Abstract
Objective: This work has aimed to develop methotrexate-conjugated pectin nanoparticle for delivering a cytotoxic drug to hepatic cancer cell.
Methods: Methotrexate was conjugated to pectin by carbodiimide chemistry. Nanoparticles of pectin conjugated with methotrexate (MTX) were then fabricated by using ionotropic gelation. The size, shape and surface charge were characterized by dynamic light scattering and microscopic method. Cytotoxicity of MTX-pectin nanoparticle was monitored by MTT assay.
Results: Methotrexate-pectin nanoparticle was successfully formulated. Drug release study indicated that MTX-NP exhibited sustained drug release at pH 7.4. Sustained release of methotrexate may enable methotrexate-pectin nanoparticle as a controlled drug delivery system. Cytotoxicity study confirmed the activity of the drug incorporated in nanoparticles. In addition, the cytotoxicity of methotrexate was increased when conjugated to pectin nanoparticles, compared to free methotrexate.
Conclusions: This study verified that pectin can deliver methotrexate to hepatic cancer cell and provide sustained drug delivery. The cytotoxicity of methotrexate was enhanced when methotrexate was conjugated to pectin indicating the improved drug delivery to cancer cell.
Introduction
Most anticancer drugs are small molecules and can diffuse into cancer cells and normal cells resulting in low efficacy and high toxicity of the drugs to normal cells (Minchinton and Tannock, Citation2006). Nanoparticles have attracted the attention for using as anti-cancer drug delivery system for a variety of reasons. Conjugation of anti-cancer drugs to nanoparticles as drug delivery system may help selectively deliver the drug to cancer cells because drug conjugated nanoparticles will enter cells via endocytosis rather than diffusion (Chavanpatil et al., Citation2006; Shi et al., Citation2008). After internalization, drug will be released from the system by the change of pH and enzymatic degradation. In addition, because of their small size, nanoparticles can easily penetrate and accumulate in tumor cells due to enhanced permeability and retention effect (Thierry, Citation2009; Malam et al., Citation2011; Hirsjärvi et al., Citation2011). Drug conjugation and encapsulation in nanoparticles can enhance the efficiency of drug delivery to cancer cells, reduce efflux of the drug, and offer to protect the drug from enzymatic degradation, which enhances toxicity of the drug and decrease viability of cancer cells.
Anti-cancer drug can be conjugated or encapsulated into nanoparticles by using various interactions including hydrophobic interaction, electrostatic interaction, and covalent bonding (Kaasgaard et al., Citation2009; Trapani et al., Citation2011; Kosasih et al., Citation2000). Polymers have been widely used to prepare nanoparticles because they possess important functional groups that can be conjugated to anti-cancer drug. The most common functional groups are carboxylic acid, hydroxyl, and amino groups. In addition, polymers can aggregate to form a cross-link that can retain and encapsulate drug with hydrophilic surface, preventing aggregation of nanoparticles. Polymers that are appropriate for nanoparticle formulation should be biocompatible and biodegradable. Pectin is a natural and hydrophilic polymer which is biocompatible. Pectin is composed of α-(1-4)-linked D-galacturonic acid and contains many carboxylic acid as a functional group that can form amide bond when reacted with amino group of anti-cancer drug (Morris et al., Citation2010). This amide bond can be easily hydrolyzed by lysosomal enzymes leading to the release of the drug from nanoparticles.
Methotrexate is an effective anti-cancer drug used for treatments of breast cancer, skin cancer, head and neck cancer, and lung cancer (Takimoto, Citation1996). However, tumors may resist to the drug in long term use by various mechanisms including reduction of drug into cells or an increase in efflux of the drug (Pastan and Gottesman, Citation1987). Polymers that have been used currently to develop delivery systems for methotrexate are including poly(amidoamine) dendrimer nanoparticles, polyether-co-polyester dendrimer, nanostructured lipid carriers (solid lipid nanocarrier), polymeric nanoparticles (methoxy poly(ethyleneglycol)-grafted chitosan copolymer), gold nanoparticles and gelatin nanoparticles (Seo et al., Citation2009; Yang et al., Citation2008; Cascone et al., Citation2002; Reddy and Murthy, Citation2004; Mukesh et al., Citation2009; Lin et al., Citation2010). These nanoparticles can increase the efficacy and reduce the toxicity of methotrexate. However, the delivery systems are limited by the fact that they have low encapsulation or conjugation efficiency resulting in a low effectiveness of the drug.
