Abstract
Therapeutic peptide and protein drugs have high specificity and activity in their functions but present challenges in their administration route, requiring development of new delivery systems to improve their bioavailability. The aim of this work was to investigate the role of N-trimethyl chitosan- (TMC-) coated liposomes in the oral administration of calcitonin. TMC with a degree of quaternization around 78% was synthesized and its mucoadhesive properties were examined in vitro using the mucin-particle method, which confirmed that TMC showed mucoadhesion comparable to that of chitosan. TMC-coated liposomes containing calcitonin were prepared and characterized as having a particle size of 262 nm, zeta potential of 35.8 mV and high entrapment efficiency (89.1%). The in vivo evaluation of mucoadhesion was carried out using confocal laser microscopy to observe the residence time and permeation extent after intragastric administration. The results showed that TMC-coated liposomes prolonged the residence time and increased the penetration effect of the liposomal system compared to non-coated liposomes. The study of pharmacological effects confirmed that TMC-coated liposomes increased the area above the blood calcium concentration-time curves (AAC) from 3.13 ± 20.50 to 448.84 ± 103.56 compared to the calcitonin solution. These results support the feasibility of TMC-coated liposomes as a new oral delivery system for peptide and protein drugs.
INTRODUCTION
Protein and peptide drugs have high specificity and activity at relative low concentrations in comparison with some chemical drugs, which make them attractive options for combatting human disease. In general, this kind of drug is most commonly administered via the parenteral route for the sake of rapid onset and high drug bioavailability. However, the oral administration route has clear advantages such as painless administration and improved patient compliance. Moreover, it is generally believed that patients would start treatment earlier with an oral therapy in comparison to a therapy requiring injections. However, hydrophilic macromolecular drugs such as peptides and proteins as well as genes are absorbed poorly due to rapid hydrolytic and enzymatic degradation in the presence of gastointestinal fluids and thereby, poor absorption through the gastrointestinal epithelium(CitationWoodley, 1994).
Although the delivery of peptide and protein drugs remains one of the major challenges in pharmaceutics, some effective approaches have been developed to solve this problem, such as the use of orally administered liposomes, and in particular the use of polymer-coated liposomes with mucoadhesive properties (CitationTakeuchi et al., 1996, CitationTakeuchi et al., 2003, CitationTakeuchi et al., 2005a, CitationTakeuchi et al., 2005b). Liposomal carriers are confirmed to be a promising drug delivery platform for oral delivery of peptide and protein drugs because they are able to protect the drugs from the adverse gastrointestinal environment and ensure controlled drug release (CitationIwanaga et al., 1997, CitationCheng et al., 2011). Liposome-based mucoadhesive formulations play an important role in oral peptide and protein drug delivery systems by prolonging the residence time of liposomal carriers and increasing the contact between carriers and mucus layers to enhance the penetration and decrease the degradation of drugs (CitationWerle and Takeuchi, 2009, CitationWerle et al., 2010, Makhlof et al., CitationJonker-venter et al., 2006, CitationTakeuchi et al., 1996).
N-trimethyl chitosan chloride (TMC), a chitosan derivative synthesized by the reductive methylation of chitosan with methyl iodide in a strongly basic environment at an elevated temperature, has fixed positive charges but superior solubility and basicity, even at low degrees of quaternization, compared to chitosan salts (CitationThanou et al., 2000, CitationKotze et al., 1997, CitationKotze et al., 1998, Citationvan der Merwe et al., 2004). It has been reported that TMC is an effective penetration enhancer of hydrophilic and/or high-molecular-weight molecules across the intestinal epithelium even in neutral environments, using the same mechanism(s) as chitosan, which might be the opening of tight junctions and the widening of the paracellular pathway (CitationJonker et al., 2002, CitationKotze et al., 1998, CitationKotze et al., 1997, CitationSinswat and tengamnuay, 2003, CitationThanou et al., 2000).
