but at all other time points, NFX-SLN was much more effective than native NFX (). Lower concentration of NFX might count for the less effectiveness of NFX-SLN at the early stages because of an incomplete release of NFX from the nanoparticles. After 12h, NFX-SLN exhibited its advantage over native NFX. Colony number of native NFX sharply increased between 36 h and 48 h and reached almost the same level as that of the controls by 72 h, indicating the exhaustion of the antibiotic. In case of NFX-SLN, the increase of bacterial colonies was much slower (). NFX-SLN remained effective for a longer period of time owing to the sustained release of the drug. The two curves representing control nanoparticles and Muller-Hinton broth were in the same trend, indicating that control nanoparticles had no antibacterial effect.
Cytotoxicity
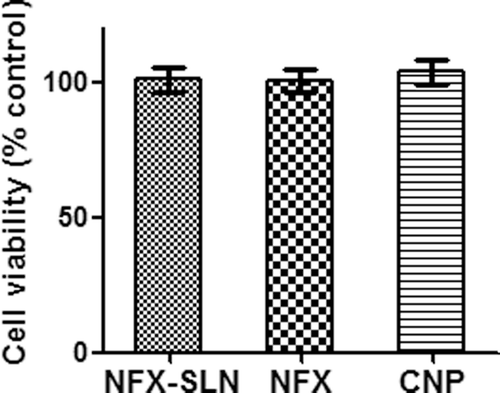
As demonstrated in , cytotoxicity study on BHK-21 cell line showed no significant differences among the NFX-SLN, native NFX and the control (P > 0.05) on average cell viability, indicating the nanoparticles were not toxic to the cells. Stearic acid and PVA are commonly used pharmaceutical lipid matrix and surfactant with good biocompatibility and low toxicity.
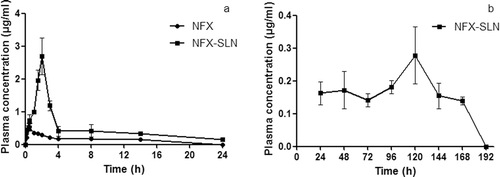
Pharmacokinetics
shows the Plasma NFX concentration vs. time plotting after oral administration of NFX-SLN or NFX, shows their pharmacokinetic parameters. At all time points, the plasma concentrations were significantly higher with NFX-SLN than with native NFX. NFX-SLN reached the peak plasma concentration (Cmax) of 2.7 ± 0.6 μg/ml at 2 h. The drug concentration decreased between 2 h and 4 h, then sustained above the MIC90 (0.12 μg/ml) in a steady trend. After fourteen hours, the plasma NFX concentration of NFX-SLN was 0.32 ± 0.03 μg/ml and then maintained over 0.139 ± 0.012 μg/ml for a period of 168 h. In contrast, the plasma drug concentration of native NFX increased rapidly to the peak level of 0.54 ± 0.16 μg/ml at 0.5 h, then declined quickly and was undetectable after 14 h. This result is similar to the in vitro release trend between NFX and NFX-SLN. The elimination half-life (t1/2) and the mean residence time (MRT) of NFX-SLN were significantly longer than those of native NFX (101.84 ± 7.52 h vs 9.23 ± 0.33 h, and 155.98 ± 5.8 h vs 14.74 ± 0.64 h), which also indicated that the NFX-SLN had a sustained release effect. The perceived advantage of SLN was the encapsulation of the drug in the solid matrix for protection and controlled release purposes, the carrier itself may exhibit certain absorption promoting effect (CitationMüller et al., 2000; Garcia et al., 2002). The lipid protects the drug from chemicals as well as enzymatic degradation, thereby delaying the in vivo metabolism and prolonging the systemic circulation time and controls the release of drug in blood (CitationLuo et al., 2006; CitationBala et al., 2004; CitationBhardwaj et al., 2005). Sustained drug release effect over a period of days or even weeks could provide a long-circulation effect in vivo (CitationPanyam & Labhasetwar 2003; CitationVasir et al., 2003). This NFX-SLN should be a promising formulation to improve the therapeutic efficacy and avoid frequent administrations of NFX for treatments needed multiple and frequent dosages, such as cancer induced neutropenia and gastrointestinal hemorrhage, and consequently reduce the adverse effects. The Cmax of NFX-SLN indicates that there was a fast release of SLN which was consistent with the in vitro initial fast release in this study. This kind of curves is desirable for sustained-release products where the initial release rate should be sufficiently rapid to ensure the therapeutic drug serum levels.
Table 4. Pharmacokinetic parameters after oral administration to SD rats (mean ± SD, n = 5).
