Abstract
Oridonin (ORI)-loaded Nanostructured lipid carriers (NLC) were prepared by emulsion–evaporation and low temperature–solidification technique, and evaluated for morphological observation, particle size, zeta potential and in vitro drug release. Next, the characteristics of biodistribution and pharmacokinetics in vivo were examined. The average particle size of resultant NLC was 245.2 nm and the zeta potential was found to be -38.77 mV. The in vivo characteristics of ORI-loaded NLC were studied after intravenous administration using Kunming strain mice as experimental animals. An ORI control solution was studied parallelly. In tested organs, the distribution of ORI-loaded NLC to liver was higher than that of free drug. ORI-loaded NLC showed higher AUC (area under tissue concentration–time curve) values and circulated in the blood stream for a longer time compared with ORI solution. These results support the potential applications of NLC for the delivery of ORI.
Introduction
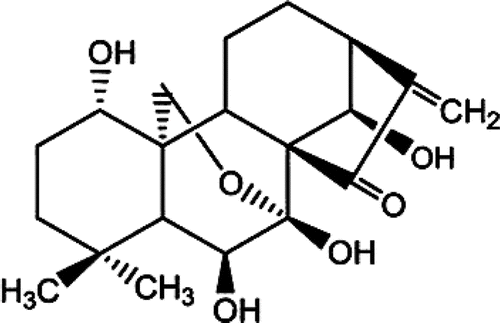
Oridonin (ORI, ), a diterpenoid compound, is isolated from the well known Chinese herb Rabdosia rubescens, which has various pharmacological and physiological effects including anti-inflammation, anti-bacterial and anti-tumor activity (CitationLi et al., 2011). The results of pharmacological research show that ORI exhibits apoptosis-inducing activities on a wide variety of cancer cells including those from liver (SMMC-7721, HepG-2), breast (MCF-7), leukemia (K562), melanoma (K1735M2) (CitationLou et al., 2011; CitationWang et al., 2011; CitationHsieh et al., 2005; CitationLou et al., 2009; CitationRen et al., 2006), etc. However, its poor water-solubility and short biological half-life limit its successful use in clinic. New pharmaceutical techniques have been applied to overcome the pharmaceutical problems, such as nanosuspensions, nanoparticles, self-microemulsion, solid dispersions (CitationGao et al., 2008; CitationXing et al., 2007; CitationZhang et al., 2008; CitationLi et al., 2011), etc.
Nanostructured lipid carriers (NLC) as new generation of lipid nanoparticles have been developed in recent years (CitationLin et al., 2007). NLC are comprised of an inner oil core surrounded by an outer solid shell and hence allow the high payload of a lipophilic drug, which are produced by controlled mixing of solid lipids with spatially incompatible liquid lipids leading to special nanostructures with improved drug incorporation and release properties (CitationMüller et al., 2002a). The resulting matrix of the lipid particles shows a melting point depression compared to the original solid lipid but the matrix is still solid at body temperature (CitationMüller et al., 2002b). The NLC could be prepared by a solvent diffusion method in an aqueous system, modified microemulsion method, microemulsion template technique, hot high-pressure homogenization method (CitationZhang et al., 2010; CitationJoshi & Patravale, 2008; CitationJoshi et al., 2008; CitationObeidat et al., 2010), etc.
It was previously reported that the NLC could improve the pharmacokinetic and biodistribution parameters compared with the free drug, and exhibit superior anti-tumor effect and good hemocompatibility (CitationLi et al., 2010). It also promoted the oral absorption of drugs with rapid metabolism in the gut and liver (CitationChen et al., 2010).
In the present work, we utilized the NLC for intravenous delivery of ORI. We presented an investigation on the feasibility of using the mixture of solid lipid- and liquid lipid-based nanoparticles to develop a novel delivery system for ORI. The physicochemical properties of obtained NLC, such as morphology, particle size, zeta potential, in vitro drug release and the in vivo characteristics in the mice were investigated. Oridonin-loaded NLC (ORI-NLC) were expected to reach the aim of enhancing its therapeutic efficacy of hepatoma through hepatic targeting and decreasing its side effects. The results of our studies contribute to the development of injection of poorly soluble drugs, and play a very important role in clinical application of ORI.
