Abstract
Context: A transdermal delivery system is warranted for repaglinide (RPG) which possesses half-life of 1 h and oral bioavailability of 56%. Ethosomes are useful tools for transdermal drug delivery.
Objectives: To prepare and evaluate ethosomes as mode for transdermal delivery of RPG.
Material and methods: Ethosomes loaded with RPG were prepared from dipalmitoyl phosphatidylcholine and ethanol by the cold method. They were characterized using Fourier transform infrared spectroscopy and differential scanning calorimetry. They were evaluated for vesicle size, entrapment efficiency and ex-vivo skin permeation. Ethosomal composition was optimized using the 32 factorial design. Gel containing optimzsed ethosomes was studied for antidiabetic activity in rats.
Result: RPG ethosomes possessing the size of 0.171–1.727 µm and entrapment efficiency of 75–92% were obtained. They demonstrated a significantly higher permeation (64–97% of the administered dose) across excised rat skin when compared to free drug and its hydro alcoholic solution. In-vivo, RPG ethosomal system caused sustained antidiabetic effect.
Discussion: The lipid and ethanol concentration affected the physicochemical attributes and performance of ethosomes. The flexible ethosomes permeated the stratum corneum and improvized the availability of RPG for antidiabetic action. They prolonged the antidiabetic effect of RPG over a significantly longer period of time in comparison with the equivalent oral dose.
Conclusion: Ethosomal system can successfully deliver RPG transdermally; sustain its effect and thus reduce its dosing frequency. Ethosomes are useful for enhancing the efficacy of RPG in the treatment of diabetes.
Introduction
Diabetes mellitus, currently, is one of the major and growing health problems worldwide and has become an important cause of prolonged ill health and early death (Jain & Saraf, Citation2009). Type 2 diabetes is particularly associated with post prandial hyperglycemia. Despite a large number of oral hypoglycemic agents being available for type 2 diabetes, these agents have a limited capacity to provide stable long-term glycemic control. Repaglinide (RPG) is an oral blood glucose-lowering drug of the meglitinide class used for the management of type 2 diabetes mellitus. RPG lowers blood glucose levels by stimulating the release of insulin from the pancreas. Dosage frequency of RPG is 0.5–4 mg, 3–4 times in a day. It shows rapid onset of action but for shorter duration, as it is rapidly eliminated from the blood stream with a half-life of approximately 1 h. The mean absolute bioavailability is about 56% because of first pass metabolism (Ambavane et al., Citation2002).
Oral administration of RPG has been criticized due to first pass metabolism and thus reduced bioavailability. Likewise, the risks and inconveniences associated with the intravenous route of administration limit formulations thereof. Since it shows rapid onset of action, it becomes an ideal candidate to control post prandial hyperglycemia. But for better control of fasting as well as post prandial blood glucose levels in type 2 diabetes, there is need to develop a sustained release delivery system of RPG. The need for sustained delivery of RPG is further justified due to the requirement of maintaining un-fluctuating plasma concentrations for the effective management of blood sugar for a long period in diabetic patients (Ambavane et al., Citation2002). In such conditions transdermal drug delivery remains the most favored mode of administration where the hepatic metabolism of RPG can be bypassed (Sheo & Ramchand, Citation2011). But, stratum corneum forms the most formidable barrier for the penetration of drug through skin. To overcome the stratum corneum barrier, the use of lipid vesicles like liposomes, niosomes, transferosomes and ethosomes (Verma, Citation2011; Jadhav et al., Citation2012) in delivery systems has attracted increasing attention in recent years. Among these, ethosomes are reported to have the potential of overcoming the stratum corneum barrier and enhance the permeability of drug through the skin.
Ethosomes are soft, malleable vesicles of nanometer to micron size, composed mainly of phospholipids, ethanol (relatively high concentration) and water (Touitou & Dayana, Citation2000; Touitoua et al., Citation2001; Elisabetta et al., Citation2004; Singh et al., Citation2010). These are novel vesicular carrier for enhanced delivery to/through skin that enables drugs to reach the deep skin layers and/or the systemic circulation (Misra & Sachin, Citation2009). It is thought that ethanol fluidizes bilayers of the stratum corneum intercellular lipid; the soft, malleable vesicles then penetrate the disorganized lipid bilayers. The lipid vesicles provide a controlled transdermal delivery system and also serve for the solubilization of poorly soluble drugs. Combination of phospholipids and high concentration of ethanol in vesicular formulations have been suggested to be responsible for deeper penetration and distribution in the skin lipid bilayers. Vesicles can incorporate both hydrophilic and lipophilic drugs (Sarath Chandran & Arun, Citation2011; Verma & Ram, Citation2011). Although ethosomal systems are conceptually sophisticated, they are characterized by simplicity in their preparation, safety and efficacy – a combination that can highly expand their application (Tiwari et al., Citation2010). Enhanced delivery of bioactive molecules through the skin and cellular membranes by means of an ethosomal carrier opens numerous challenges and opportunities for the research and future development of novel improved therapies (Elsayed et al., Citation2007; Kundlik et al., Citation2010; Verma, Citation2011).