Figure 6. Plasma NFX concentration vs. time plotting after oral administration of NFX-SLN or NFX: (a) within 24 h; (b) from 24 h to 168 h (mean ± SD, n = 5). NFX-SLN: norfloxacin-nanoparticles; NFX: native norfloxacin.
The AUC0-inf of NFX-SLN and native NFX were 57.81 ± 6.89 and 4.8 ± 0.14 respectively. SLN enhanced the oral bioavailability of NFX as reflected by AUC0-inf by 12 folds. The mechanisms of oral uptake include diffusion of particles through mucus and accessibility to enterocyte surface, epithelial interaction, cellular trafficking, exocytosis and systemic dissemination (CitationHou et al., 2009). Nanoparticles are preferentially absorbed via “M-cells” in the Peyer’’s patches by the process of endocytosis (lymphoid uptake), thereby delivering the drug loaded particles directly into systemic circulation through the lymphatics and circumventing the first-pass metabolism (CitationJani et al., 1992; CitationDesai et al., 1996). Lymphatic uptake is influenced by lipid nature, chain length, and hydrophobicity of nanoparticles (CitationHussain et al., 2001; CitationNordskog et al., 2001). The stearic acid has a long chain fatty acid, which increased in lymphatic uptake of NFX. The size of SLN in the range of 20–500 nm allows the efficient uptake in intestine, particularly in the lymphoid sections of this tissue (CitationYuan et al., 2007). Due to their small particle size, SLN might exhibit bioadhesion to the gastrointestinal tract wall or enter the intervillar spaces thus increasing their residence time in the gastrointestinal tract (CitationVasir et al., 2003). This increase in adhesion will result in enhanced bioavailability (CitationLuo et al., 2006). Bioavailability enhancement helps to lower dosage levels (CitationMunoz et al., 1994) and thus decreases toxicity or side effects. The enhanced bioavailability of NFX suggests that the NFX-SLN could be a useful formulation for treatment of diseases specifically depended on high dosage such as treatment of urinary tract infections.
Conclusions
Norfloxacin streaic acid-SLN prepared by hot homogenization and ultrasonic technique had good sustained release effect and stability. The SLN effectively enhanced the bioavailability and antibacterial activity of NFX. It could be a promising system for oral delivery of NFX.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aleem O, Kucheka B, Pore Y, Late S. (2008). Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J Pharm Biomed Anal. 47, 535–540.
- Andersson MI, MacGowan AP. (2003). Development of the quinolones. J Antimicrob Chemother. 5, 1–11.
- Arredondo GJL, Figueroa DR, Rosas A. (2004). Comparison of short-term treatment regimen of ciprofloxacin versus long-term treatment regimens of trimethoprim/sulfamethoxazole or norfloxacin for uncomplicated lower urinary tract infections: a randomized, multicentre, open-label, prospective study. J Antimicrob Chemother. 54, 840–843.
- Bala I, Hariharan S, Kumar MN. (2004). PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carr Syst. 21, 387–422.
- Beaulac C, Clément-Major S, Hawari J, Lagacé J. (1996). Eradication of mucoid Pseudomonas aeruginosa with £uid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob Agents Chemo-ther. 40, 665–669.
- Beaulac C, Sachetelli S, Laglce J. (1998). In-vitro bactericidal efficacy of sub-MIC concentration of liposome-encapsulated antibiotic against gram-negative and gram-positive bacteria. J Antimicrob Chemother. 41, 35–41.
- Bhardwaj V, Hariharan S, Bala I, Lamprecht A, Kumar N, Panchagnula R, Kumar MNV. (2005). Pharmaceutical aspects of polymeric nanoparticles for oral delivery. J Biomed Nanotechnol. 1, 235–258.
- Boerema JB, Saene HKV. (1986). Norfloxacin treatment in complicated urinary tract infection, Scand. J Infect Dis. Suppl. 48, 20–26.
- Breda SA, Jimenez-Kairuz AF, Manzo RH, Olivera ME. (2009). Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int J Pharm. 371, 106–113.
- Brown SA. (1996). Fluoroquinolones in animal health. J Vet Pharmacol Therap. 19, 1–14.
- Carratalá J, Fernández-Sevilla A, Tubau FE, Callis M, Gudiol F. (1995). Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clin Infect Dis. 20, 557–560.
- Carratala J, Roson B, Fernandez-Sevilla A, Alcaide F, Gudiol F. (1998). Bacteremic pneumonia in neutropenic patients with cancer causes, empirical antibiotic therapy, and outcome. Arch Intern Med. 158, 868–872.
- Charman WN. (2000). Lipids, lipophilic drugs, and oral drug delivery–some emerging concepts. J Pharm Sci. 89, 967–978.