It was the aim of the current study to develop a novel liposomal system for intestinal transmucosal administration of high-molecular-weight drugs such as peptides or proteins. In this study, salmon calcitonin-loaded liposomes coated with TMC were prepared and characterized in terms of particle size and zeta potential. The mucoadhesive properties of TMC were investigated in vitro and compared with those of chitosan. The mucoadhesive properties of TMC-coated liposomes in a rat intestinal segment after intragastric administration were also evaluated by means of confocal laser scanning microscopy (CLSM), and compared with that of non-coated liposomes. Moreover, in vivo experiments were conducted to investigate the pharmacological effect of salmon calcitonin-loaded TMC-coated liposomes.
Material and methods
Materials
Medium-molecular-weight chitosan (CS, 200kDa), 1-methyl-2-pyrrolidinone, cholesterol (Chol), dicetylphosphate (DCP), mucin powder (Mw. 100 × 104~1000 × 104) and coumarin-6 were purchased from Sigma. Sodium iodide and sodium chloride were obtained from Osaka Kishida Chemical Company (Japan). Methyl iodide was purchased from Nacalai Tosque (Kyoto, Japan). Distearoyl phosphatidylcholine (DSPC) was obtained from Nippon Oil and Fats. Calcitonin was kindly provided by Asahi Chemical Co., Japan. All reagents were of analytical grade.
Synthesis and characterization of TMC
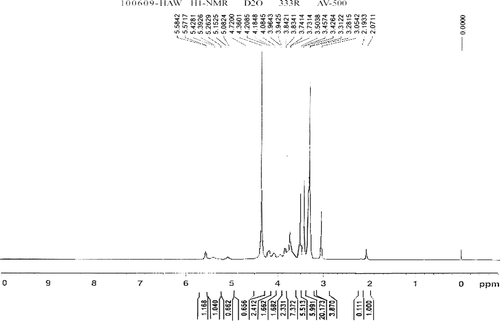
TMC with about 78% quaternization was synthesized using the two-step method previously reported (CitationSIEVAL et al., 1998). Briefly, in the first step, medium-molecular-weight chitosan (CS) was dispersed in a solution of 1-methyl-2-pyrrolidinone and sodium iodide with stirring, then both sodium hydroxide and methyl iodide were added to the solution at 60 °C to start the reaction for 1h. First, a product was precipitated with ethanol and washed with ether. Then, the product obtained in the first step was reacted in the same method with minor adjustments. The product was separated from ether by centrifugation and dissolved in NaCl (10%, w/v) solution to exchange I− with Cl−. The polymer solution was dialyzed against deionized water and lyophilized to get a white floccular product with an 80% quantitative yield and a 78.10% substitution rate. The 1H-NMR spectrum was obtained in D2O using a 500MHz spectrum (AV500, Bruker, Denmark) and the degree of quaternization and the degrees of 3- or 6- O-methylation of TMC were calculated according to the following equations:
where [CH3] 3 is the integral of the chemical shift of the trimethyl amino group at 3.3 ppm; [CH3] is the integral of the chemical shift of methyl substitution for 3- or 6- hydroxyl groups at 3.5 ppm or 3.4 ppm, respectively and [H] is the integral of the 1H peaks between 4.7 and 5.7 ppm (CitationPolnok et al., 2004).
Preparation of non-coated liposomes and polymer-coated liposomes
We prepared liposomes as described previously (CitationTAKEUCHI et al., 2005a), but with minor adjustments. Anionic small unilamellar vesicles (SUV) were prepared by the thin film hydration method. DSPC, DCP and Chol (molar ratio: 8:2:1) were dissolved in an adequate amount of chloroform; then a thin film lipid layer was obtained by evaporating the organic solvent for 3h at 40 °C under vacuum. The obtained lipid film layer was dried overnight in a vacuum oven to remove the chloroform completely. The lipid film layer was hydrated with calcitonin phosphate buffer solution by repeated gentle heating and vortexing, and the final concentration of calcitonin was 40 μg/ml. The obtained suspension of multilamellar vesicles was probe sonicated in ice water for 10 min to obtain small unilamellar vesicles (SUV).