Conjugation and encapsulation of methotrexate in nanoparticles have been studied. Iron oxide nanoparticles which surface of these nanoparticles was modified to have amino group can be conjugated with carboxylic acid of methotrexate using carbodiimide chemistry. In addition, methotrexate was successfully conjugated to dendrimer of polyamidoamine and to gold nanoparticles (Chen et al., Citation2007; van Haandel and Stobaugh, Citation2010). Encapsulation of nanoparticles prepared from chitosan, gelatin, poly(butylcyanoacrylate), calcium phosphate and lipid were reported (Seo et al., Citation2009; Yang et al., Citation2008; Cascone et al., Citation2002; Reddy and Murthy, Citation2004; Mukesh et al., Citation2009; Lin et al., Citation2010).
This study aims to develop pectin based nanoparticles by using ionotropic gelation method. Carboxylic acids of pectin were conjugated to amine group of methotrexate by using carbodiimide chemistry, followed by fabrication of drug loaded nanoparticles. The conjugation efficiency and in vitro release study were reported. The IC50 of MTX-NP, pectin nanoparticles (NP) compared to free methotrexate (MTX) were investigated in HepG2 hepatic cell line.
Materials and methods
Materials
(Non-amidated) High-methoxylated pectin from citrus was purchased from P. C Drug, Thailand. 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Thermo Fisher scientific, Inc. IL, USA. Methotrexate was obtained from Sigma and 2-mercaptoethanol was purchased from Merck.
Methods
Determination of degree of esterification by titrimetric method
Degree of esterification of pectin was determined by titrimetric method of Food Chemical Codex (1981). Dried pectin (500 mg) was transferred into Erlenmeyer flask and moistened with alcohol. Carbon dioxide-free water (30 ml) was added. The sample was swirled until completely hydrated. Phenolphthalein TS (2 drops) was added. Then pectin was titrated with 0.1N sodium hydroxide, the volume of sodium hydroxide used to reach the equivalent point was recorded as V1 (initial titer). The 0.1N sodium hydroxide (2 ml) was added. The flask was shaken vigorously and allowed to stand for 10 min followed by the addition of 2 ml of 0.5N hydrochloric acid. The flask was shaken vigorously until the pink color disappeared. Phenolphthalein TS (2 drops) was added and pectin sample was titrated again. The volume of 0.1N sodium hydroxide was recorded and known as V2 (saponification titer).
Pectin nanoparticle preparation and characterization
Pectin nanoparticles were formulated using an ionotropic gelation method. In brief, pectin was dissolved in purified water (20 mg/ml). Magnesium chloride solution (10 mg/ml) was slowly transferred to pectin solution. Then, sodium hydrogen carbonate solution (10 mg/ml) was added into the mixture. The ratio of the solutions of pectin, MgCl2 and NaHCO3 is 2:2:1. The solution was mixed for 30 min at room temperature. Pectin nanoparticles were collected by centrifugation (13,000 RPM, 10 min) and washed with purified water.
Conjugation of methotrexate to pectin nanoparticles
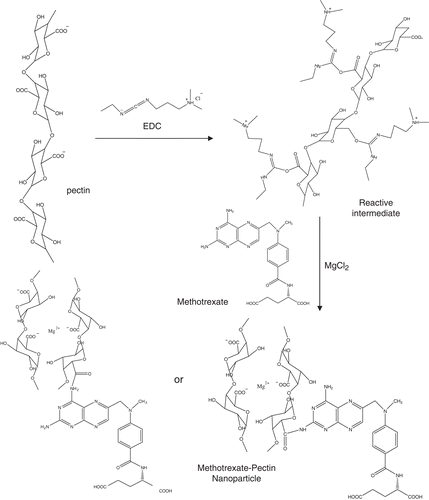
The conjugation reaction of methotrexate to pectin was performed via carbodiimide reaction and illustrated in . Pectin solution (20 mg/ml) was incubated with 100 mM 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) and 100 mM NHS (sulfo-NHS) for 15 min at room temperature. The 2-mercaptothanol (10 µl) was added to inactivate EDC. The activated carboxyl terminus of pectin was allowed to react with the amino terminus of methotrexate (0.03 mg/ml) at least 12 h at room temperature. Then, MgCl2 (10 mg/ml) was slowly added into methotrexate conjugated pectin followed by NaHCO3 (10 mg/ml). The mixture was stirred for 30 min at room temperature. Conjugated nanoparticled were then collected by centrifugation (13,000 rpm, 10 min) and washed three times with purified water. The size and charge of nanoparticles and MTX-NP were characterized by using dynamic light scattering (Zetasizer nanoseries, Malvern instruments).
