Abstract
In order to improve brain uptake of nanoparticles following nasal administration, odorranalectin (OL), the smallest lectin with much less immunogenicity than other members of lectin family, was conjugated to the surface of poly (ethylene glycol)-poly (lactic-co-glycolic acid) (PEG-PLGA) nanoparticles (NP) in this study. The bioactivity of OLconjugated to the nanoparticles was verified by haemagglutination tests.Tissue distribution of OL-modified and unmodified nanoparticles (OL-NP and NP) wasevaluated following intranasal (i.n.) administration by in vivo fluorescence imaging technique using DiR as a tracer, comparing with that of unmodified nanoparticles after intravenous (i.v.) injection. Besides, the nasal toxicity of OL-NP was evaluated on Calu-3 cell lines, toad palate and rat nasal mucosa.The results of TEM examination and dynamic light scattering showed a generally spherical shape of OL-NP with an average volume-based diameter around 90 nm. The haemagglutination test proved thatOL retained its haemagglutination activity when conjugated to nanoparticles. The brain targeting indexes of NP andOL-NPfollowing i.n. administrationand NP following i.v. injection were 5.8, 11.6 and 0.08, respectively.Thus,i.n. administration demonstratedmuch better brain targetingefficiency thani.v. injection, and OL modificationfacilitatedthe nose-to-brain delivery of nanoparticles.Moreover, the toxicityassessment suggested good safety of OL-NPboth in vitro and in vivo.In summary, odorranalectin-conjugated nanoparticlecould be potentially used as a nose-to-brain drug deliverycarrierfor thetreatment of CNS diseases.
1. Introduction
Becausethe blood-brain barrier (BBB) of the patients with central nervous system (CNS) disorder is almost undamaged (CitationKreuter et al., 2002), drugs have to cross a set of tight connective membranes to enterCNS from blood. This problem has seriously hindered the development of therapy for CNS disease, especially using macromolecular drugs(CitationCollister & Albensi, 2005; CitationPardridge, 2005; CitationTyler & Federoff, 2006; CitationKabanov & Gendelman, 2007). Therefore, a drug delivery system that could efficiently deliver drugs into brain is urgently needed.
Drugs couldbe directly deliveredinto CNSbypassingthe BBB via intranasal (i.n.) administration, whichis considered as an attractive alternative to traditional injection therapy for CNS disorders, such as Parkinson’s disease or Alzheimer’s disease(CitationKissel & Werner, 1998). Nevertheless, there are several limitations, including enzymatic degradation, mucociliary clearance in the nasal cavity and poor mucosal membrane permeability (CitationLuessen et al., 1996), that result in low bioavailability and limited therapeutic effects. We have found that modification on nanoparticles with lectins, such as wheat germ agglutinin (WGA) (CitationGao et al., 2006)or ulex europeus agglutinin I (UEA-I) (CitationGao et al., 2007),could efficiently improve nose-to-brain delivery of nanoparticles, because the lectins could prolong the residence time byspecifically recognizing sugar molecules and binding the glycosylated nasal mucosa.However,most lectins, because of large molecular weight, have potent red cell agglutination activity and high immunogenicity (CitationLiu et al., 2004), which may hamper their applications as targeting molecules.
Odorranalectin (OL), a peptide identified recently from skin secretions of Odorrana grahami, was proved to be the smallest peptide to date with lectin-like activity. It is a 17-amino-acids peptide with a molecular weight of 1.8 kDa whichpresents extremely low immunogenicity, about one eighth of that of WGA in mice (CitationLi et al., 2008). Besides, it can specifically bind L-fucose which widely locates on the olfactory epithelium of nasal mucosa (CitationLundh, Brockstedt & Kristensson, 1989). Thus, OL is very likely to be a targeting molecule for nose-to-brain drug delivery with low toxicity.
Therefore, our aim was to prepare OL-conjugated nanoparticles and to investigate their potential use in enhancing brain drug delivery following i.n. administration.To this end, OL-NP was prepared by double emulsion method using the mixture of synthesized PEG-PLGA and OL-conjugated PEG-PLGA (OL-PEG-PLGA).Delivery to brainand distribution inother tissueswere studied by in vivo fluorescence imaging technique using DiR as a fluorescent probe. In addition, the toxicity of OL-NP wasevaluated both in vitro and in vivo using Calu-3 cell lines, toad palate model and rat nasal mucosa model.