For the polymer coating of the liposomes, appropriate amounts of TMC and chitosan were dissolved in phosphate buffer (66.67 mM, pH 6.8) and acetate buffer (100 mM, pH 4.4), respectively. The coating process was performed by mixing an aliquot of the described SUV suspension with the same volume of polymer solutions, followed by vortexing and incubation at 10 °C for 30 min, so that the final concentrations of lipids and polymers were half those of the original suspensions. And the non-coated liposomes (non-Lips) were mixed with the same volume of corresponding buffer to adjust the concentration to that of the coated liposomes.
Coumarin-6 (C-6) labeled liposomes for the in vivo mucoadhesion property studies were prepared with adjustment in the preparation of lipid film. The lipid film was prepared with DSPC, DCP, Chol and C-6 (molar ratio of DSPC:DCP: Chol was still 8:2:1 and the final concentration of C-6 was 25 μg/ml), and the other operations were the same as described above.
Characterization of non-coated liposomes and polymer-coated liposomes
The size and zeta potential of liposomes were determined using dynamic light scattering (Zetasizer 3000 HSa, Malvern, Worcestershire, UK), with a suspension prepared by adding 200 μl of the liposomal suspension to 4 ml of purified water.
The calcitonin-loaded liposomes were ultracentrifuged at 231 000×g and 4 °C for 45 min and the calcitonin concentration of the supernatant was analyzed by a commercially available micro BCA™ protein assay kit (Pierce, USA). The entrapment efficiency (E.E%) was calculated by the following equation:
where Aini is the initial amount of calcitonin and Asup is the amount of calcitonin in the supernatant portion.
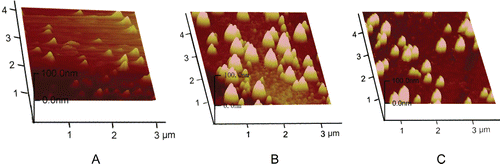
The surface properties of non-Lips, chitosan-coated liposomes (CS-Lips) and TMC-coated liposomes (TMC-Lips) were observed by an atomic force microscopy (AFM) (Veeco diNanoScope V, USA). Explorer atomic force microscope was a tapping mode, using high resonant frequency, pyramidal cantilevers with silicon probes having force constants of 20 N/m. Scan speed was set at 2 Hz. The samples were diluted to a proper concentration and dropped onto the surface of a freshly cleaved mica plates, and then dried exposing to air at room temperature.
Mucoadhesive properties of polymers using the ss-mucin particle method
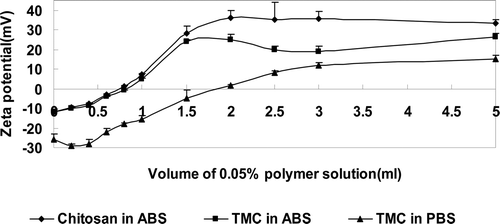
The interaction between polymer and mucin particles was determined by the ss-mucin (submicron sized mucin) particle method developed by Takeuchi et al. (CitationTAKEUCHI et al., 2005b, CitationTHONGBORISUTE and TAKEUCHI, 2008). The ss-mucin suspension was obtained by applying probe sonication to a coarse mucin suspension. Briefly, 1 ml of ss-mucin suspension at a concentration of 0.5% (w/v) was mixed with different volumes of 0.05% polymer solutions, then incubated at 37 °C for 1h. The zeta potential value was measured by a zetasizer (Malvern). All experiments were performed in triplicate.