- Davda J, Labhasetwar V. (2002). Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 233, 51–59.
- Desai MP, Labhasetwar V, Amidon GL, Levy RJ. (1996). Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 13, 1838–1845.
- Eandi M, Viano I, Nola FD, Leone L, Genazzani E (1983). Pharmacokinetics of norfloxacin in healthy volunteers and patients with renal and hepatic damage. Eur. J. Clin. Microbiol. 2, 253–259.
- Esmaeili F, Hosseini-Nasr M, Rad-Malekshahi M, Samadi N, Atyabi F, Dinarvaand R. (2007). Preparation and antibacterial activity evaluation of rifampicin-loaded poly-lactide-co-glycolide nanoparticles. Nanomedicine Nanotechnology, Biology and Medicine. 3, 161–167.
- Fernández J, Arbol LR, Gómez C. (2006). Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 131, 1049–1056.
- Ferrero L, Cameron B, Crouzet J. (1995). Analysis of gyrA and gyrB mutations in stepwise-selected ciprofloxacin-resistant mutants of staphylococcus aureus. Antimicrob Agents Chemother. 39, 1554–1558.
- Gao F, Yang P, Xie J, Wang HF. (1995). Synthesis, characterization and antibacterial activity of novel Fe(III), Co(II), and Zn(II) complexes with norfloxacin. J Inorg Biochem. 60, 61–67.
- Garcia-Fuentes M, Torres D, Alonso MJ. (2002). Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloids Surf B: Biointerfaces. 27, 159–168.
- Gips M, Soback, S. (1996). Norfloxacin nicotinate pharmacokinetics in unwearied and weaned calves. J Vet Pharmacol Ther. 19, 130–134.
- Guyot M, Fawaz F, Bildet J, Bonini FAM, Lagueny. (1995). Physicochemical characterization and dissolution of norfloxacin cyclodextrin inclusion compounds and PEG solid dispersions. Int J Pharm. 123, 53–63.
- Han C, Qi CM, Zhao BK, Cao J, Xie SY, Wang S, Zhou WZ. (2009). Hydrogenated castor oil nanoparticles as carriers for the subcutaneous administration of tilmicosin: in vitro and in vivo studies. J Vet Pharmacol Ther. 32, 116–123.
- Han YX, Wu X, Yang JH, Sun SN. (2005). The fluorescence characteristic of the yttrium–norfloxacin system and its analytical application. J Pharm Biomed Anal, 38, 528–531.
- Hans H, Li SG. (1991). Norfloxacin, next term the first of a new class of fluoroquinolone antimicrobials, revisited. Int J Antimicrob Agents. 1, 3–28.
- Hooper DC, Wolfson JS. (1985). The Fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 28, 716–721.
- Hou LL, Zhao XB, Ma YK, Zhai GX, Li LB, Lou HX. (2009). Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Controlled Release. 133, 238–244.
- Hu FQ, Yuan H, Zhang HH, Fang M. (2002). Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization–selected. Int J Pharm. 239, 121–128.
- Hussain N, Jaitley V, Alexander TF. (2001). Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 50, 107–142.
- Jani PU, McCarthy DE, Florence AT. (1992). Nanosphere and microsphere uptake via Peyer’’s patches: observation of the rate of uptake in rat after a single oral dose. Int J Pharm. 86, 239–246.
- Jenninga V, Schäfer-Kortingb M, Gohla S. (2000). Vitamin A–loaded solid lipid nanoparticles for topical use: drug release properties. J Controlled Release. 66, 115–126.
- Kalaria DR, Sharma G, Beniwal V, Kumar MNVR. (2008). Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res. 26, 492–501.
- Lavy E, Ziv G, Glickman A. (1995). Intravenous disposition kinetics, oral and intramuscular bioavailability and urinary excretion of norfloxacin nicotinate in donkeys. J Vet Pharmacol Ther. 18, 101–107.
- Levy MY, Schutze W, Fuhrer C, Benita S. (1994). Characterization of diazepam submicron emulsion interface: role of oleic acid. J Microencapsul. 11, 79–92.
- Li HL, Zhao XB, Ma YK, Zhai GX, Li LB, Lou HX. (2009). Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Controlled Release. 133, 238–244.
- Li XF, Carter SJ, Dovichi NJ. (2000). Non-aqueous capillary electrophoresis of tamoxifen and its acid hydrolysis products. J Chromatogra A. 895, 81–85.
- Luo YF, Chen DW, Ren LX, Zhao XL, Qin J. (2006). Solid lipid nanoparticles for enhancing vinpocetine’’s oral bioavailability. J Controlled Release. 114, 53–59.