2. Materials and methods
2.1 Materials
Methoxy poly (ethylene glycol)-poly (lactic-co-glycolic acid) (PEG-PLGA) (MW 2 kDa-30 kDa) was obtained from university of Electronical Science and Technology (Sichuan, China). OL-conjugated PEG-PLGA (OL-PEG-PLGA) was synthesized in our laboratory.DiR wasbought from Caliper Life Sciences (Woodland Hills, CA, USA).Rabbit anti-nerve-specific enolase polyclonal antibody and SABC Kit werepurchased from Boster (Hubei, China). 3,3N-Diaminobenzidine Tertrahydrochloride (DAB) waspurchased from Vector Laboratories (Burlingame, CA, USA). All the other chemicals were analytical reagent grades and used without further purification.
Sprague-Dawley (SD) rats (160–180 g, ♀) and toads (30–40g) were obtained from Experimental Animal Center of Fudan University and maintained at 22 ± 2 °C with access to food and water ad libitum. The animals used for the experiment were treated according to the protocols evaluated and approved by the ethical committee of Fudan University.
2.2 Preparation and characterization of NP/DiR and OL-NP/DiR
2.2.1 Preparation of NP/DiR and OL-NP/DiR
DiR-loaded nanoparticles (NP/DiR)were prepared with the polymer of PEG-PLGA using double emulsion method with minor revision (CitationTobio et al., 1998). DiR-loaded OL-conjugated nanoparticles (OL-NP/DiR) were prepared in the same procedure as NP/DiR except that the polymer ratio of PEG-PLGA to OL-PEG-PLGA was 24:1 (n/n). In brief, 50 μl of water was emulsified in 1ml of dichloromethane solution containing25 mg of polymers and 0.4 mg of DiR by sonication (200W, 20 s) using a probe sonicator (Scientz Biotechnology Co. Ltd., China). The primary emulsion was then emulsified in 2ml of sodium cholate aqueous solution (1%, w/v) using the same supersonic conditionas before to produce the w/o/w double emulsion. The double emulsion was then added into 20 ml of sodium cholate aqueous solution (0.5%, w/v) under moderate magnetic stirring. After 10 minutes stirring, dichloromethane was removed from the system to form nanoparticles using a rotary evaporator (R-200, Büchi, Germany). Nanoparticlesuspensionwasconcentrated by centrifugation at 10,000 g for 40 minand subjected to a sepharose CL-4B columnto remove the sodium cholate and unentrapped DiR.
Blank NP and OL-NPwere prepared for toxicity evaluationin the same procedure as above mentioned, except that DiR was not added.
2.2.2 Characterization of morphology and particle size
The morphological examination was carried out by transmission electron microscope (TEM, H-600, Hitachi, Japan). The mean diameters of the nanoparticles were determined by dynamic light scattering (DLS) using a Particle Sizer (NICOMP™380 ZLS, Santa Barbara, CA, USA).
2.2.3 Haemagglutination test of OL-NP/DiR
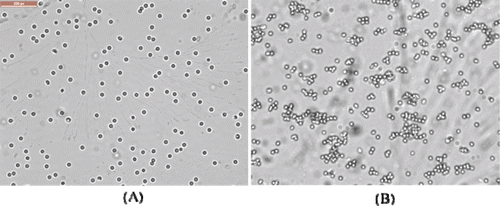
The bioactivity of OL on the surface of nanoparticles was confirmed by a haemagglutination test (CitationGao, Tao, Lu et al., 2006). OL-NP and NPwereincubated with 1% (v/v) fresh red blood cell suspensions of rat at 37 °C for 30 min. The suspensions were observed under a microscope (Leica DMI4000 BTS100, Germany).
2.2.4 Determination of encapsulating efficiency and loading capacity
To determine the encapsulating efficiency of DiR in nanoparticles, the supernatant was collected after centrifugation at 10,000 g for 40 min and properly diluted for content determination of DiR by a luminescence spectrometer (LS55 Perkin Elmer branch company, Shanghai, China). The drug encapsulation efficiency and loading capacity were calculated as indicated below. The analyses wereperformed in triplicate.