Mucoadhesive properties of TMC-coated liposomes in rat intestines using the CLSM method
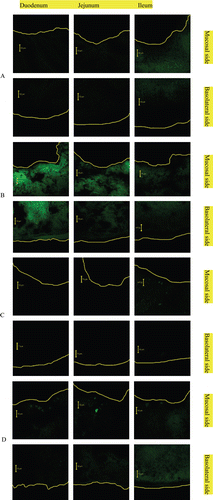
The coumarin-6 labeled non-coated and TMC-coated liposomes were prepared as described above. One ml of each sample was intragastrically administered to 10-week-old rats that had been fasted for 48 h before the experiment (final concentration of TMC was 0.5% and final concentration of C-6 was 25 μg/ml). One ml of water was intragastrically administered to control rats. Rats were sacrificed at each time point, and the intestine was excised and divided into the duodenum, jejunum and ileum. Samples were fixed with O.C.T compound (Tissue-Tek) and frozen, then sliced into sections of 10-μm thickness using a crytostat (LEICA). Samples were detected by confocal laser scanning microscope (LSM510, CarlZeiss Jena, Germany) at an excitation wavelength of 488nm. The excitation beam was delivered to the specimen via a Zeiss Apochromat 63× oil immersion objective (numerical aperture 1.4). Emitted fluorescence was captured using LSM510 software (release 3.2, CarlZeiss).
Pharmacological effect of calcitonin-loaded polymer-coated liposomes
All animal experiments for the current study were approved by the animal welfare commission of Gifu Pharmaceutical University. Non-coated or polymer-coated calcitonin-loaded liposomes (final polymer concentration was 0.5%) as well as calcitonin solution as a control were intragastrically administrated to 10-week-old male Wistar rats which were fasted 24 h before the experiment and had free access to water. The dose of calcitonin was 500 IU/kg rat, which equal to 100 μg/kg rat. Rats were divided into 4 cohorts of n = 6, respectively, which received either 1 ml of calcitonin solution, 1 ml of non-coated liposomes (non-Lips), or 1 ml of CS-coated or TMC-coated liposomes (CS-Lips or TMC-Lips). Blood samples (200 μl) were taken from the ocular venous plexus at each time point (0.5, 1, 2, 4, 8, 12 and 24 h). Blood calcium levels were determined by a commercially available calcium kit (Calcium C-Test, Wako, Wako Pure Chemicals, Japan), and the area above the plasma calcium concentration versus time curve (AAC) was calculated using trapezoidal rule by Microcal Origin, version 6.0.
Statistical evaluation
All the results were expressed as mean values±SD. The Student’s t test was applied to study the significance of difference between two groups, while the one-way analysis of variance (ANOVA) followed by Tukey–Kramer test was used in the case of multiple comparison.
Results
Synthesis of TMC
The 1H-NMR spectra of TMC is presented in . In , the peak at 3.3 ppm is attributed to the trimethyl amino group, with the signals at 3.4 and 3.5 ppm assigned to the 6- and 3- hydroxylmethyl groups, respectively. The degree of quaternization was 78.10% and the degrees of 3-O-methylation and 6-O-methylation were 64.03% and 69.58%, respectively.
Characterization of liposomes
shows the particle size, zeta potential and E.E% of all the liposomal systems including non-Lips CS-Lips and TMC-Lips. The anionic SUV serving as the core liposomes were prepared as described previously (CitationTakeuchi et al., 2005a). After coating with chitosan or TMC, the initial negative zeta potential of the core liposomes of around -60 mV switched to +46.4 or +35.8 mV, respectively, which both indicated a successful coating after mixing with positively charged polymer solution. The non-coated liposome had a high E.E% and the coating process didn’t decrease the E.E% of calcitonin.
Table 1. Physicochemical properties of calcitonin-loaded liposomes.
The morphologies of liposomes were observed by AFM. shows the 3D surface plots of non-Lips, CS-Lips and TMC-Lips, respectively. The observed size of three kinds of liposomes was similar to the results obtained from the DLS determination. From a relatively uniform size distribution of non-Lips was observed. showed the particle size of CS-Lip was increased significantly and the distribution of size also increased, which in terms of exist of particles with different size. showed that the particle size of TMC-Lips was bigger than non-Lips but smaller than CS-Lips and the size distribution was also better than CS-Lips.
