Abstract
The purpose of this study is to increase the lag time and prevent release of budesonide, a corticosteroid drug used in Crohn’s disease for the first 5 h and efficiently deliver it to the colon. Eudragit S100 spray-coated capsules and pulsatile systems using tablet plugs of cellulose acetate butyrate (CAB), HPMC K4M, guar gum, and pectin were prepared. Eudragit S100-coated capsules released 80.62% after 5 h. In pulsatile systems, decreasing the ratio of the polymer significantly increased the rate and extent of drug release. Spray-coating with EUD S100 decreased the extent of drug release to 48.41%, 69.94%, 80.58%, and 45.23% in CAB, HPMC K4M, pectin, and guar gum, respectively; however, the entire amount was released in the target area. In the presence of bacterial enzymes, selected formulas showed nearly 100% release. X-ray imaging performed to monitor the capsules throughout the GIT in human volunteers of the capsules and spray-coated pulsatile systems with 25% guar gum in the plug showed bursting in the transverse and ascending colon, respectively. Both formulations showed marked reduction in induced rabbit colitis model.
Introduction
Crohn’s disease is a chronic inflammatory bowel disease primarily affecting the terminal ileum and proximal colon. Patients having inflammatory bowel disease including Crohn’s disease and ulcerative colitis are increasing yearly (CitationRönnblom et al., 2010; CitationWilson et al., 2010; CitationZheng et al., 2010; CitationShin et al., 2011). National Cooperative Crohn’s Disease Study (NCCDS) and the European Cooperative Crohn’s Disease Study (ECCDS) trials have found that corticosteroids have shown a statistically significant improvement and effectiveness for the treatment of the disease compared to aminosalicylates (CitationBaker, 2001; CitationFeagan & Sandborn, 2002; CitationKlotz & Schwab, 2005). Enemas and suppositories are not effective for extensive proximal ulcerative colitis and Crohn’s disease; in addition, traditional oral dosage forms for colon-specific diseases are absorbed before reaching the colon target sites (CitationYu et al., 1995). To reduce their adverse effects, these agents should be targeted to the colon by modified release formulations. Site-specific targeting of drugs to the colon has been tried by several different approaches, i.e. pH control (CitationIbekwe et al., 2006a), time-control (CitationSawada et al., 2003), pressure-control (CitationJeong et al., 2001), pro-drug, and colon-specific-polymer (CitationTozaki et al., 1997; CitationKakoulides et al., 1998).
BUD, a second generation glucocorticoid, exhibits high affinity to the corticosteroid receptors with a high ratio of topical to systemic anti-inflammatory activity. It is commercially available in the market in the form of enteric-coated preparations mainly for the treatment of small intestine active Crohn’s disease. However, it was found that less than 5% of the drug was available beyond the ileum and caecum (CitationEdsbäcker et al., 2003). Previous studies have reported microsphere delivery systems of BUD (CitationRodriguez et al; 2001; CitationKrishnamachari et al., 2007). However, their large scale manufacturing requires a lot of technological advancement and skills.
In a previous study we tried to prevent BUD release in the stomach and small intestine, and to deliver drug specifically to the colon by using a compression coating technique (CitationYehia et al., 2009). As a result of this study, coating of BUD with 75% pectin protected the drug in pH 1.2, however 8.02% was released in pH 6.8 and 91.00% was released in pH 7.4 in the presence of pectionolytic enzyme. In this study we aimed at increasing the lag time and preventing drug release in either pH 1.2 or 6.8 for the first 5 h and to efficiently deliver the entire drug to the colon. Pulsatile release systems were formulated to undergo a lag-time of pre-determined span of time of no release, followed by a rapid and complete release of loaded drugs. This approach is based on the principle of delaying the time of drug release until the system transits from mouth to colon. A lag-time of 5 h is usually considered sufficient where the gastric transit time of single-unit non-disintegrating dosage forms has been reported to vary from 15 min to 3 h (CitationKaus et al., 1984) and the small intestine transit is ~ 3–4 h, which is relatively constant and hardly affected by the nature of formulation administered (CitationHinton et al., 1969). This system offers many advantages over conventional oral drug delivery systems like patient compliance, reduced dosage, reduced dosage frequency, avoidance of side-effects, avoidance of peak and valley fluctuation, and nearly constant drug level at the target site (CitationWilding et al., 1992).