Materials and methods
Materials
Oridonin (99%) was purchased from Shanxi Xuhuang Biotechnology Co. Ltd. (China). Monostearin (MS) was obtained from Beijing Chemical Reagent Co. Ltd. (China). Caprylic/capric triglycerides (CT) were provided by Tieling Beiya pharmaceutical oil Co. Ltd. (China). Pluronic F68 was purchased from Sigma (USA). Lecithin was obtained from Beijing Shuangxuan Microbic Medium Co., Ltd. (China). Methanol was of high performance liquid chromatography (HPLC) grade. All other reagents and solvents were of analytical reagent grade.
Preparation of NLC
ORI-NLC was prepared by the emulsion–evaporation and low temperature–solidification technique described previously (CitationDai et al., 2010). Briefly, 200 mg lipid (consisting of MS and CT), lecithin and 5 wt% drug relative to lipid were mixed and dissolved into a mixture of acetone (5 ml) and ethanol (5 ml) in a water bath at 70°C to obtain a clear lipid phase. As for the preparation of NLC, the total amount of liquid lipid (CT) was 20 wt% relative to lipid. At the same time, an aqueous surfactant solution had been prepared and heated at the same temperature. The resultant hot organic solution was then injected into hot surfactant solution using a mechanical agitate (ETS-D4 stirrer, IKA, Germany) at 1000 rpm for 4 h. The NLC dispersions were obtained by dispersing warm o/w nanoemulsion into icy distilled water (0°C) under mild mechanical mixing at a ratio of 1:3 (nanoemulsion:water, v/v). For long-term stability, the prepared ORI-NLC dispersions were freeze-dried with a FD5-series freeze dryer (SIM, USA).
Morphology of freeze-dried NLC
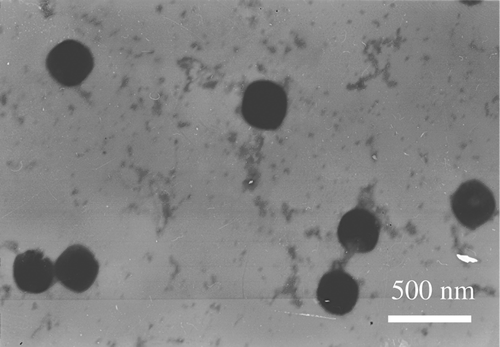
The morphological observation of ORI-NLC was performed by transmission electron microscopy (TEM, H-7000, Hitachi, Tokyo, Japan) using a negative-staining method. A drop of diluted NLC suspension was spread on a Cu grid and negatively stained with 2% (w/v) phosphotungstic acid for 30 s. Samples were allowed to dry at room temperature before being observed under the TEM.
Evaluation of particle size, zeta potential
The particle size and zeta potential of ORI-NLC dispersions were measured by a Zetasizer (3000HS, Malvern Instruments, Malvern, UK). Prior to the measurement all samples were diluted with double distilled water to have a suitable concentration. Each sample was determined in triplicate.
In vitro release assay
The release studies were carried out as following. Three batches of freeze-dried powders (containing 20 mg ORI, respectively) were placed in 200 ml pH 7.4 phosphate buffer solution at 37°C. The revolution speed of the paddle was maintained at a rate of 100 rpm; 2 ml dispersions were withdrawn out at different time intervals and the same volume of fresh dissolution medium was placed to maintain constant volume (200 ml). Withdrawn samples were added into Amicon Ultra-4 ultrafiltration device and centrifuged at 4000 rpm for 15 min. The filtrates were filtered with 0.22 μm filter membrane and then were determined by HPLC (1100, Agilent Technologies, Santara Clara, CA, USA), and detection conditions were set as follows: Kromasil C18 column (150 mm × 4.60 mm, 5 μm); mobile phase: methanol/water (52:48, v/v); flow rate of the mobile phase: 1.0 ml/min; measured wavelength: 242 nm.
Animals
Kunming strain mice (25 ± 2 g), supplied by the Experimental Animal Center of Shandong University (Jinan, China), were used for in vivo studies. At first, the animals were acclimatized at a temperature of 25 ± 2°C and a relative humidity of 70 ± 5% under natural light/dark conditions for 1 week. Rodent diet and water were provided ad libitum. Prior to experiment the animals were kept under fasting overnight but had free access to water. All experimental procedures are abided by the ethics and regulations of animal experiments of Pharmaceutical Sciences, Shandong University, China.