In vitro mucoadhesion studies
The zeta potentials of mucin particles were changed when the polymer solutions were added. The relation between the zeta potential and the amount of polymer solution is shown in the . In the acetate buffer, the zeta potential of ss-mucin particles changed in each case when the ss-mucin particle suspensions were mixed with solutions of chitosan and TMC. In the phosphate buffer, the zeta potential of ss-mucin particles also changed when mixed with the solution of TMC. These results suggest that both chitosan and TMC have high affinities to mucin particles to cover their surfaces in the acidic condition, while TMC retains its affinity to mucin particles even in a neutral environment.
In vivo mucoadhesion studies
The CLSM images of the muco-penetrative behaviors of non-Lips and TMC-Lips through the intestine are shown in . From the images we can see that the muco-penetrative behaviors depend on the time course and the type of samples. In both non-Lips and TMC-Lips, the fluorescence of coumarin-6 at 4 h was significantly decreased. At both time points, the coumarin-6 intensity of TMC-Lips in each intestinal segment was conspicuously stronger than that of non-Lips. This result demonstrated that fluorescence-labeled TMC-coated liposomes had prolonged retention time and better penetrative behaviors than fluorescence-labeled uncoated liposomes.
Pharmacological effect studies
The time-dependent blood calcium levels in the 24 h after intragastric administration of calcitonin solution or the calcitonin-loaded liposomal formulation are shown in . The blood calcium profile of the calcitonin solution was smooth, which meant that calcitonin solution itself had hardly any decreasing effect on blood calcium level. The profile of non-Lips showed a slight decrease of the blood calcium level, while both CS-Lips and TMC-Lips showed significant decreases of blood calcium levels. The pharmacokinetic parameters after oral administration of calcitonin solution, non-Lips, CS-Lips and TMC-Lips are summarized in . The most pronounced decrease of blood calcium level of calcitonin-loaded liposomal systems occurred after 0.5 h. Especially, the lowest blood calcium level of TMC-Lips was at 4 h. In both CS-Lips and TMC-Lips, the blood calcium level was still depressed at 24 h. The areas above the blood calcium concentration-time curve (AAC) of those formulations are shown in . The TMC-Lips dramatically increased the AAC after intragastric administration compared to both calcitonin solution and non-Lips, as did the CS-Lips (p<0.01). There was no significant difference in AAC after oral administration between TMC-Lips and CS-Lips, but the blood calcium levels of TMC-Lips were significantly higher than those of CS-Lips at both the 4-h and 8-h time points.
Figure 5. Blood calcium profile after intragastric administration of calcitonin solution, calcitonin-loaded non-Lips, calcitonin-loaded CS-Lips and calcitonin-loaded TMC-Lips (means±SEM, n=4-6).
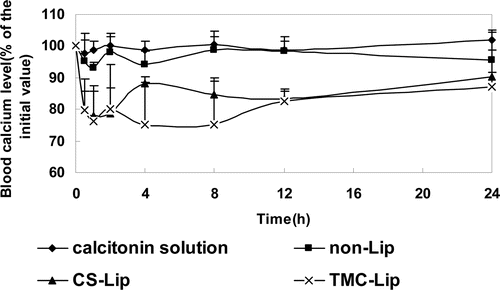
Figure 6. Area above the blood-calcium-concentration time curve (AAC) after intragastric administration of calcitonin solution, calcitonin-loaded non-Lips, calcitonin-loaded CS-lips and calcitonin-loaded TMC-Lips (means±SEM, n=4-6, **differs P<0.01 as compared with calcitonin solution).
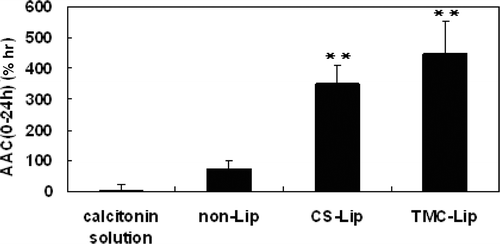
Table 2. Pharmacokinetic parameters after oral administration of various liposomal systems containing calcitonin.