Materials and methods
Materials
Budesonide Batch No.: 5309MO-00905 was supplied by Medical Union Pharmaceutical (MUP Co.; Cairo, Egypt), Anhydrous lactose was obtained from Meggle (Wasserburg, Germany), Eudragit S100 (EUD S100) and Eudragit L100 (EUD L100) were from Rohm GmbH and Co. KG, pharma polymer (Darmstadt, Germany), Hydroxypropyl methyl cellulose K4M (HPMC) was from Aqualon (Wilmington, DE, USA), Cellulose acetate butyrate (CAB) was from Eastman Chemical (Kingsport, TN), Pectin was from Sigma Chemical Co. (St. Louis, MO), Guar gum was from Aldrich (WI), Pectinex 3XL (3000 FDU/ml) was from Fluka Chemicals (St. Gallen, Switzerland), Galactomannanase enzyme from Aspergillus niger was from Fluka Chemicals (St. Gallen, Switzerland), Acetonitrile, HPLC grade was from Fisher Scientific UK limited (Loughborough, UK), and Trinitrobenzene sulfonic acid (TNBS) was from Sigma Chemical Co. Commercially available capsules were used (Entocort™ TM EC capsules, AstraZeneca, Wilmington, DE, USA)
Preparation of BUD spray-coated capsules
BUD spray-coated capsules were prepared. The body of hard gelatin capsule size 4 (Arab caps co., Cairo, Egypt) was filled with a powder mixture of 3 mg BUD and 57 mg anhydrous lactose. The capsule caps and bodies were sealed using 5% ethyl cellulose solution. Then the prepared capsules were spray-coated with different polymers viz. EUD S100, EUD L100, and ethyl cellulose. The coating solution was prepared by dissolving EUD S100 and EUD L100 in ethanol (12.5%w/v) in the presence of PEG 400 as a plasticizer. Ethyl cellulose was dissolved in ethanol (5%w/v). The prepared capsules were put in a coating pan rotating with a speed of 30 rpm (Yuyu Mediimpex P., Mumbai, India), then sprayed with the coating solution with a rate of 4 ml/min under a stream of hot air for solvent evaporation. Spraying was continued until a 20 mg increase in capsule weight occurred. The codes of the different coating solutions are shown in . The prepared capsules were subjected to quality control tests.
Table 1. Compositions of different spray-coated and pulsatile capsules.
Preparation of BUD pulsincap systems
Hard gelatin capsule bodies size 4 were filled with 3 mg BUD and 57 mg lactose. The capsule bodies were plugged with tablet plugs. These tablet plugs were prepared using time-dependent and enzyme-dependent polymers. The polymers were mixed with anhydrous lactose using a mortar and pestle. Then, powder mixtures were compressed using punches and dies set with diameter 5.15 mm to be fitted to capsules bodies. Ethyl cellulose (5%w/v) solution in ethanol was used for sealing tablet plugs to capsules bodies. Then the capsule caps were sealed to the bodies using a brush dipped in 5% ethyl cellulose solution. The prepared capsules’ bodies were coated by dipping in 5%w/v ethyl cellulose solution in ethanol (3-times, each for 30 s). Drying was done using a stream of hot air at 80°C for solvent evaporation. Then the capsules were placed in a coating pan rotating with speed 30 rpm, sprayed with Eudragit S100 solution as previously discussed. The codes of the different prepared pulsincap systems is shown in .
The prepared tablet plugs were tested for weight variation, diameter, thickness, hardness, and friability.
In vitro drug release studies
BUD release from the spray-coated capsules, pulsincap systems, as well as the commercial BUD capsules was assessed by dissolution testing using the USP Dissolution Tester, Apparatus I (Rotating basket), at a rotation speed of 50 rpm maintained at 37.0 ± 0.5°C (USP Dissolution Tester, Hanson Research Corporation, Chatsworth, CA, USA). In order to maintain sink condition, the release study was performed in 250 ml 0.1 N HCl for 2 h, followed by 250 ml phosphate buffer (pH 6.8) for another 3 h, and finally 250 ml phosphate buffer (pH 7.4) until the end of the 24 h to simulate the pHs pertaining to stomach, proximal and middle small intestine (duodenum and jejunum), and distal small intestine (ileum), respectively. One milliliter of dissolution medium was withdrawn at 2, 3, 5, 6, 8, 12, 20, and 24 h time intervals and replaced with an equal volume of media. The collected media was filtered through 0.45 µm membrane, and the concentration of dissolved drug was measured using HPLC. The analysis was carried out using a Shimadzu HPLC system with UV-Visible detector: SPD-10A, Liquid chromatogram: LC-10AD, Integrator: C-R6A, Injector (20 µL): 6E, (Shimadzu, Japan), Hypersil C18 column with dimensions (mm): 150 × 4.6, particle size: 5 µm, Thermo Electron Corporation at wavelength 247 nm. The volume of injection was 20 µl; the mobile phase consisted of acetonitrile–water (40:60). The flow-rate was 1.5 ml/min.
In order to evaluate enzyme triggered drug release of pectin and guar gum selected formulas, 1 ml pectinolytic enzyme for the former (CitationTurkoglu & Ugurlu, 2002), and 0.05 mg/ml galactomannanase for the later (CitationWong et al., 1997) were added to the phosphate buffer solution pH 7.4 to simulate the degradation of the polymers by the microflora.
In order to compare between the prepared formulas the amount released between the 5th and 24th h for each formula after starting the release was calculated. This amount was taken as a measure for the tested formula if it hit the target area in the colon or it failed.
In order to determine a drug release mechanism from the prepared tablets, the release kinetic data (up to 60% release) were analyzed by the following equation (CitationRitger & Peppas, 1987).
where, Mt, M∞, k, and n are the amount of drug released at time t, the total amount of drug in tablet, a constant, and the exponent for the release kinetics, respectively. The value of n for a tablet, n = 0.45 for Fickian (Case I) release, >0.45 but <0.89 for non-Fickian (Anomalous) release, and 0.89 for Case II (Zero order) release and >0.89 for super case II.