Morphology characterization of MTX-NP
Transmission electron microscopy (TEM) was used to investigate the morphology of the MTX-NP. MTX-NP was treated with a phosphotungstic acid aqueous solution (1%) for positive staining. A drop of the mixture solution was then mounted on formvar coated copper grid. After 1 min, the excess solution was removed by touching the grid edge using a filter paper. The sample was stained again by the above procedure and was dried at room temperature for about 12 h. The dried samples were then examined using transmission electron microscope (JEOL, JEM-2100, Japan).
Determination of encapsulation efficiency
The encapsulation efficiency was determined indirect method. After MTX-NP was formed in the suspension, 1 ml of MTX-NP suspension was sampled and centrifuged at 13,000 rpm for 10 min. The supernatant was collected and amount of free methotrexate was quantified by UV spectrophotometer at 372 nm (UV-1601 UV-Visible spectrometry Shimadzu, Japan).
Where the drug at initial is the amount of MTX added in the carbodiimide reaction, and the drug loss refers to the amount of free MTX in the supernatant
In vitro release study of methotrexate conjugated to nanoparticles
The release study of methotrexate from particles has been investigated according to the following procedure (Ho et al., Citation2008). Twenty milligrams of MTX loaded pectin particles were resuspended in 2.6 ml of pH 7.4 phosphate buffer saline buffer in capped vial. Samples were incubated at 37°C. At the certain time intervals, 350 µl of suspension was sampled and centrifuged (13,000 rpm, 10 min). Then, 350 µl of the supernatant was collected and replaced with 350 µl of fresh PBS buffer. The amount of released methotrexate in the supernatant was determined using UV spectroscopy at 372 nm (UV-1601 UV-Visible spectrometry Shimadzu, Japan).
Where amount of released MTX was determined by UV spectroscopy and amount of total MTX in pectin NP was the amount of MTX encapsulated in pectin nanoparticle.
Cell culture
HepG2 (human hepatocyte carcinoma cells) was maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1:100 penicillin-streptomycin (10,000 units/ml) at 37°C and 5% CO2.
MTT assay (cytotoxicity assay)
HepG2 cells (10,000 cells/well) were cultured in 96-well culture plates, in a total volume of 200 µl of DMEM supplemented with 1% FBS plus penicillin/streptomycin. Cells were treated with PBS (vehicle control) and samples at various concentrations for 24 h. Both treatment and control groups were performed in 2 replicate wells. The relative number of viable cells was then determined at 24 h after incubation, by adding 1 mg/ml of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) and incubating the cell further for 4 h. The formazan crystals formed were then solubilized with DMSO. The absorption value of the solution at 595 nm directly represents relative cell numbers. The cell decreasing percent relative to the control group was then determined. The percentage of cell viability was calculated according to the following equation. The IC50 of free methotrexate, methotrexate conjugated nanoparticle and pectin nanoparticle were determined by analyzing the graph plotted between log concentration and % inhibition of cell growth and analyzed by dose response curve analysis and statistical analysis using GraphPad Prism software.
Statistical analysis
Statistical evaluation of data was performed using an analysis of variance (one-way ANOVA). Newman-Keuls was used as a post hoc test to assess the significance of differences. To compare the significance of the difference between the means of two groups, the t-test was performed; in all cases, a value of p < 0.05 was accepted as significant.
Results
Degree of esterification of pectin
Pectin is a polysaccharide consisting of D-galacturonic acid units. Some of the carboxylic acid groups are esterified with methanol. The remaining free acid was determined by acid-base titration method. The result of titration has shown that the average degree of esterification of pectin used for preparation of nanoparticle was 57.6 ± 1.8%, suggesting that carboxylic acids exist in almost half of all galaturonic acid and that pectin can be used to conjugate with methotrexate by using carbodiimide chemistry.