Discussion
Chitosan is one of the most useful materials for offering mucoadhesive properties to particulate drug carriers such as liposomes. Drug absorption enhancement is another promising characteristic of chitosan. It has been reported that the positive charge of chitosan is partly responsible for these specific properties. However, the quaternary amino group is more favorable for showing positive charges than the tertiary amino group that chitosan possesses. In this sense, TMC is expected as an alternative to chitosan. Some research groups have reported that TMC with only 36% quaternization can increase the absorption of hydrophilic model compounds such as mannitol and PEG4000 across intestinal epithelia and nasal mucosa at physiological pH. And TMC with higher degrees of quaternization also increased its permeation-enhancing effect (CitationAmidi et al., 2010, CitationJonker et al., 2002).
The particle size and zeta potential of calcitonin-loaded liposomes increased after coating with chitosan solution (pH 4.4) and TMC solution (pH 6.8), indicating a successful coating process in both cases. However, the zeta potential and particle size of chitosan-coated liposomes were slightly higher than those of TMC-coated liposomes. The chitosan-coating process occurred under acidic conditions, in which the amino groups of chitosan were mostly protonated to result in a higher positive charge on molecules. By contrast, the TMC-coating process occurred under neutral conditions. The remaining amino groups of TMC could not be protonated, which resulted in a neutral charge. And the quaternary ammonium groups were responsible for the positivity under a neutral medium. So the zeta potential of TMC was slightly lower in comparison to that of the chitosan itself.
To study the in vitro mucoadhesion, the ss-mucin-particle method was used to detect the mucoadhesive properties of polymers by measuring the change in zeta potential of ss-mucin particles. As shown in , the mucoadhesion process can be broken down into three stages: the contact stage, consolidation stage and saturation stage. The contact stage of all the samples was from a volume of 0 to 0.4 ml. In this stage, intimate surface contact between the bioadhesive polymers and mucus tissue was established, so that the zeta potential of mucin didn’t increase apparently with the amount of polymer solution added. In ABS, the consolidation stage of chitosan was from 0.4 to 2 ml while that of TMC was from 0.4 to 1.5 ml; and in PBS, the consolidation stage of TMC was from 0.4 to 3 ml while the chitosan experiment in PBS failed because of chitosan’s poor solubility in a neutral environment. In the consolidation stage, there was a significant increase of the zeta potential of mucin with increasing amounts of polymer solution. Both the mucoadhesive polymer and mucus tissue interdiffused or interpenetrated to a certain extent and formed secondary chemical bonds, such as electrostatic interaction, hydrophobic interaction, hydrogen bonding and Van Der Waals forces to consolidate and strengthen the adhesive joint, leading to prolonged adhesion (CitationSmart, 2005). The last stage was the saturation stage, in which the amounts of polymer solution added did not affect the zeta potential of mucin because the mucin surface was saturated with the polymer. The results indicated that TMC showed high affinity to mucin in both acidic and neutral conditions whereas the interaction between chitosan and mucin only occurred in an acidic medium.
Takeuchi et al. have already demonstrated that chitosan-coated liposomes could penetrate into the mucosa and achieve prolonged retention time (CitationTakeuchi et al., 2005a). In this study, the images of confocal laser scanning microscopy also confirmed that TMC coating of liposomes led to prolonged residence time and increased the permeation effect of the liposomal system compared with non-coated liposomes. In Werle and Takeuchi’s study, confocal laser microscopy studies showed comparable mucoadhesive properties of chitosan–aprotinin-coated MLV and chitosan-coated MLV with the final dose of coumarin-6 of 50 μg (CitationWerle and takeuchi, 2009). To tell the differences between non-Lips and TMC-Lips clearly, the final dose of coumarin-6 in this study was a half that in Werle and Takeuchi’s study. It was reported that TMC exhibits mucoadhesive properties on the mucus gel layer covering the small intestine not only for the electrostatic interaction between positively charged TMC and negatively charged mucosal surfaces but also for other non-covalent bonds such as hydrogen bonds and hydrophobic bonds (CitationJintapattanakit et al., 2009).