In vivo studies in mice
The in vivo studies of ORI-NLC were compared with ORI solution. Since ORI was practically insoluble in water, its solution was prepared by dissolving ORI in ethanol/1,2-propanediol/normal saline (at optimal rates) mixture solvent. The concentration of ORI solution was 2 mg/ml. The Kunming strain mice were randomly divided into two groups. ORI-NLC and ORI solution were intravenously administrated via tail vein at a dose of 25 mg/kg to mice. At determined time points (0.25, 0.5, 1, 2, 5, 8, 12, 24, 36, 48 h), the blood of mice was collected from postorbital vein into heparinized tubes and was centrifuged to get the plasma samples. Tissues of interest (heart, liver, spleen, lung, and kidney) were collected immediately after lightly rinsed with normal saline and dried with tissue paper. The plasma and tissue samples were frozen at −20°C until analysis.
Plasma and tissue sample analysis
Liquid–liquid plasma extraction procedure was as follows: 500 μl plasma and 5 ml acetic ether was added into a 10 ml glass screw-capped conical tube, and then vortexed for 5 min followed by sonicating for 2 min. After centrifuging at 4000 r/min for 15 min, the organic layer was transferred to another tube and evaporated under a light stream of nitrogen at 40°C. The residue was redissolved by 300 μl methanol and filtered through a 0.22 μm filter; 20 μl of the filtrate was injected for HPLC analysis.
Tissue samples were weighed accurately and homogenized using a glass tissue homogenizer after addition of 1 ml physiologic saline. Tissue homogenates were processed similarly as plasma samples. The plasma and tissue samples were all analyzed by HPLC according to the same detection conditions as described above. The detection limit was 0.03 mg/kg for each tissue.
Statistics
Statistical significance was determined using Student’s t test with p < 0.05 indicating significant difference. Pharmacokinetic parameters were obtained using drug and statistics (DAS) version 2.1.1 software (Mathematical Pharmacology Professional Committee of China, Shanghai, China).
Results and discussion
Characterization of ORI-NLC
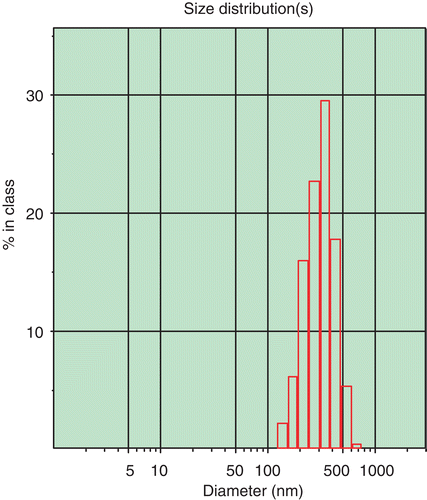
The resultant suspension was transparent in appearance with opalescence of blue, and shows the TEM image of ORI-NLC. As can be seen in , the NLC were in round shape and non-adherent among each other. The mean size of ORI-NLC was 245.2 ± 6.8 nm (n = 3). The size distribution of ORI-NLC was shown in , which displayed that all the particles were smaller than 691.4 nm, in which 90% were less than 436.2 nm.
The analysis of the zeta potential, the electric potential at the plane of shear, is a useful method to predict the physical stability of nanoparticles during storage. In this study, the zeta potential of NLC was −38.77±1.63 mV (n = 3), which attributed to the application of amphoteric surfactants (lecithin) in the formulation. The long-term stability of nanoparticles also related to the use of Pluronic F68 as the stabilizers. Pluronic F68 had the typical characteristics of surfactants and self-associated into large micelles, in case the sterically stabilizing layer is sufficiently thick (CitationZhang et al., 2010; CitationMüller & Jacobs, 2002).
In vitro release of drug
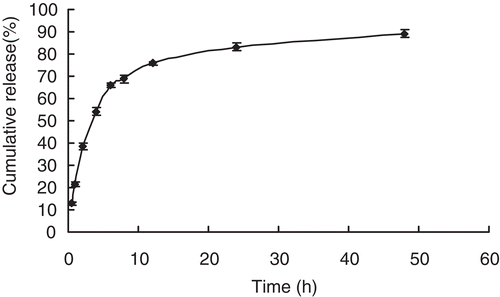
showed the in vitro drug release behaviours of ORI-NLC. In order to mimic the in vivo condition, 0.1 M phosphate buffer (pH 7.4) was chosen as the release medium. According to the results of HPLC analysis, the release profile showed that about 89.2% released during a total of 48 h, and the drug release behavior of ORI from the NLC exhibited a biphasic pattern with an initial burst effect followed by a slower continuous release (CitationHu et al., 2005). A possible explanation of the fast release was normally attributed to the enrichment of drug in the outer region of the NLC or drug deposition on the particle surface, which might occur during the solidification of the NLC during the cooling period after homogenization at 70–90°C. Such enrichment needs a short diffusion path to the release medium. In contrast to a fast precipitation, a slower and sustained release of ORI was followed, that means formation of large crystals is preferred. This period might be attributed to diffusion of the ORI entrapped within the core of the nanoparticles (Citationzur Mühlen et al., 1998).