In vivo studies
X-ray imaging in human
An x-ray imaging technique was used to monitor the capsules throughout the GIT. Nine healthy male volunteers, with a mean age of 29 years (range 22–40) and 50–80 kg body weight, have participated in the study. They were non-alcoholics, non-smokers, and have not taken any drugs. The protocol of the study was reviewed and approved by the Ethical Committee of the Faculty of Medicine, Cairo University. The purpose of the study was fully explained to the volunteers and they had given their written consent. Each subject ingested barium sulfate containing formulas orally with 200 ml water, after an overnight fast. The capsules were visualized using plain X-ray. Abdominal radiographs were taken after 20 min, 3, 5, 8, and 24 h. The volunteers were served with food; 2 h (breakfast) and 4 h (lunch) after the administration.
In vivo study in rabbits
Induction of experimental colitis Colitis was induced in rabbits following the method described by CitationDepoortere et al. (1999). Fifteen adult male rabbits weighing 2.5–3 kg were used throughout the study. The animals were randomly divided into five groups, each consisting of three animals: Group I: normal control group. Group II: induced colitis group. Group III: animals treated with F2 spray-coated with EUD S100. Group IV: animals treated with F17 (Pulsincap using tablet plug containing 25% Guar gum and spray-coated with 20 mg EUD S100). Group V: animals treated with commercial BUD capsules. The study protocol was reviewed and approved by the ethical committee (CARAS), Faculty of Pharmacy, Cairo University. Briefly, all rabbits except the control group were fasted for 24 h with free access to water before experimentation. A rubber catheter was inserted 15 cm into the colon and inflated with 3 cm of air. Gentle withdrawal of catheter caused fecal pellets in the distal colon and rectum to be expelled by muscle action. Colitis was induced by slow intra-rectal administration of 2 ml containing 135 mg/kg 2,4,6-trinitrobenzenesulfonic acid (TNBS) in 50% ethanol. The rabbits were housed for 3 days with no treatment to maintain the development of a full inflammatory bowel disease. The animals of groups III, IV, and V received one capsule once daily for 5 continuous days by gastric intubations. Animals were sacrificed 24 h after the last drug administration.
Histopathological study Two tissue samples were excised from each colon and were maintained in 10% (v/v) formalin in saline for histopathological evaluation. Different sections of ~5 µm were stained with hematoxylin and eosin. Then the histologic damage was evaluated.
Myeloperoxidase activity The activity of MPO can be used for evaluating the degree of inflammation in the intestine. The distal colon specimen (200 mg) was minced in a beaker containing 10-times its amount of water for injection on ice, transferred to a test tube, and homogenized (Potter E1 Vejhem glass homogenizer, Warsaw, Poland) three times for 30 s each; 0.5 ml of the homogenate was mixed with 0.5 ml of HTAB buffer (0.5% HTAB in 50 mM phosphate buffer, pH 6.0) and the mixture was sonicated for 10 s and centrifuged at 10,000 rpm for 15 min. Then the supernatant was assayed for MPO activity. MPO activity was measured spectrophotometrically (UV-1601 PC spectrophotometer, Shimadzu, Kyoto, Japan): 0.1 ml of supernatant was combined with 2.9 ml containing 0.167 mg/ml O-diansidine hydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was measured every minute for 4 min. One unit of MPO activity is defined as the amount which degrades 1 mmol of the peroxide per minute at 25°C (CitationCui et al., 1994). The results were expressed as the mean ± standard deviation of the mean.
Statistical analysis
One-way ANOVA test was performed to test the significance of the difference of the obtained results using the SPSS program.
Results and discussion
Spray-coated capsules
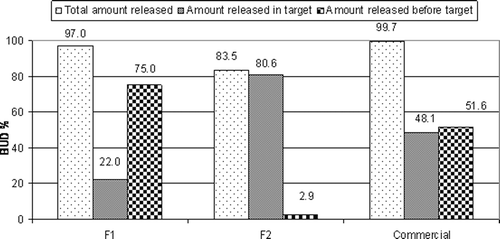
All prepared capsules met the USP 27 requirements for quality control tests. shows the in vitro release of the spray-coated capsules as well as the commercial BUD capsules. All the prepared capsules dissolved during the release study except F3 coated with ethyl cellulose, where it remained intact all over the release time. The commercial capsules released 11.09% of the drug in pH 1.2, 51.56% after 5 h in pH 6.8, and almost the rest of the drug (cumulative percentage released reached 99.70%) in phosphate buffer (pH 7.4) after 24 h. F1 spray-coated with EUD L100 showed a very low release in the first 3 h (2.91%), however a rapid release (75.04%) occurred in the 5th h. This might have occured due to the pH-dependent solubility of EUD L100 in pH 6. After 24 h, F1 released 97.00%. F2 spray-coated with EUD S100 was protected in pH 1.2 and 6.8 where it released only 2.88% in the first 5 h. In pH 7.4 there was a lag time of nearly 45 min, and this was in agreement with CitationIbekwe et al. (2006a), who observed a 30 min lag time in pH 7.4 during the release of Prednisolone from Eudragit S100-coated tablet. The cumulative percentage released after 24 h was 83.50%.