In the previous study, we demonstrated that chitosan-coated liposomes were more effective than calcitonin solution and non-coated liposomes (CitationTakeuchi et al., 2005a, CitationThongborisute et al., 2006). In this study we investigated the pharmacological effect of TMC-coated liposomes via the oral route. The AAC of calcitonin solution itself was almost zero, due to attack by the gastric acidic environment and enzymes from the alimentary canal. For the protection of calcitonin by incorporation into liposomes and prolonged residence time, the AACs of polymer-coated liposomal systems were significantly increased. It was observed that the blood calcium levels of the chitosan- and TMC-coated liposomes before the 4-h time point after oral administration were not significantly different, whereas the blood calcium levels of TMC-coated liposomes were significantly lower than that of chitosan-coated liposomes after the 4-h time point. This effect might due to the better solubility of TMC in neutral environments. In the upper intestinal segment of rats, intestinal fluids provide a weakly acidic environment, in which both chitosan and TMC dissolved well to achieve mucoadhesion and penetration enhancement. However, the chitosan did not dissolve well in the lower intestinal segment because of the increasing pH of the intestinal fluid, so the TMC-coated liposome showed a better effect on the decrease of blood calcium level after the 4-h time point. Chitosan, a potentially useful polymer for hydrophilic macromolecules—not only for peptides and proteins, but also for siRNA and pDNA (CitationKatas and Alpar, 2006, CitationMao et al., 2010)—is limited by its pH-dependent solubility. Commercially available chitosan cannot be dissolved above a pH of 5.6, which becomes a limitation for its use in oral drug delivery. TMC, with a higher degree of quaternization, has better solubility and higher mucoadhesive properties. Thus, TMC-coated liposome might be a promising alternative to chitosan as an effective delivery system for oral administration of protein and peptide drugs.
Conclusion
Based on the obtained results, TMC with a high degree of quaternization exhibited good mucoadhesive properties both in vitro and in vivo. TMC-coated liposomes were successfully prepared, and the properties of the novel system were investigated. It was demonstrated that calcitonin-loaded TMC-coated liposomes had a high pharmacological effect of calcitonin after oral administration. Thus, we can draw the conclusion that TMC-coated liposomes have the potential to be developed as a new oral delivery system for peptide and protein drugs.
Acknowledgement
The first author wishes to express her appreciation to her professors and colleagues at both Gifu Pharmaceutical University and China Pharmaceutical University. Part of this work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Sciences and Technology (Monbukagakusho) of Japan (21390011).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- AMIDI, M., MASTROBATTISTA, E., JISKOOT, W. & HENNINK, W. E. (2010) Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev, 62, 59–82.
- CHENG, H. C., CHANG, C. Y., HSIEH, F. I., YEH, J. J., CHIEN, M. Y., PAN, R. N., DENG, M. C. & LIU, D. Z. (2011) Effects of tremella-alginate-liposome encapsulation on oral delivery of inactivated H5N3 vaccine. J Microencapsul, 28, 55–61.
- IWANAGA, K., ONO, H., NARIOKA, K., MORIMOTO, K., KAKEMI, M., YAMASHITA, S., NANGO, M. & OKU, N. (1997) Oral delivery of insulin by using surface coating liposomes: Improvement of stability of insulin in GI tract International Journal of Pharmaceutics, 157, 8.
- JINTAPATTANAKIT, A., JUNYAPRASERT, V. B. & KISSEL, T. (2009) The role of mucoadhesion of trimethyl chitosan and PEGylated trimethyl chitosan nanocomplexes in insulin uptake. J Pharm Sci, 98, 4818–30.
- JONKER-VENTER, C., SNYMAN, D., JANSE VAN RENSBURG C., JORDAAN, E., SCHULTZ, C., STEENEKAMP, J. H., HAMMAN J. H. & KOTZE, A. F. (2006) Low molecular weight quaternised chitosan (11): in vitro assessment of absorption enhancing properties. Pharmazie, 61, 301–5.
- JONKER, C., HAMMAN, J. H. & KOTZE, A. F. (2002) Intestinal paracellular permeation enhancement with quaternised chitosan: in situ and in vitro evaluation. Int J Pharm, 238, 205–13.