The main aim of this work was to study the usefulness of ethosomes for transdermal delivery of RPG in the treatment of type 2 diabetes mellitus. The transdermal delivery of ethosomes offers many advantages over oral systems that include improving the systemic bioavailability of drug by avoiding first pass metabolism and thus providing a sustained, controlled drug delivery (Godin & Touitou, Citation2005). Hence, we attempted to develop RPG ethosome for transdermal delivery to reduce the dosing frequency, to sustain the drug release, avoid the hepatic first-pass metabolism – factors which might eventually increase the bioavailability of RPG.
Methods
Preparation of RPG ethosomes
Ethosomes were prepared by the cold method with modification (Kumar et al., Citation2010; Jadhav et al., Citation2012). Appropriate amount of drug (10 mg) and dipalmitoyl phosphatidyl choline (DPPC) dissolved in sufficient amount of ethanol (maintained at 30 °C) was injected into a closed vessel containing water at 30 °C with the help of syringe. Mixture was stirred for about 2 min with magnetic stirrer and sonicated for 5 min using the probe sonicator (BMI-599, Biomedica, New Delhi, India).
Vesicle characterization
Vesicle shape
Ethosome vesicles were visualized under Motic microscope (BI Advanced, Ahmedabad, India) under 40× magnification.
Vesicle size
Vesicle size of RPG ethosomes was determined using the computerized inspection system, i.e. MALVERN 2000SM (Malvern Instruments Ltd., Worcestershire, UK). The vesicular suspensions were diluted with water and measurements were carried out in triplicate. The vesicle size has been expressed as d (0.9) µm.
Determination of entrapment efficiency
Entrapment efficiency was measured by the micro centrifugation technique (Misra & Sachin, Citation2009). The ethosomal sample was subjected to centrifugation for 15 min at 1500 rpm. After centrifugation the supernatant and pellets were separated and analyzed for the amount of drug using U.V. Spectrophotometer (Jasco V-530, Jasco International Co. Ltd., Tokyo, Japan). The entrapment efficiency was calculated using the following equation:
where T = theoretical amount of drug that was added and C = amount of T drug detected after dissolving the vesicles.
Fourier transform infrared spectroscopy
The Fourier transform infrared spectroscopy (FTIR) analysis of RPG, blank ethosomes and RPG-loaded ethosomes was carried out by using FTIR (Jasco M4100, JASCO). Dried potassium bromide was placed in the sample holder of FTIR and blank sample was run. Then mixtures were prepared in potassium bromide 1:5 w/w ratios of drug, blank ethosomes and drug-loaded ethosomes. FTIR scans were obtained at a resolution of 2 cm−1 from 4000 to 400 cm−1.
Differential scanning calorimetry
The thermal behavior of RPG and its ethosomes was determined using DSC (STAR SW 9.20, Mettler, Zurich, Switzerland) by heating at a rate of 10 °C/min within a temperature range of 20–300 °C.
Preparation of RPG ethosomal gel
A 1.5% w/w Carbopol 940 gel base was selected as a vehicle for the RPG ethosomes. Ethosomes loaded with RPG ethosomes were incorporated into the gel base by slow stirring until a homogeneous ethosomal gel was achieved. The pH was then adjusted to neutral using triethanolamine and stirred slowly. The viscosity of ethosomal gel was determined using a Brookfield digital viscometer at a shear rate of 80 rpm at room temperature. Its pH was measured with a pH meter (Ashoniya & Chauhan, Citation2001).
Ex-vivo drug permeation study
Permeation of ethosomal RPG was studied across excised rat skin mounted on a Franz diffusion cell (PermeGear, Hellertown, PA) with an effective permeation area and receptor cell volume of 3.14 cm2 and 20 ml, respectively. Phosphate buffer pH 7.4 maintained at 37 ± 0.5 °C was the medium for diffusion. Samples withdrawn (replaced with equivalent amount of fresh medium) at different time intervals were analyzed for drug content spectrophotometrically. The cumulative drug permeated was determined using PCP Disso software. Flux was determined directly as the slope of the curve between the steady-state values of the amount of drug permeated versus time in hours. Similar permeation experiments were put up for the aqueous suspension and hydroalcoholic solution of RPG.