Pectin nanoparticle preparation and characterization
Pectin nanoparticles were prepared using an ionotropic gelation method. Magnesium chloride possessing divalent cation was added to provide positive charge to form polyelectrolyte complexes with pectin that has negative charge of carboxylic acid groups. Magnesium chloride was selected to yield cationic charge to cross-link with anionic charge of pectin and to obtain spherical nanoparticle (Opanasopit et al., Citation2008). The unbound carboxylic acid groups will form a hydrophilic shell and allow conjugation of the drug molecule in the drug loading step. According to Yu et al., spherical shape of nanoparticles were achieved when NaHCO3 were added during nanoparticle preparation (Yu et al., Citation2009). Therefore, NaHCO3 solution was included into the mixture to provide carbonic anions (CO3−) interacting with magnesium ion by electrostatic interaction and stabilize the spherical shape of nanoparticles (Yu et al., Citation2009). In addition, it has been reported that the size of nanoparticles formed via the electrostatic interaction were reduced when magnesium cation was used compared to other positively charge cations such as calcium cation (Opanasopit et al., Citation2008). In this study, the optimal concentrations of pectin and magnesium chloride were adjusted to allow nanoparticles with acceptable size distribution and highly negative charge. However, the size distribution of pectin nanoparticles formulated by ionotropic gelation was considered more widely, compared to nanoparticles produced by other methods such as solvent precipitation. This might be attributed to the entrapment of various amounts of magnesium ions in the core of pectin nanoparticles.
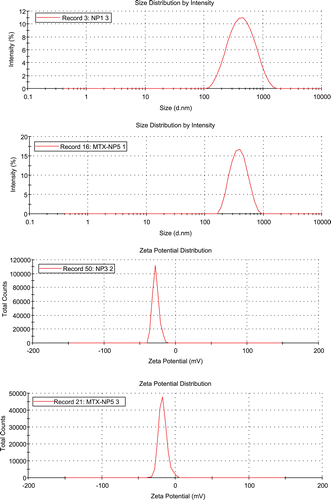
The diameter of pectin nanoparticles was approximately 390 nm with a low polydispersity suggesting an acceptable size distribution (). The zeta potential value was about −27 mV (). This result suggested that the carboxylic acid groups on the outer layer of nanoparticles yielded the negatively charged nanoparticles and may provide the stabilization of nanoparticles from aggregation. The mean diameter and zeta potential of methotrexate conjugated nanoparticle were approximately 370 nm and −17 mV, respectively, indicating that the size of MTX-nanoparticles were not significantly influenced by the coupling of the drug to pectin.
Table 1. Nanoparticle properties at specified formulation points.
Conjugation of methotrexate on the surface of pectin nanoparticles
The efficiency of conjugation reaction between the carboxyl of pectin and the amino group of methotrexate was evaluated. The amount of free methotrexate remaining in the reaction medium after carboddiimide reaction and nanoparticle separation was quantified by using UV-Vis spectrometry. The conjugation efficiency of methotrexate with pectin nanoparticles was acceptable at 54.9 ± 11.3%. The charge of methotrexate loaded nanoparticles decreased compared to the unloaded pectin nanoparticles suggesting that the carboxylic acid groups of pectin were conjugated with methotrexate.
Morphology of methotrexate-pectin nanoparticle
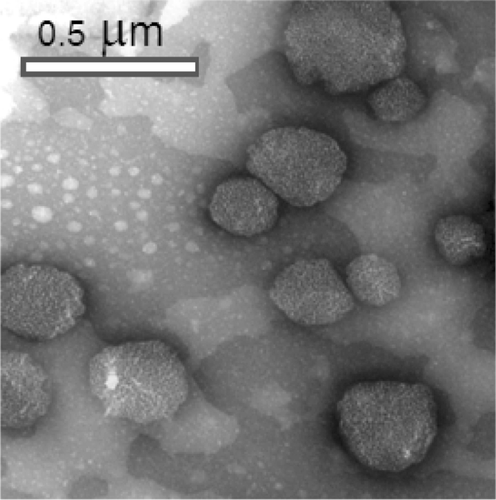
The average particle size characterized by transmission electron microscope was found to be around 300–400 nm, which is in agreement with the size obtained from dynamic light scattering measurements. The TEM picture showed that MTX-NP were spherical in shape ().