- KATAS, H. & ALPAR, H. O. (2006) Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release, 115, 216–25.
- KOTZE, A. F., LUESSEN, H. L., DE LEEUW, B. J., DE BOER A. G., VERHOEF J. C. & JUNGINGER, H. E. (1998) Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J Control Release, 51, 35–46.
- KOTZE, A. F., LUESSEN, H. L., DE LEEUW B. J., DE BOER B. G., VERHOEF, J. C. & JUNGINGER, H. E. (1997) N-trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: in vitro evaluation in intestinal epithelial cells (Caco-2). Pharm Res, 14, 1197–202.
- MAKHLOF, A., FUJIMOTO, S., TOZUKA, Y. & TAKEUCHI, H. (XXXX) In vitro and in vivo evaluation of WGA-carbopol modified liposomes as carriers for oral peptide delivery. Eur J Pharm Biopharm.
- MAO, S., SUN, W. & KISSEL, T. (2010) Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev, 62, 12–27.
- POLNOK, A., BORCHARD, G., VERHOEF, J. C., SARISUTA, N. & JUNGINGER, H. E. (2004) Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur J Pharm Biopharm, 57, 77–83.
- SIEVAL, A. B., THANOU, M., KOTZ, A. F., VERHOEF, J. C., BRUSSEE, J. & JUNGINGER, H. E. (1998) Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Pollym, 36, 157–165.
- SINSWAT, P. & TENGAMNUAY, P. (2003) Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats: comparison with hydroxypropyl- and dimethyl-beta-cyclodextrins. Int J Pharm, 257, 15–22.
- SMART, J. D. (2005) The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev, 57, 1556–68.
- TAKEUCHI, H., MATSUI, Y., SUGIHARA, H., YAMAMOTO, H. & KAWASHIMA, Y. (2005a) Effectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugs. Int J Pharm, 303, 160–70.
- TAKEUCHI, H., MATSUI, Y., YAMAMOTO, H. & KAWASHIMA, Y. (2003) Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J Control Release, 86, 235–42.
- TAKEUCHI, H., THONGBORISUTE, J., MATSUI, Y., SUGIHARA, H., YAMAMOTO, H. & KAWASHIMA, Y. (2005b) Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv Drug Deliv Rev, 57, 1583–94.
- TAKEUCHI, H., YAMAMOTO, H., NIWA, T., HINO, T. & KAWASHIMA, Y. (1996) Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm Res, 13, 896–901.
- THANOU, M. M., KOTZE, A. F., SCHARRINGHAUSEN, T., LUESSEN, H. L., DE BOER A. G., VERHOEF, J. C. & JUNGINGER, H. E. (2000) Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal caco-2 cell monolayers. J Control Release, 64, 15–25.
- THONGBORISUTE, J. & TAKEUCHI, H. (2008) Evaluation of mucoadhesiveness of polymers by BIACORE method and mucin-particle method. Int J Pharm, 354, 204–9.
- THONGBORISUTE, J., TSURUTA, A., KAWABATA, Y. & TAKEUCHI, H. (2006) The effect of particle structure of chitosan-coated liposomes and type of chitosan on oral delivery of calcitonin. J Drug Target, 14, 147–54.
- VAN DER MERWE S. M., VERHOEF, J. C., VERHEIJDEN, J. H., KOTZE, A. F. & JUNGINGER, H. E. (2004) Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur J Pharm Biopharm, 58, 225–35.
- WERLE, M., MAKHLOF, A. & TAKEUCHI, H. (2010) Carbopol-lectin conjugate coated liposomes for oral peptide delivery. Chem Pharm Bull (Tokyo), 58, 432–4.
- WERLE, M. & TAKEUCHI, H. (2009) Chitosan-aprotinin coated liposomes for oral peptide delivery: Development, characterisation and in vivo evaluation. Int J Pharm, 370, 26–32.
- WOODLEY, J. F. (1994) Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst, 11, 61–95.