Figure 1. Release profile of BUD from spray coated capsules in 0.1N HCl for 2 hours, phosphate buffer (pH 6.8) for another 3 hours and phosphate buffer (pH 7.4) till the end of 24 hours.
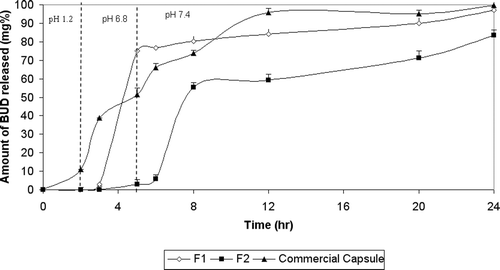
By fitting the release data up to 60% of BUD to Korsmeyer-Peppas model, the values of n for formulas F1 and F2 were less than 0.45, indicating Fickian diffusion.
shows that the extent of drug release in the target area (amount released between 5th and 24th h) from F2 (80.62%) was significantly higher (p < 0.05) than both F1 and commercial capsules.
Pulsincap systems
All prepared tablet plugs met the USP 27 requirements for weight variation and hardness. The prepared tablets friability was within the compendial limits. The thickness of tablets was in the range 0.23–0.27 mm, and their mean diameter was 5.15 mm.
Time-dependent polymers
From the release study of spray-coated capsules it was found that capsules spray-coated with ethyl cellulose remained intact all over the release time, so capsule bodies of all the prepared pulsincap systems were dip-coated in ethyl cellulose to remain intact the whole release time.
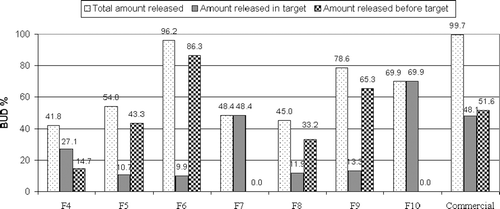
shows the release of BUD from pulsincap systems using CAB as time-dependent polymers in the tablet plugs. F4 with tablet plug formed of 50% CAB showed almost the same drug release as the commercial capsule in pH 1.2 (12.40% and 11.90%, respectively). However, a significant decrease (p < 0.05) in the amount of drug release was observed in pH 6.8 and 7.4. The amount of drug released after 5 h in pH 6.8 was 14.74% and 51.56%, and the cumulative amount of drug release after 24 h in pH 7.4 was 41.84% and 99.70% for F4 and commercial capsule, respectively. Incorporation of an effervescent mixture enhanced the drug release rate and extent where the percentages of drug released from F5 at 2, 5, and 24 h were 23.42%, 43.32%, and 53.97% compared to 12.40%, 14.74%, and 41.84%, respectively, in the case of F4. Decreasing the amount of CAB to 25% increased the drug release, where F6 showed a very rapid release all over the 24 h compared to F4. It released 56.96% in the first 2 h, 86.34% after 5 h, and 96.21% after 24 h. Spray-coating this formula with EUD S100 caused a marked retardation in drug release, where F7 released no drug in 0.1 N HCl, phosphate buffer (pH 6.8), and during the first hour in phosphate buffer (pH 7.4), it released only 48.41% of the drug after 24 h.
Figure 3. Release profile of BUD from pulsatile capsules using time dependent polymers in tablet plugs in 0.1N HCl for 2 hours, phosphate buffer (pH 6.8) for another 3 hrs and phosphate buffer (pH 7.4) till the end of 24 hours.
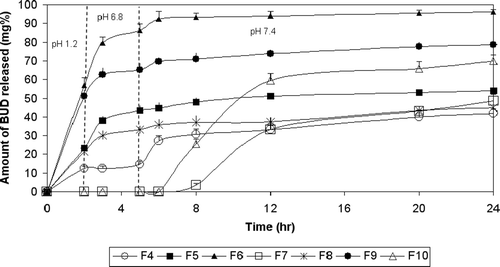
The increase in the rate and extent of drug release in the case of F5 compared to F4 might be attributed to the presence of an effervescent mixture (citric acid and sodium bicarbonate) which produced carbon dioxide gas when water penetrated into the capsule through the tablet plug. The pressure of the CO2 gas either caused the push-out of the plug or rupture of some of the hydrated plug material, thus resulting in shorter lag times and release of the drug thereafter.
Decreasing the percentage of CAB in F6 resulted in faster drug release. This might be due to the hydrophobic character of BUD which hindered its delivery through the hydrophobic tablet plug. Therefore, the diffusion of BUD through the polymer wall became the controlling-step of drug release (CitationRodriguez et al., 1998). Upon spray-coating the system entirely with EUD S100, drug release was delayed with a lag time of 6 h. This might be due to the pH-dependent solubility of the coat. However, the extent of drug release was only 49.12% due to the aforementioned hydrophobicity of CAB polymer.