2.3 Biodistribution of nanoparticles in vivo
Biodistribution of NP and OL-NP in major tissuesfollowing i.n. administration to SD rats was investigated by in vivo imaging system (CRi, Woburn, MA, USA) using DiR as a tracer.Thirty-sixratswere divided into three groups(0.4 g/kg, i.p.). Group 1 and Group 2 were intranasally given NP/DiR and OL-NP/DiR, respectively, whileGroup 3 was intravenously given NP/DiR. For the i.n. administration, rats wereanesthetized with 10% hydral and set in a supine position. Corresponding preparations were administered at the openings of nostrils using a polyethylene 10 (PE 10) tube attached to a microliter syringe. The procedure was performed gently and slowly, allowing the rats to inhale all the preparations. Preparations foreach rat contained 80 μg of DiR and were suspended in 20 μlof double distilled water (10 μl each nostril).For thei.v. administration, preparations in the same dose as Group 1 and 2 were suspended in 0.6 ml of saline andgiven toeachrat of Group 3 via caudal vein. Rats were sacrificed by perfusion-fixation. Blood and major tissuesincluding brains, hearts, livers, spleens, lungs, and kidneys were collected for image acquirement at 1, 3, 6 and 12 hours after administration.The excitationwavelength for imaging was 770 nm and the exposure time was 0.5 to 30s depending on different groups and tissues.Fromthe luminescent images, regions of interest were drawn,and the integrated bioluminescence flux (counts/s)of DiR was calculated utilizing Maestro Software v2.10.
2.4 Toxicity assessment of OL-NP
2.4. 1 In vitro cytotoxicity
MTT cell viability assaywas performed on Calu-3 cell lines to evaluate the cytotoxicity of OL-NP in vitro. Cells were incubated with NP and OL-NP suspensions at the concentration of 1.37 to 3,000 μg/ml and OL solution at the concentration of 0.152 to 333μg/ml,respectively, in DMEM containing 20% FBS for 4 h at 37 °C.Cell viability was expressed as a percentage of the control (untreated) cells.
2.4.2 In vivo mucosa toxicity
The mucosa toxicity evaluation was conducted using toad palate and rat nasal mucosa. In the experiment on toad palate, the upper palate mucosa of toadsin each group was respectively exposed to 0.5 ml of saline, NP (25mg/ml), OL-NP (25mg/ml) and1% sodium deoxycholate solution (positive control) for 1 hour, rinsed with saline, and dissected and examined under an optical microscope (37XB, Shanghai Optical Instrument Company, Shanghai, China). The duration time of the ciliary movement was recorded and pictures were taken by a camera (μ740, Olympus, Japan).
In the experiment on rat nasal mucosa, 10 μl of NP, OL-NP (25 mg/ml) or saline was administrated successivelyfor oneweek to the nostrils of each group(n = 3) usinga polyethylene 10 (PE 10) tube attached to a microliter syringe. The rats were sacrificedand the nasal mucosa was harvestedat 24 hours after the last administration. Immunohistochemical staining was performed on paraffin sections (5-μm thick) of nasal mucosafor neuron-specific enolase (NSE) examination. In brief, sections were incubated with rabbit anti-nerve-specific enolase polyclonal antibody, performed with SABC kit, and finally stained with DAB.Images of sections were obtained using a microscope(Leica DMI4000 B TS100, Germany).
3. Results and discussion
3.1 Characterization of NP/DiR and OL-NP/DiR
3.1.1 Morphology and particle size
Transmission electron micrographs showed that NP/DiR and OL-NP/DiR were generally spherical with narrowsize distributions (). The particle size is an important factoraffectingnanoparticle endocytosis by nasal mucosa. It is generally controlled under 200 nm for drug delivery(CitationVila et al., 2005). In this study, the volume-based diameters of resulting nanoparticles were around 90 nm, which was advantageous for mucosa transport (, Column 2).
Figure 1. Transmission electron micrographs of DiR-loaded nanoparticles (NPs/DiR) (A) andOL-conjugated nanoparticles (OL-NPs/DiR) (B). (Bar = 100 nm)
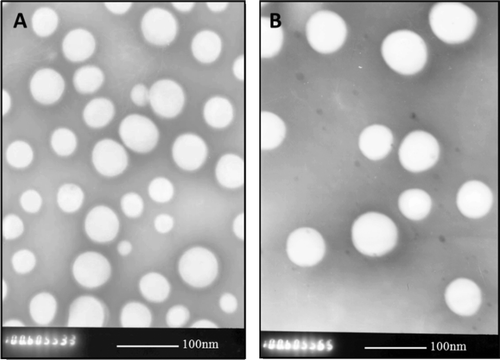
Table 1. Nanoparticle size, polydispersity index (PI), encapsulating efficiency and loading capacity of NPs and OL-NPs loaded with or without DiR. (Mean ± S.E.M., n = 3)
3.1.2 Haemagglutination test
The results of haemagglutination test showed that saline and NP/DiR had no haemagglutination activity, whereas OL-NP/DiR had, suggesting that OL maintained its haemagglutination activity after being conjugated tothe nanoparticles ().