Biodistribution study
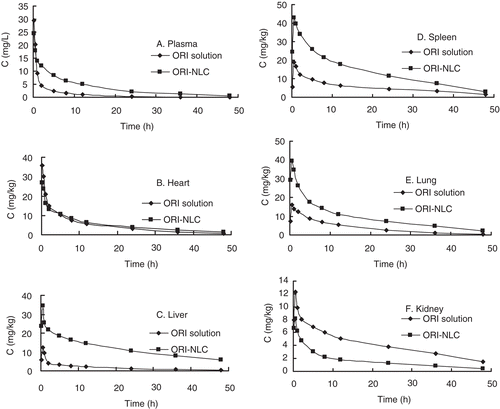
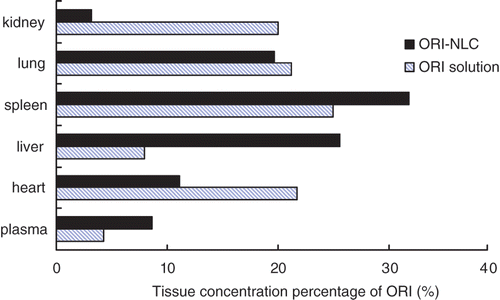
The intra- and inter-day precisions of ORI are within 10% in plasma and different tissues of mice. The intra- and inter-day recovery was ranged from 93.4% to 97.9% in tested tissues. The lowest limit of quantification (LOQ) of ORI was 0.07 mg/L and the standard curves having ORI concentrations ranging from 0.1 to 40 mg/L exhibited good linearity (R > 0.990). The tissue and serum ORI concentration–time curves after intravenous administration of ORI-loaded NLC and ORI solution were shown in . The AUC, mean residence time (MRT) and re (targeting parameter) values of tested organs are given in . As deduced from , AUC values of ORI-NLC in liver was 8.6-fold greater than that of ORI solution, which can be attributed to smaller size of particles (CitationTiwari & Pathak, 2011). Meanwhile the AUC values in heart, kidney and lung decreased when compared to free drug, which was helpful to reduce the tissue toxicity. showed ORI tissue concentration percentage of plasma, heart liver, spleen, lung and kidney at 12 h following i.v. administration of ORI solution and ORI-NLC. For ORI-NLC, 25.6% and 31.8% of ORI were distributed in liver and spleen, which was higher than the ORI solution. The results directly indicated that ORI-NLC could remain in liver and spleen for a longer time that was beneficial to therapy of liver and spleen disease.
Table 1. AUC, MRT and re values in different tissues of mice after i.v. administration of ORI-Sol and ORI-NLC with dose of 25 mg/kg.
Pharmacokinetic study
Mean pharmacokinetic parameters for ORI solution and ORI-NLC are shown in . As shown in , AUC values for plasma with ORI-loaded NLC were found about three-fold higher than ORI solution, and the MRT value of ORI-NLC was significantly longer than ORI solution. The small size of nanoparticles and slow release of drug from NLC might result in considerably higher blood level of loaded drug for a prolonged period as compared to ORI solution (CitationManjunath & Venkateswarlu, 2005). The higher concentration and the longer residence time of drug in blood could contribute to the opportunities for drug to contact with tumor tissues, thus promoting the conveyance of drugs to tumors.
Table 2. Pharmacokinetic parameters of two-compartmental model following i.v. administration of ORI-Sol and ORI-NLC to mice with dose of 25 mg/kg.
Conclusions
The purpose of this study was to assess the feasibility of using NLC to promote the therapeutic efficacy of ORI. ORI-loaded NLC with improved drug incorporation was prepared and characterized in vitro and in vivo. The physicochemical properties and release rate showed that the controlled adjustment of drug release was achieved. Compared with ORI solution, ORI-NLC showed higher AUC values and a prolonged residence time of drug in the blood circulation. The tissue distribution study showed that ORI-NLC after intravenous administration showed significantly higher accumulation in liver as compared to the free solution. These results suggested that the nanostructured lipid carriers can improve the clinical efficacy of ORI.