In vitro release study of methotrexate conjugated nanoparticles
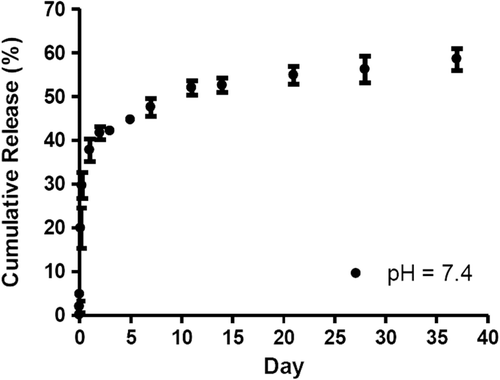
In general, encapsulation efficiency of drug loaded in nanoparticles fabricated from pectin is low and the drug release rate is fast because pectin is hydrophilic and prone to be hydrolyzed (Yu et al., Citation2009). In this study, the release of methotrexate from pectin nanoparticles was determined in phosphate buffer at pH 7.4, representing the physiological pH. At pH 7.4, methotrexate was in the ionized form and freely soluble in water (Javadzadeh and Hamishehkar, Citation2011). The release of methotrexate was about 30 ± 3.0% in the first 6 h of the study followed by the slow and sustained release (). The release of the drug was prolonged for 37 days with 60% release. In the first 6 h, the drug physically entrapped in the nanoparticle was released due to the swelling of pectin and the diffusion the drug. Then the release was resulted from the hydrolysis of amide bond conjugated between pectin and methotrexate.
Cytotoxicity of methotrexate conjugated nanoparticles, unconjugated nanoparticles and methotrexate in HepG2 cell line
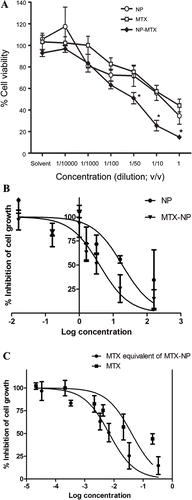
The cytotoxic effects of methotrexate and methotrexate conjugated pectin nanoparticles on HepG2 cells were performed by MTT assay (). The results demonstrated that free MTX and methotrexate-pectin nanoparticle (MTX-NP) exhibited cytotoxicity with respect to HepG2 cells. The percentage of cell viability decreases in relation to the increasing in nanoparticle concentration. MTX-NP at concentrations of 3.2, 16 and 160 mg/ml displayed significantly higher cytotoxicity against hepG2 cell line after 24 h incubation compared to free MTX and unconjugated NP (). The MTT assay suggested that high concentration of unconjugated pectin nanoparticle was moderately cytotoxic. The IC50 values of free methotrexate (MTX), and methotrexate conjugated to pectin nanoparticle were 40 µg/ml and 7 µg/ml, respectively (). The concentration of methotrexate conjugated to pectin was calculated from the drug loading and encapsulation efficiency. The IC50 values reported for methotrexate conjugated nanoparticle (MTX-NP) and unconjugated nanoparticle (NP) were shown at 4.0 and 18.8 mg/ml, respectively (). The results of cytotoxicity assay revealed that methotrexate conjugated pectin nanoparticle (MTX-NP) decreased the IC50 value relative to the free drug suggesting that methotrexate-pectin nanoparticle (MTX-NP) significantly improved the cytotoxicity of methotrexate against HepG2 cell line.
Figure 5. (A) Cytotoxicity profile of methotrexate, pectin nanoparticles, and methotrexate-pectin nanoparticles on HepG2 cells after 24 h incubation as measured by MTT assay (n = 3). *, p < 0.05 vs NP or MTX (B) Dose response curve plotted between % inhibition of cell growth and log concentration of NP and MTX-NP (C) Dose response curve plotted between % inhibition of cell growth and log MTX equivalent concentration.