3.1.3 Encapsulating efficiency and loading capacity of DiR
The encapsulation efficiency and loading capacity of NP/DiR and OL-NP/DiR were shown in (Column 4 and 5).They showed similar encapsulating efficienciesaround 60%, suggesting that DiR could be efficiently encapsulated intoPEG-PLGA nanoparticles.
3.2 In vivo distribution study of nanoparticles
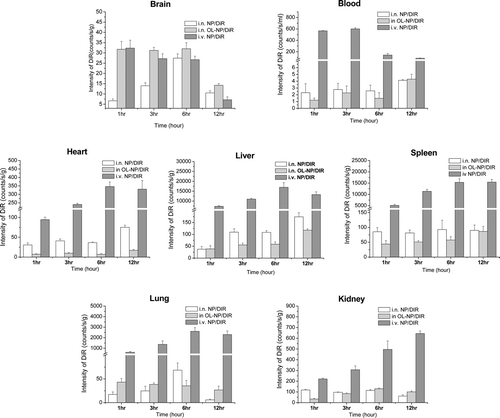
Recently, near-infrared fluorescence imaging technique has been widely used in biological, medical, and clinical research due to its high sensitivity, non-invasiveness, convenient and efficientdetermination (CitationNtziachristos, Bremer & Weissleder, 2003; CitationMei et al., 2010; CitationZhan et al., 2010).To evaluatebrain delivery and tissue distribution of nanoparticles,fluorescent marker DiR was incorporated to trace the nanoparticles in different tissues in vivo. In our previous study, less than 2% of DiR released from the nanoparticles within 24 h, suggesting that the florescence of DiR could represent nanoparticles inthe study in vivo.The semi-quantitative function of in vivo imaging system software makes it easy to get quantitative data from fluorescence images. However, because tissues vary widely on shape and density, the quantitative analysis is only suitable for evaluating distribution in the same tissues or the ratios of two different tissues. So fluorescence data of the same kind of tissues and the ratios of brain/bloodfrom different groups were compared here.Within the two i.n. groups, DiRintensity in brains of OL-NP groupwas higher than those of NP group, and heart distribution of DiR of OL-NP group was lower than that of NP group at all the time points. There was no significantly or regular difference of DiR intensity between the two i.n. groups in other tissues (). As to i.v. group, except for brain, the fluorescence intensities in liver, spleen, lung, kidney and blood were much higher than those of i.n. groups. To compare the brain targeting efficiency of the three groups, the targeting index (TI)was calculated by dividingthe AUC0-12 hof brain by that of blood (, the last column). The results showed that the TI values of NP and OL-NP weresignificantly enlarged by 73 folds and 146 folds, respectively, compared with i.v. injected NP,suggesting thati.n.administration isa good way to delivery drug to CNS with little systemic adverse effect to other tissues, and that OL modification canfacilitatethe nose-to-brain delivery of nanoparticles.
Table 2. AUC0-12hof tissues and blood, and brain targeting indexes (TI, AUCBrain/AUCBlood) followingi.n. administration of NPs/DiR (i.n. NP) or OL-NPs/DiR (i.n. OL-NP) compared withi.v. administration of NPs/DiR (i.v. NP).
Figure 3. Fluorescence intensity of DiR in different tissues and blood at different time points following intranasal administration of NPs/DiR (white stands) and OL-NPs/DiR (light grey stands), and intravenousinjection of NPs/DiR (dark gray stands). All data are representedas the mean±S.E.M. (n = 3).
It was reported that nasal mucosa was composed of respiratorymucosa and olfactory mucosa. Under the former there are plenty of vascular, through which preparations can be absorbed into systemic circulation, and from the latterpreparations can be directly delivered to CNS, bypassing the BBB(CitationIllum, 2000).The two regions presented different lectin-binding profiles (CitationGao et al., 2007),andOL could specially bind L-fucose, which is widely distributed on the olfactory epithelium (CitationLundh, Brockstedt & Kristensson, 1989; CitationGao, Chen, Tao et al., 2007). The characteristic of OL would facilitate drug absorption into olfactory mucosa and delivery through olfactory pathway to brain,decreasing the systemic absorption from the respiration mucosa.