By fitting of BUD release data to Korsmeyer-Peppas model, the n value for F5 was less than 0.45, indicating Fickian transport. The n values for F4 and F7 were in the range 0.45 <n ≤ 0.89, indicating anomalous (non-Fickian) transport. In the case of F6, there was not enough data for fitting to this model.
shows the release of BUD from as pulsincap system using HPMC as time-dependent polymers in the tablet plug. The percentages of drug released from F8 prepared using 50% HPMC at 2, 5and 24 h were 21.44%, 33.17%, and 45.03%, respectively. F9 prepared using 25% HPMC showed very rapid release all over the 24 h. It released 51.23%, 65.30%, and 78.59% after 2, 5, and 24 h, respectively. In the case of F10 prepared using 25% HPMC and spray-coated with EUD S100, no drug was released in 0.1 N HCl solution and phosphate buffer (pH 6.8) solution and during the first hour in phosphate buffer (pH 7.4). It released 69.94% of the drug after 24 h.
Decreasing the percent of HPMC in F9 fastened the drug release. This might be due to the lower degree of polymer entanglement formation of a weaker gel layer which allowed easier drug outward diffusion (CitationMcConville et al., 2004). In order to optimize drug release in the target area, the system was entirely coated with EUD S100, which delayed drug release (F10) due to the pH-dependent solubility of the coat. The lag time was 6 h. The extent of drug release was 69.95%, which is considered a promising percentage.
Fitting BUD data to Korsmeyer-Peppas model showed that the n-values for F8 was less than 0.45, indicating Fickian transport. The n-value for F10 was in the range 0.45 < n ≤ 0.89, indicating anomalous (non-Fickian) transport. In the case of F9, there was not enough data for fitting to this model.
The extent of drug release in the target area from pulsincap systems prepared using time-dependent polymers in tablet plugs is shown in . It is obvious that the extent of drug release from both F7 (48.42%) and F10 (69.95) was significantly higher (p < 0.05) than other time-dependent polymers.
Enzyme-dependent tablet plugs
shows the release of BUD from pulsincap system using Pectin as an enzyme-dependent polymer in the formulation of the tablet plug. F11 prepared using 100% pectin showed a very slow release rate as it released only 19.05% after 24 h. Decreasing the amount of pectin to 75% and 50% in F12 and F13, respectively, enhanced the drug release. F12 and F13 released 29.58% and 25.54% of the drug in pH 1.2, respectively. In phosphate buffer pH 6.8 and 7.4, F13 exhibited a higher extent of the drug release than F12, where the cumulative percentage of the drug released in pH 6.8 was 64.18% and 58.17%, and in pH 7.4 was 83.51% and 79.84% for F13 and F12, respectively. Spray-coating the system prepared with 50% pectin (F14) caused no drug release in the first 12 h; however, 80.58% of the drug was released after 24 h.
Figure 5. Release profile of BUD from pulsatile capsules using enzyme dependent polymers in tablet plugs in 0.1N HCl for 2 hours, phosphate buffer (pH 6.8) for another 3 hrs and phosphate buffer (pH 7.4) till the end of 24 hours.
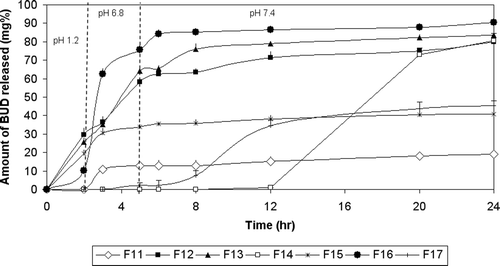
Decreasing the concentration of pectin increased the rate and extent of drug release due to hydration and swelling of pectin followed by formation of a viscous gel layer that slows down diffusion of fluids towards the capsule core. When water reached the core, drug release took place by diffusion which is supported by mechanical erosion of the swollen polymer (CitationKrishnaiah et al., 2002). The drug release of F14 was delayed due to the pH-dependent solubility of the EUD S100 coat. Lag time was 8 h, which is considered the highest one compared to the other spray-coated pulsincap systems. The extent of drug release was 80.58%.
The n values for F11 and F13 were less than 0.45, indicating Fickian transport. The n-value for F12 was in the range 0.45 <n ≤ 0.89, indicating anomalous (non-Fickian) transport. In the case of F14, there was not enough data for fitting to this model.
Coming to the release of BUD from a pulsincap system using Guar gum as enzyme-dependent polymers in the formulation of the tablet plug (). F15 prepared using 50% guar gum released 19.32%, 33.75%, and 40.84% in pH 1.2, 6.8, and 7.4, respectively. Decreasing the polymer content to 25% in F16 enhanced the extent of drug release in both pH 6.8 and 7.4 to 75.79% and 90.33%, respectively. Spray-coating this pulsincap formula prevented drug release for the first 3 h (F17), it released only 2.36% of the drug in pH 6.8. The cumulative percentage of the drug released was 45.23% at the end of the release.
Decreasing the percent of Guar gum resulted in faster drug release due to the formation of a weaker gel layer which allows easier drug outward diffusion. Coating the system with EUD S100 delayed the drug release for 3 h. The extent of drug release was only 45.23%. This indicates the formation of a swollen stiff gel of Guar gum containing plug which limits the rate and extent of drug release (CitationMastiholimath et al., 2007).