Discussion
Hydrophilic polymer matrix systems are widely used to improve the efficacy and control the release of the drug. Pectin is a heteropolysaccharide which is composed of galacturonic acid at the backbone of polymer and constitutes of galatose, arabinose, and rhamnose as side chains (Morris et al., Citation2010). The degree of esterification is defined as the number of moles of methanol per 100 moles of galacturonic acid (Morris et al., Citation2010). In the present study, the degree of esterification of citrus pectin was characterized by titration method and found to be about 57%, which is considered as a highly methoxyl pectin and suggested that there are carboxylic acid groups available for further modification. The carboxylic acids of pectin were activated with carbodiimide coupling reagent and successfully conjugated with amino groups of methotrexate. The amide bond formation between amino group of MTX and carboxylic acid group of pectin did not affect the activity of the drug. The size and shape of MTX-NP were regulated by the cross-linking of polymer with cations. With particle size of 370 nm, nanoparticles may be taken up via carveolar-mediated internalization. However, the pathway of cellular uptake of nanoparticle is influenced by the interaction of nanoparticles with cells and the cell type (Lu et al., Citation2009). In this study, methotrexate was efficiently encapsulated in the nanoparticle. The fabricated MTX-NP obtained a desirable drug release profile with no obvious initial burst effect and controlled drug release. The methotrexate-nanoparticle presented a slow rate of delivery, followed by a rather constant rate over the subsequent days, indicating an interaction between the amino group of the drug and the carboxylic acid of pectin nanoparticle. The in vitro cytotoxicity of free MTX, MTX-NP and unloaded nanoparticle revealed that unloaded pectin nanoparticles showed cytotoxicity at 4–5 times greater concentration compared to MTX-NP, suggesting that the cytotoxicity of MTX-NP was mostly resulted from the encapsulated MTX. In addition, MTX-NP exhibited a more potent cytotoxicity than free MTX. This result suggests that MTX-NP may enhance the delivery of nanoparticle loaded with a significant amount of MTX and increased the cytotoxicity, compared to free MTX which enter the cells by diffusion. We hypothesized that MTX-NP may enhance the cytotoxicity of MTX by accumulating MTX to high level in hepG2 cells due to the uptake of NP loaded with MTX. The uptake of MTX-NP may yield the greater extent of MTX and more rapid accumulation of drug in the cells. However, the uptake of MTX-NP by hepG2 cell line has to be further investigated. Several studies have shown that pectin itself can suppress tumor and inhibit metastasis in animal studies (Heitman et al., Citation1992; Platt and Raz, Citation1992; Pienta et al., Citation1995; Nangia-Makker et al., Citation2002). Furthermore, pectin can also induce apoptosis in cancer cells (Jackson et al., Citation2007; Chang et al., Citation1997; Avivi-Green et al., Citation2000a; Avivi-Green et al., Citation2000b; Kossoy et al., Citation2001; Olano-Martin et al., Citation2003; Chauhan et al., Citation2005). Here, we showed the IC50 of unconjugated pectin NP was up to 19 mg/ml. These results indicated that pectin nanoparticle may be used as a potential anti-cancer drug delivery system.
Conclusions
In summary, MTX conjugated pectin nanoparticle as anti-cancer drug delivery system was developed with an efficient conjugation strategy. The results showed that MTX-NP can effectively sustain release of the drug and enhance the MTX delivery to hepG2 cells in vitro. Pectin nanoparticle may serve as a potential carrier for tumor drug delivery.
Acknowledgements
The authors acknowledge support from Faculty of Pharmacy, Srinakharinwirot University.
Declaration of interest
The authors report no conflicts of interest.
References
- Avivi-Green C, Madar Z, Schwartz B. Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int J Mol Med 2000a;6:689–698.
- Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res 2000b;12:83–95.
- Cascone MG, Lazzeri L, Carmignani C, Zhu Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J Mater Sci Mater Med 2002;13:523–526.
- Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis 1997;18:721–730.
- Chauhan D, Li G, Podar K, Hideshima T, Neri P, He D et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res 2005;65:8350–8358.
- Chavanpatil MD, Khdair A, Panyam J. Nanoparticles for cellular drug delivery: mechanisms and factors influencing delivery. J Nanosci Nanotechnol 2006;6:2651–2663.
- Chen YH, Tsai CY, Huang PY, Chang MY, Cheng PC, Chou CH et al. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharm 2007;4:713–722.
- Heitman DW, Hardman WE, Cameron IL. Dietary supplementation with pectin and guar gum on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Carcinogenesis 1992;13:815–818.
- Hirsjärvi S, Passirani C, Benoit JP. Passive and active tumour targeting with nanocarriers. Curr Drug Discov Technol 2011;8:188–196.
- Ho ML, Fu YC, Wang GJ, Chen HT, Chang JK, Tsai TH et al. Controlled release carrier of BSA made by W/O/W emulsion method containing PLGA and hydroxyapatite. J Control Release 2008;128:142–148.