- Kaplowitz L, Vishniavsky N, Evans T. (1987). Norfloxacin in the treatment of uncomplicated gonococcal infections. Am J Med, 82, 35–39.
- Maeda H. (2001). The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 41, 189–207.
- Manjunath K, Venkateswarlu V. (2005). Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Controlled Release. 107, 215–228.
- Misra R, Acharya S, Dilnawaz F, Sahoo SK. (2009). Sustained antibacterial activity of doxycycline-loaded poly(D,L-lactide-co-glycolide) and poly(ϵ-caprolactone) nanoparticles. Nanomedicine. 4, 519–530.
- Mühlen AZ, Schwarz C, Mehnert W. (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery–Drug release and release mechanism. Eur J Pharm Biopharm. 45, 149–155.
- Müller RH, Keck CM. (2004). Challenges and solutions for the delivery of biotech drugs–a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 113, 151–170.
- Müller RH, Mäder K, Gohla S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 50, 161–177.
- Munoz A, Guichard JP, Reginault P. (1994). Micronised fenofibrate. Atherosclerosis. 110, 45–48.
- Nordskog BK, Phan CT, Nutting DF. (2001). An examination of the factors affecting intestinal lymphatic transport of dietary lipids. Adv Drug Deliv Rev. 50, 21–44.
- Pandey R, Khuller GK (2005). Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis. 85, 227–234.
- Pandey R, Sharma S, Khuller GK (2005). Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis. 85,415–420.
- Panyam J, Labhasetwar V. (2003). Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Del Rev. 55, 329–347.
- Pittman W, Moon JO, Hamrick LC, Cox CE, Clark J, Childs S, Pizzuti D, Fredericks J, Clair PS. (1993). Randomized double-blind trial of high-and low-dose fleroxacin versus norfloxacin for complicated urinary tract infection. Am J Med. 94, 101–104.
- Porter CJH, Charman WN. (2001). In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev. 50, 127–147.
- Preheim LC, Cuevas TA, Roccaforte JS, Mellencamp MA, Bittner MJ. (1987). Oral use of ciprofloxacin in the treatment of elderly patients with complicated urinary tract infections due to trimethoprim sulfamethoxazole-resistant bacteria. Amer J Med. 82, 295–297.
- Sachetelli S, Khalil H, Chen T, Beaulac C, Sénéchal S, Lagacé J. (2000). Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim Biophys Acta. 1463, 254–266.
- Smith CR. (1987). The adverse effects of fluoroquinolones, J Antimicrob Chemoth. 19, 709–712
- Üner M. (2006). Preparation, characterization and physico-chemical properties of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Their benefits as colloidal drug carrier systems.Pharmazie. 61, 375–386.
- Vasir JK, Tambwekar K, Garg S. (2003). Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 255, 13–32.
- Verhoef J, Rozenberg-Arska M. (1989). Prevention of bacterial and fungal infections in granulocytopenic patients. Eur J Cancer. 25, 1345–1350.
- Wella M, Nabera KG, Kinzig-Schippersb M, Sörgelb F (1998). Urinary bactericidal activity and pharmacokinetics of enoxacin versus norfloxacin and ciprofloxacin in healthy volunteers after a single oral dose. Int J Antimicrob Ag. 10, 31–38.
- Wolfson JS, Hooper DC. (1988). Norfloxacin: a new targeted fluoroquinolone antimicrobial agent. Ann Intern Med. 108, 238–251.
- Xie SY, Pan BL, Wang M, Zhu LY, Wang FH, Dong Z, Wang XF, Zhou WZ.(2010). Formulation, characterization and pharmacokinetics of praziquantel-loaded hydrogenated castor oil-solid lipid nanoparticles, Nanomedicine.5, 693–701
- Xie SY, Wang SL, Zhao BK, Han C, Wang M, Zhou WZ. (2008). Effect of PLGA as a polymeric emulsifier on preparation of hydrophilic protein-loaded solid lipid nanoparticles. Colloids Surf B: Biointerfaces. 67, 199–204.
- Xie SY, Wang SL, Zhu LY, Wang FH, Zhou WZ. (2009). The effect of glycolic acid monomer ratio on the emulsifying activity of PLGA in preparation of protein-loaded SLN. Colloids Surf B: Biointerfaces. 74, 358–361
- Yuan H, Chen J, Du YZ, Hu FQ, Zeng S, Zhao HL. (2007). Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf B: Biointerface. 58,157–164.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. (2008). Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin Pharmacol Ther. 83, 761–769.
- Zhang Q, Yie GQ, Li Y, Yang Q, Nagai T. (2000). Studies on the cyclosporin a loaded stearic acid nanoparticles. Int J Pharm. 200, 153–159.