The n values for F15 and F17 were less than 0.45, indicating Fickian transport. In the case of F16, there was not enough data for fitting to this model.
The extent of drug release in the target area () could be arranged in descending order as follows: F14 (80.58%) >F17 (42.87%) >F12 (21.67%) >F13 (19.33%) >F16 (14.54%) >F15 (7.09%) >F11 (6.31%).
Figure 6. Percentage of BUD released before the target area, in the target area and total percentage released after 24 hrs from pulsatile capsules using enzyme dependent polymers in tablet plugs.
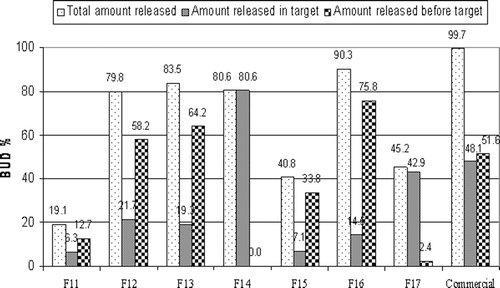
The percentage of drug released in the target area of F14 and F17 are significally higher (p < 0.05) than the amount released from other formulas with plugs containing enzyme-dependent polymers so their release was repeated in the presence of pectinolytic and galactomannase enzymes, respectively.
In the presence of pectinolytic enzyme during dissolution of F14 (figure not shown), there was no drug release through the first 6 h in target area followed by a significant increase (p < 0.05) from 80.52% to 99.37% (F14E). This might be due to increased destruction of pectin chains which might have led to faster erosion rate (CitationTurkoglu & Ugurlu, 2002). On the other hand in the presence of galactomannase enzyme during dissolution of F17, no drug release was observed during the first 8 h. However, drug release percent in target area significantly increased (p < 0.05) from 42.86% to 99.34% (F17E). This might be due to the cleavage of the β-1,4 bond in the mannose backbone of guar gum by galactomannanase enzyme (CitationGliko-Kabir et al., 2000).
Based on the amount of drug released in the target area, F2 was selected from spray-coated capsules, and F14 and F17 were selected from pulsincap systems with plug containing enzyme dependent polymers for further in vivo studies.
In vivo studies
X-ray imaging
BUD is poorly absorbed from the colon and it is generally agreed that it acts locally to produce its anti-inflammatory effect on the colon. Therefore, we solely aimed at monitoring the selected formulas throughout the GIT at different time intervals to see whether they reached the colon or not in healthy volunteers.
Abdominal radiographs showed that the formulas remained intact in the stomach in all the subjects. The transit time of the formulas throughout the GI tract was variable.
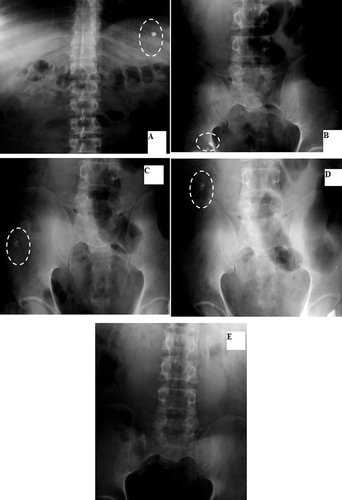
and show that F2 was intact in the stomach in the first 20 min. After 3 h, it burst in the transverse colon (). However, in a previous study of the gastrointestinal performance of tablets coated with Eudragit S100, disintegration was variable between the ileo-caecal junction and ascending colon (CitationIbekwe et al., 2006b). In another study (CitationSchellekens et al., 2010), coated capsules enabled specific and reliable drug delivery in the ileocolonic region in healthy volunteers.
Figure 7. Localization of F2 throughout the GIT: A- Stomach after 20 min. B- Transverse colon after 3 hrs.
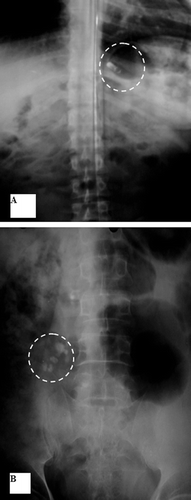
Table 2. Position of different formulas at different time intervals as detected by x-ray imaging.
These in vivo results went parallel with the in vitro release profile where F2 was protected in pH 1.2 and pH 6.8.
F14 remained intact in the stomach and ileum after 20 min and 3 h, respectively ( and and ). It passed the colon () and stayed intact in the rectum after 8 h (). Again, the X-ray imaging reflects a strong correlation with the in vitro release as F14 remained intact until 12 h in various pH.
Figure 8. Localization of F14 throughout the GIT: A: Stomach after 20 min. B: Ileum after 3 hrs. C: Transverse colon after 5 hrs. D: Rectum after 8 hrs.