3.3 Toxicity of OL-NP in vitro and in vivo
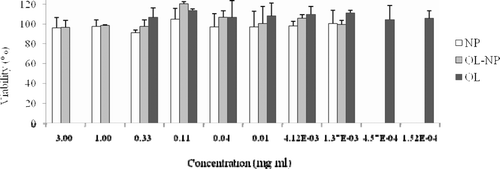
Thehuman lung adenocarcinoma Calu-3 cell line, has similar properties as the serous cell of the upper airway(CitationWitschi & Mrsny, 1999) and usually used as the membrane model for drug transmission test across nasal mucosa(CitationAmidi et al., 2006; CitationSeki et al., 2007). Thus,Calu-3 cell linewas used in this study to evaluate the in vitro cytotoxicity of OL and OL-NP. The results showed that OL peptideand OL-NP did not present any toxic effects on Calu-3 cells since cell viability was around 100% in both OL and OL-NP group, even at the highest concentration (0.33mg/ml of OL or3 mg/ml of OL-NP) ().
Figure 4. In vitro cytotoxicity of NP, OL-NP at concentrations ranging from 1.37 to 3,000 μg/ml and OL from0.152 to 333μg/ml on calu-3 cells. Data represented the mean±S.D. n = 3.
In vivo toxicity data should be more convincing,which was also investigated in this study using toads and rats. Since cilia contributeto the body’s primary nonspecific defense mechanism by propelling potentially hazardous substances, the components of preparations intended for nasal delivery should not adversely affect the clearance system, especially in the chronic therapy. Thus, cilia toxicitiesof NP and OL-NP wereevaluated using toad palate model. As shown in the microphotographs of toad palates, most of cilia werein good order on the edge of the mucosa treated with NP or OL-NP for 1 h (, and ). In contrast, mostof the cilia fell off from the edge of palate mucosa treated with 1% sodium deoxycholate solution (, ). In the OL-NP and NP groups, the average lasting times ofcilia movement were 11.12 and 10.82 h, respectively, which were comparable with thatof the negative control (11.75 h) (shown in ). The resultssuggested thatOL-NP hadnegligible toxicity on mucosa cilia.
Table 3. Influence of NP and OL-NP on the duration time of ciliary movement on palate mucosa of toads. Data are represented as the mean±S.D. (n = 3).
Figure 5. Optical microscopic images of mucosa cilia of toad palate (A-D) and expression of NSE in rat septal mucosa (E-H).The mucosa cilia of toad palate were treated withnegative control (saline, cilia on the mucosa was intact, dense and beat actively after 1 h treatment) (A), NPs (similar phenomenon with that of saline visualized after 1 h treatment) (B), OL-NPs (similar phenomenon with that of saline visualized after 1 htreatment) (C), and positive control (1% deoxysodium cholatesolution, no cilia on the mucosa observed) (D). Cilia were indicated by arrow. (400 magnification) Neuron-specific enolase (NSE) was stained by immunological technique. Expressions of NSE in rat septal mucosa following i.n. administration of 10μlof 25 mg/ml NPs (G) and OL-NPs (H) to the nostrils of rats for one week, which were similar with that following i.n. administration of 10μl of NaCl (F). Negative control stained without primary antibody was shown in (E). The positive signals were shown with arrows (600 magnification).
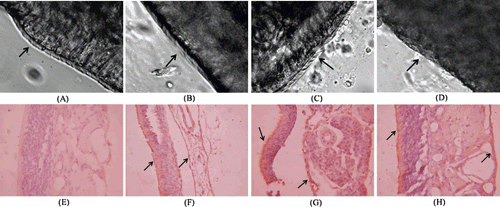
Positive immunoreactivity for neuron-specific enolase (NSE) is a reliable marker of olfactory receptor cells (CitationYamagishi et al., 1989). Pellier and Astic(CitationPellier & Astic, 1994)previously demonstrated a rapid disappearance of NSE immunoreactivity after degeneration of olfactory receptor cells. Thus, NSE could indicate the amount of olfactory nerves in nasal mucosa. As shown in , there was no obvious reduction of NSE immunoreactivity in rat nasal mucosa treated with OL-NP compared with other groups, suggesting that OL-NPdid not cause degeneration of olfactory nerves.