Declaration of interest
This work was supported by the National Basic Research Program of China (973 Program), No. 2009CB930 300, and the National Nature Science Foundation of China, No.30472130.
References
- Chen CC, Tsai TH, Huang ZR, Fang JY. (2010). Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: physicochemical characterization and pharmacokinetics. Eur J Pharm Biopharm 74:474–482.
- Dai W, Zhang D, Duan C, Jia L, Wang Y, Feng F, Zhang Q. (2010). Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul 27:234–241.
- Gao L, Zhang D, Chen M, Duan C, Dai W, Jia L, Zhao W. (2008). Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int J Pharm 355:321–327.
- Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. (2005). Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochem Biophys Res Commun 337:224–231.
- Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. (2005). Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B Biointerfaces 45:167–173.
- Joshi M, Pathak S, Sharma S, Patravale V. (2008). Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int J Pharm 364:119–126.
- Joshi M, Patravale V. (2008). Nanostructured lipid carrier (NLC) based gel of celecoxib. Int J Pharm 346:124–132.
- Li CY, Wang EQ, Cheng Y, Bao JK. (2011). Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol 43:701–704.
- Li F, Weng Y, Wang L, He H, Yang J, Tang X. (2010). The efficacy and safety of bufadienolides-loaded nanostructured lipid carriers. Int J Pharm 393:203–211.
- Li S, Liu Y, Liu T, Zhao L, Zhao J, Feng N. (2011). Development and in-vivo assessment of the bioavailability of oridonin solid dispersions by the gas anti-solvent technique. Int J Pharm 411:172–177.
- Lin XH, Li XW, Zheng LQ, Yu L, Zhang QQ, Liu WC. (2007). Preparation and characterization of monocaprate nanostructured lipid carriers. Colloid Surface A. 311:106–111.
- Lou H, Gao L, Wei X, Zhang Z, Zheng D, Zhang D, Zhang X, Li Y, Zhang Q. (2011). Oridonin nanosuspension enhances anti-tumor efficacy in SMMC-7721 cells and H22 tumor bearing mice. Colloids Surf B Biointerfaces 87:319–325.
- Lou H, Zhang X, Gao L, Feng F, Wang J, Wei X, Yu Z, Zhang D, Zhang Q. (2009). In vitro and in vivo antitumor activity of oridonin nanosuspension. Int J Pharm 379:181–186.
- Manjunath K, Venkateswarlu V. (2005). Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release 107:215–228.
- zur Mühlen A, Schwarz C, Mehnert W. (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm 45:149–155.
- Müller RH, Jacobs C. (2002). Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm 237:151–161.
- Müller RH, Radtke M, Wissing SA. (2002a). Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 242:121–128.
- Müller RH, Radtke M, Wissing SA. (2002b). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54 Suppl 1:S131–S155.
- Obeidat WM, Schwabe K, Müller RH, Keck CM. (2010). Preservation of nanostructured lipid carriers (NLC). Eur J Pharm Biopharm 76:56–67.
- Ren KK, Wang HZ, Xie LP, Chen DW, Liu X, Sun J, Nie YC, Zhang RQ. (2006). The effects of oridonin on cell growth, cell cycle, cell migration and differentiation in melanoma cells. J Ethnopharmacol 103:176–180.
- Tiwari R, Pathak K. (2011). Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm 415:232–243.
- Wang H, Ye Y, Pan SY, Zhu GY, Li YW, Fong DW, Yu ZL. (2011). Proteomic identification of proteins involved in the anticancer activities of oridonin in HepG2 cells. Phytomedicine 18:163–169.
- Xing J, Zhang D, Tan T. (2007). Studies on the oridonin-loaded poly(D,L-lactic acid) nanoparticles in vitro and in vivo. Int J Biol Macromol 40:153–158.
- Zhang P, Liu Y, Feng N, Xu J. (2008). Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm 355:269–276.
- Zhang XY, Liu JP, Qiao H, Liu H, Ni JM, Zhang WL, Shi YB. (2010). Formulation optimization of dihydroartemisinin nanostructured lipid carrier using response surface methodology. Powder Technol 197:120–128.