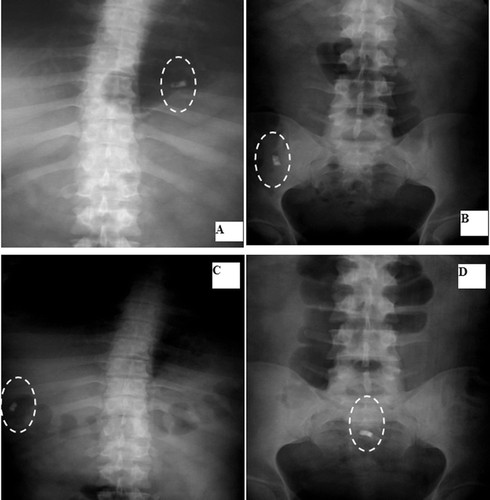
and and show that F17 remained intact in the stomach and ileum at time intervals of 20 min and 3 h, respectively. After 5 h, it opened, leaving the insoluble body coat containing a little BaSO4 in the ascending colon and transverse colon at time intervals of 5 and 8 h, respectively ( and ). This was in accordance with its in vitro release profile.
Histopathological evaluation
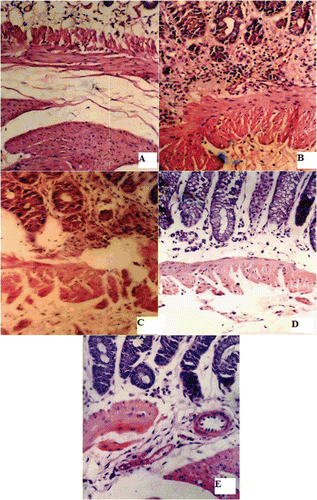
The colon of the control group, shown in , is formed of mucosa, submucosa, musculosa, and serosa. The mucosa is differentiated into glands, connective tissue core, and muscularis mucosa. In the case of the colitis group, the glandular epithelium showed edema but was still continuous. The connective tissue core showed mononuclear infiltration until the muscularis mucosa (). Furthermore, vasculitis with perivascular cellular infiltration is evident. revealed that there was marked reduction of inflammatory cells observed in the connective tissue core of groups III, IV, and V.
Myeloperoxidase activity
MPO activity, which is an important quantitative index for colonic inflammation, was determined in terms of U/g tissue weight. MPO activity for the normal control group was 3.57 ± 0.26 U/g tissue weight. However MPO activity of the induced colitis group was found to be 9.16 ± 1.52 U/g tissue weight. This MPO was significantly decreased (p < 0.05) to 3.80 ± 0.37, 4.58 ± 0.60, and 4.68 ± 0.33 U/g tissue weight in the case of commercial capsule-treated group, F2 and F17, respectively.
Conclusion
The in vitro release study as well as gastrointestinal performance in human volunteers using x-ray imaging revealed that both EUD S100 spray-coated BUD capsules and EUD S100 spray-coated pulstaile capsules using 25% guar gum in the tablet plug delayed BUD release and delivered it specifically to the colon without being opened in the stomach or ileum.
Declaration of Interest
This work was done in the Faculty of Pharmacy, Cairo University. There is no conflict of interest.
References
- Baker, D.E. (2001). Budesonide modified release capsules. Rev Gastroenterol Disord. 1:147–55.
- Cui, N., Friend, D.R., Fedorak, R.N. (1994). A budesonide prodrug accelerates treatment of colitis in rats. Gut. 3510:1439–46.
- Depoortere, I., Van Assche, G., Thijs, T., Geboes, K., Peeters, T.L. (1999). Differential changes in ACh-, motilin-, substance P-, and K(+)-induced contractility in rabbit colitis. Am J Physiol. 277:61–8.
- Edsbäcker, S., Bengtsson, B., Larsson, P., Lundin, P., Nilsson, A., Ulmius, J., Wollmer, P. (2003). A pharmacoscintigraphic evaluation of oral budesonide given as controlled-release (Entocort) capsules. Aliment Pharmacol Ther. 15:525–36.
- Feagan, B.G., Sandborn, W.J. (2002). Initial therapy for mild to moderate Crohn’s disease: Mesalmaine or budesonide? Rev Gastroenterol Disord. 2:s9–s15.
- Gliko-Kabir, I., Yagen, B., Baluom, M., Rubinstein, A. (2000). Phosphated crosslinked guar for colon-specific drug delivery. II. In vitro and in vivo evaluation in the rat. J Contr Rel. 63:129–34.
- Hinton, J.M., Lennard-Jonnes, J.E., Young, A.C. (1969). A new method for studying gut transit times using radioopaque markers. Gut. 10:842.
- Ibekwe, V.C., Fadda, H.M., Parsons, G.E., Basit, A.W. (2006a). A comparative in vitro assessment of the drug release performance of pH-responsive polymers for ileo-colonic delivery. Int J Pharm. 308:52–60.
- Ibekwe, V.C., Liu, F., Fadda, H., Khela, M.K., Evans, D.F., Parsons, G.E., Basit, A.W. (2006b). An Investigation into the in vivo performance variability of pH responsive polymers for ileo-colonic drug delivery using gamma scintigraphy in humans. J Pharm Sci. 95:2760–6.
- Jeong, Y.I., Ohno, T., Hu, Z., Yoshikawa, Y., Shibata, N., Nagata, S., Takada, K. (2001). Evaluation of an intestinal pressure-controlled colon delivery capsules prepared by a dipping method. J Contr Rel. 71:175–82.