- Jackson CL, Dreaden TM, Theobald LK, Tran NM, Beal TL, Eid M et al. Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure. Glycobiology 2007;17:805–819.
- Javadzadeh Y, Hamishehkar H. Enhancing percutaneous delivery of methotrexate using different types of surfactants. Colloids Surf B Biointerfaces 2011;82:422–426.
- Kaasgaard T, Andresen TL, Jensen SS, Holte RO, Jensen LT, Jørgensen K. Liposomes containing alkylated methotrexate analogues for phospholipase A(2) mediated tumor targeted drug delivery. Chem Phys Lipids 2009;157:94–103.
- Kosasih A, Bowman BJ, Wigent RJ, Ofner CM 3rd. Characterization and in vitro release of methotrexate from gelatin/methotrexate conjugates formed using different preparation variables. Int J Pharm 2000;204:81–89.
- Kossoy G, Ben-Hur H, Stark A, Zusman I, Madar Z. Effects of a 15% orange-pulp diet on tumorigenesis and immune response in rats with colon tumors. Oncol Rep 2001;8:1387–1391.
- Lin YK, Huang ZR, Zhuo RZ, Fang JY. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int J Nanomedicine 2010;5:117–128.
- Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009;5:1408–1413.
- Malam Y, Lim EJ, Seifalian AM. Current trends in the application of nanoparticles in drug delivery. Curr Med Chem 2011;18:1067–1078.
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583–592.
- Morris G, Kök S, Harding S, Adams G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev 2010;27:257–284.
- Mukesh U, Kulkarni V, Tushar R, Murthy RS. Methotrexate loaded self stabilized calcium phosphate nanoparticles: a novel inorganic carrier for intracellular drug delivery. J Biomed Nanotechnol 2009;5:99–105.
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst 2002;94:1854–1862.
- Olano-Martin E, Rimbach GH, Gibson GR, Rastall RA. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res 2003;23:341–346.
- Opanasopit P, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Ruktanonchai U. Development and characterization of pectinate micro/nanoparticles for gene delivery. AAPS PharmSciTech 2008;9:67–74.
- Pastan I, Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med 1987;316:1388–1393.
- Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst 1995;87:348–353.
- Platt D, Raz A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J Natl Cancer Inst 1992;84:438–442.
- Reddy LH, Murthy RR. Influence of polymerization technique and experimental variables on the particle properties and release kinetics of methotrexate from poly(butylcyanoacrylate) nanoparticles. Acta Pharm 2004;54:103–118.
- Seo DH, Jeong YI, Kim DG, Jang MJ, Jang MK, Nah JW. Methotrexate-incorporated polymeric nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan. Colloids Surf B Biointerfaces 2009;69:157–163.
- Shi W, Wang J, Fan X, Gao H. Size and shape effects on diffusion and absorption of colloidal particles near a partially absorbing sphere: implications for uptake of nanoparticles in animal cells. Phys Rev E Stat Nonlin Soft Matter Phys 2008;78:061914.
- Takimoto CH. New Antifolates: Pharmacology and Clinical Applications. Oncologist 1996;1:68–81.
- Thierry B. Drug nanocarriers and functional nanoparticles: applications in cancer therapy. Curr Drug Deliv 2009;6:391–403.
- Trapani A, Denora N, Iacobellis G, Sitterberg J, Bakowsky U, Kissel T. Methotrexate-loaded chitosan- and glycol chitosan-based nanoparticles: a promising strategy for the administration of the anticancer drug to brain tumors. AAPS PharmSciTech 2011;12:1302–1311.
- van Haandel L, Stobaugh JF. Bioanalytical method development for a generation 5 polyamidoamine folic acid methotrexate conjugated nanoparticle. Anal Bioanal Chem 2010;397:1841–1852.
- Yang X, Zhang Q, Wang Y, Chen H, Zhang H, Gao F et al. Self-aggregated nanoparticles from methoxy poly(ethylene glycol)-modified chitosan: synthesis; characterization; aggregation and methotrexate release in vitro. Colloids Surf B Biointerfaces 2008;61:125–131.
- Yu CY, Cao H, Zhang XC, Zhou FZ, Cheng SX, Zhang XZ et al. Hybrid nanospheres and vesicles based on pectin as drug carriers. Langmuir 2009;25:11720–11726.