In summary, OL-conjugated nanoparticles (OL-NP) weredeveloped as anovel nose-to-brain drug delivery system. DiR was incorporated into OL-NP by double emulsion method and used as an inflorescence marker to investigate the tissue distribution of OL-NP in vivo.Nanoparticles following intranasal administration had much higher brain targeting efficiencythan intravenous injection. Moreover, OL modification could further enhance the nanoparticle delivery from nose to brain.The in vitro and in vivo toxicity study indicatedthat OL-NP had negligible toxicity on nasal mucosa. Thus, the OL-modified nanoparticlesare an effective and promising brain targeting delivery system for the therapy of CNS diseases.
Acknowledgements
This work was supported by the grant from National Basic Research Program (2007CB935800) of China (973 Program) and National Science and Technology Major Project (2009ZX09310-006).
Declaration of interest
The authors report no declarations of interest.
References
- Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W. (2006). Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release, 111, 107–116.
- Collister KA, Albensi BC. (2005). Potential therapeutic targets in the NF-kappaB pathway for Alzheimer’s disease. Drug News Perspect, 18, 623–629.
- Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, Jiang X. (2007). UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int J Pharm, 340, 207–215.
- Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, Fu S. (2006). Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials, 27, 3482–3490.
- Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, Rong Z, Chen H, Jiang X. (2007). Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release, 121, 156–167.
- Illum L. (2000). Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci, 11, 1–18.
- Kabanov AV, Gendelman HE. (2007). Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci, 32, 1054–1082.
- Kissel T, Werner U. (1998). Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human nasal epithelium. J Control Release, 53, 195–203.
- Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. (2002). Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target, 10, 317–325.
- Li J, Wu H, Hong J, Xu X, Yang H, Wu B, Wang Y, Zhu J, Lai R, Jiang X, Lin D, Prescott MC, Rees HH. (2008). Odorranalectin is a small peptide lectin with potential for drug delivery and targeting. PLoS One, 3, e2381.
- Liu WK, Sze SC, Ho JC, Liu BP, Yu MC. (2004). Wheat germ lectin induces G2/M arrest in mouse L929 fibroblasts. J Cell Biochem, 91, 1159–1173.
- Luessen HL, de Leeuw BJ, Langemeyer MW, de Boer AB, Verhoef JC, Junginger HE. (1996). Mucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm Res, 13, 1668–1672.
- Lundh B, Brockstedt U, Kristensson K. (1989). Lectin-binding pattern of neuroepithelial and respiratory epithelial cells in the mouse nasal cavity. Histochem J, 21, 33–43.
- Mei H, Shi W, Pang Z, Wang H, Lu W, Jiang X, Deng J, Guo T, Hu Y. (2010). EGFP-EGF1 protein-conjugated PEG-PLA nanoparticles for tissue factor targeted drug delivery. Biomaterials, 31, 5619–5626.
- Ntziachristos V, Bremer C, Weissleder R. (2003). Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol, 13, 195–208.
- Pardridge WM. (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2, 3–14.
- Pellier V, Astic L. (1994). Histochemical and immunocytochemical study of the migration of neurons from the rat olfactory placode. Cell Tissue Res, 275, 587–598.
- Seki T, Kanbayashi H, Chono S, Tabata Y, Morimoto K. (2007). Effects of a sperminated gelatin on the nasal absorption of insulin. Int J Pharm, 338, 213–218.
- Tobio M, Gref R, Sanchez A, Langer R, Alonso MJ. (1998). Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm Res, 15, 270–275.
- Tyler CM, Federoff HJ. (2006). CNS gene therapy and a nexus of complexity: systems and biology at a crossroads. Cell Transplant, 15, 267–273.
- Vila A, Sanchez A, Evora C, Soriano I, McCallion O, Alonso MJ. (2005). PLA-PEG particles as nasal protein carriers: the influence of the particle size. Int J Pharm, 292, 43–52.
- Witschi C, Mrsny RJ. (1999). In vitro evaluation of microparticles and polymer gels for use as nasal platforms for protein delivery. Pharm Res, 16, 382–390.
- Yamagishi M, Nakamura H, Takahashi S, Nakano Y, Iwanaga T. (1989). Olfactory receptor cells: immunocytochemistry for nervous system-specific proteins and re-evaluation of their precursor cells. Arch Histol Cytol, 52 Suppl, 375–381.
- Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. (2010). Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release, 143, 136–142.