- Kakoulides, E.P., Smart, J.D., Tsibouklis, J. (1998). Azocrosslinked poly (acrylic acid) for colonic delivery and adhesion specificity: Synthesis and characterization. J Contr Rel. 52:291–300.
- Kaus, L.C., Fell, J.T., Sharma, H., Taylor, D.C. (1984). The intestinal transit of a single non-disintegrating object. Int J Pharm. 14:143–8.
- Klotz, U., Schwab, M. (2005). Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 57:267–79.
- Krishnaiah, Y.S., Satyanarayana, V., Dinesh Kumar, B., Karthikeyan, R.S. (2002). In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur J Pharm Sci. 16:185–92.
- Krishnamachari, Y., Madan, P., Lin, S. (2007). Development of pH- and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. Int J Pharm. 338:238–47.
- Mastiholimath, V.S., Dandagi, P.M., Jain, S.S., Gadad, A.P., Kulkarni, A.R. (2007). Time and pH dependent colon specific, pulsatile delivery of theophylline for nocturnal asthma. Int J Pharm. 328:49–56.
- McConville, J.T., Ross, A.C., Chambers, A.R., Smith, G., Florence, A.J., Stevens, H.N.E. (2004). The effect of wet granulation on the erosion behaviour of an HPMC–lactose tablet, used as a rate-controlling component in a pulsatile drug delivery capsule formulation. Eur J Pharm Biopharm. 57:541–9.
- Ritger, P.L., Peppas, N.A. (1987). A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Contr Rel. 5:37–42.
- Rodriguez, M., Antunez, J.A., Taboada, C., Seijo, B., Torres, D. (2001). Colon-specific delivery of budesonide from microencapsulated cellulosic cores: evaluation of the efficacy against colonic inflammation in rats. J Pharm Pharmacol. 53:1207–1215.
- Rodriguez, M., Vila-Jato, J.L., Torres, D. (1998). Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J Contr Rel. 55:67–77.
- Rönnblom, A., Samuelsson, S.M., Ekbom, A. (2010). Ulcerative colitis in the county of Uppsala 1945–2007: Incidence and clinical characteristics. J Crohns Colitis. 4:532–6.
- Sawada, T., Sako, K., Fukui, M., Yokohamaa, S., Hayashi, M. (2003). A new index, the core erosion ratio, of compression-coated timed-release tablets predicts the bioavailability of acetaminophen. Int J Pharm. 265:55–63.
- Schellekens, R.C.A., Stellaard, F., Olsder, G.G., Woerdenbag, H.J., Frijlink, H.W., Kosterink, J.G.W. (2010). Oral ileocolonic drug delivery by the colopulse-system: A bioavailability study in healthy volunteers. J Contr Rel. 146:334–40.
- Shin, D.H., Sinn, D.H., Kim, Y.H., Kim, J.Y., Chang, D.K., Kim, E.J., Ryu, H.Y., Song, H.U., Kim, I.Y., Kim do, H., Kim, Y.Y., Kim, S.H., Seo, Y.B., Hwang, K.W., Kim, J.J. (2011). Increasing incidence of inflammatory bowel disease among young men in Korea between 2003 and 2008. Dig Dis Sci. 56:1154–9.
- Tozaki, H., Komoike, J., Tada, C., Maruyama, T., Terabe, A., Suzuki, T., Yamamoto, A., Muranishi, S. (1997). Chitosan capsules for colon-specific drug delivery: Improvement of insulin absorption from the rat colon. J Pharm Sci. 86:1016–21.
- Turkoglu, M., Ugurlu, T. (2002). In vitro evaluation of pectin-HPMC compression coated 5-aminosalicylic acid tablets for colonic delivery. Eur J Pharm Biopharm. 53:65–73.
- Wilding, I.R., Davis, S.S., Bakhshaee, M., Stevens, H.N., Sparrow, R.A., Brennan, J. (1992). Gastrointestinal transit and systemic absorption of captopril from a pulsed-release formulation. Pharm Res. 9:654–7.
- Wilson, J., Hair, C., Knight, R., Catto-Smith, A., Bell, S., Kamm, M., Desmond, P., McNeil, J., Connell, W. (2010). High incidence of inflammatory bowel disease in Australia: A prospective population-based Australian incidence study. Inflamm Bowel Dis. 16:1550–6.
- Wong, D., Larrabee, S., Clifford, K., Tremblay, J., Friend, D. (1997). USP dissolution apparatus III (reciprocating cylinder) for screening of guar-based colonic delivery formulations. J Contr Rel. 47:173–9.
- Yehia, S.A., Elshafeey, A.H., Sayed, I., Shehata, A.H. (2009). Optimization of budesonoide compression-coated tablets for colonic delivery. AAPS PharmSciTec. 10:147–57.
- Yu, D.K., Morrill, B., Eichmeier, L.S. (1995). Pharmacokinetics of 5-aminosalicylic acid in man. Eur J Clin Pharmacol. 48:273–7.
- Zheng, J.J., Zhu, X.S., Huangfu, Z., Shi, X.H., Guo, Z.R. (2010). Prevalence and incidence rates of Crohn’s disease in mainland China: A meta-analysis of 55 years of research. J Dig Dis. 11:161–6.