In-vivo study
The antidiabetic activity of the prepared ethosomal RPG was studied in comparison to the oral administration of free drug. The experimental procedure and protocol were reviewed and approved by the Institutional Animal Ethical Committee (IAEC) of Modern College of Pharmacy, Nigdi, Pune, constituted under Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India (Approval no. MCP/IAEC/48/2011). Ethical guidelines were strictly followed during all the experiments. Wistar albino rats (200–260 gm) obtained from the animal house of Modern College of Pharmacy, Nigdi, Pune, were placed randomly in polypropylene cages (four per cage) with paddy husk as bedding. Standard laboratory conditions of temperature 24 ± 2 °C, relative humidity 55 ± 5% and 12:12 h light:dark cycle were maintained throughout all the experiments. Animals were divided into four groups, each containing six rats. The dilute alloxan solution was prepared by dissolving 1 g of alloxan in 20 ml normal saline (i.e. 50 mg/ml concentration). Group I was administered with a normal saline solution which was a normal control group. Group II–IV rats were made diabetic by a single i.p. injection of 0.6 ml alloxan monohydrate solution to each rat (i.e. 120 mg/kg body weight dose). After 2 days, induction of diabetes in rats was confirmed by measuring blood glucose levels in rats using glucometer (Accu Check Active, Roche Diagnostics, Indianapolis, IN) using glucose strips. After successful induction of diabetes in rats, Group II was administered with the normal saline solution which was a diabetic control group. Pure RPG suspension (4 mg/kg body weight of rat) was administered orally using oral feeding needle to Group III, which was a reference-treated group. RPG ethosomal gel (equivalent to a dose requirement of 4 mg/kg RPG) was applied topically to Group IV rats, which was an ethosomal gel-treated group. Blood glucose estimation was done immediately after 0 (baseline), 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 15, 18, 21, 24 h using glucometer (Accu Check Active) using glucose strips.
Statistical analysis
A prior knowledge and understanding of the process and the process variables during the preliminary experimentation brought into light that DPPC and ethanol influenced the ethosomal characteristics and performance. To optimize their concentrations, a 32 factorial design was adopted, wherein the dependent variables were the vesicle size, entrapment efficiency and ex-vivo drug permeation. The responses obtained of the nine runs were subjected to multiple regression analysis using “Design Expert®” software (Stat-Ease, Inc., Minneapolis, MN) (version DX 8.0.5.2) and fitted in the following regression equation:
where Y is the dependent variable, β0 is the arithmetic mean response of the nine runs, β1 and β2 are the estimated coefficients for the independent factors X1 and X2, respectively. β11, β22 and β12 are the estimated coefficients for the interaction X1X1, X2X2 and X1X2, respectively. The main effects terms (X1 and X2) represent the average result of changing one factor at a time from its low to high value. The interaction terms (X1X2) show how the response changes when two factors are simultaneously changed. The polynomial terms (X1X1, X2X2) are included to investigate non-linearity. The effects of the variables are interpreted considering the magnitude of coefficient and the mathematical sign it carries. The adequacy of fitted model was checked by the analysis of variance (ANOVA). The main and interaction effects were represented as response surface curves too.
Each sample was tested in triplicate for all the experiments. The results of all experiments were expressed as mean ± SD. In all tests, values of p < 0.05 were regarded as significant.
The results of in-vivo antidiabetic study were compared by the Dunnett Multiple Comparisons Test model using Graphpad Instat (GraphPad Software, Inc., La Jolla, CA) (version V3.01) software.
Results and discussion
Having realized the need for a transdermal delivery system for RPG, this study was undertaken to investigate the utility of ethosomes to fulfill the said need. Ethosomes comprising of DPPC and ethanol () were employed as carriers for transdermal delivery of RPG. The cold method of preparation yielded predominantly spherical vesicles; few oval and oblong vesicles could also be seen.
Table 1. Composition of batches of ethosomes as per 32 full factorial design.
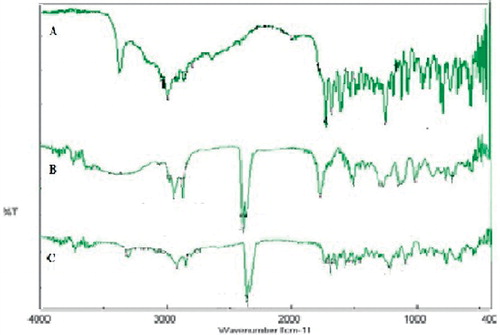
FTIR spectroscopy of RPG, RPG-loaded ethosomes and blank ethosomes are depicted in . The spectrum of RPG shows characteristics absorption bands at 3305.39 cm−1 of N–H stretch, 1633.41 cm−1 of N–H bend and 2933.2 cm−1 C–H2 stretch. Spectrum of blank ethosomes shows intense peaks at 2364.3 cm−1 for tertiary amine and other low-intensity peaks at 1733.69 cm−1 of C=O, 2858.96 cm−1 of C–H stretch and 2911.02 cm−1 of CH2 stretch. The RPG-loaded ethosome spectrum demonstrated peaks at 1645 cm−1(N–H bend), 1681.22 cm−1 (C=O), 2364.3 cm−1 for tertiary amine, 2837.74 cm−1 (C–H stretch), 2921.63 cm−1 (C–H2 stretch) and 3317.93 cm−1 (N–H stretch). Peaks observed in the spectrum of RPG and individual spectrum of blank ethosomes are retained in the FTIR spectrum of the drug-loaded ethosome but showed slight shift in the position of peaks toward higher wave number, as shown in . This suggested a possibility of slight interaction between RPG and the ethosomal contents.
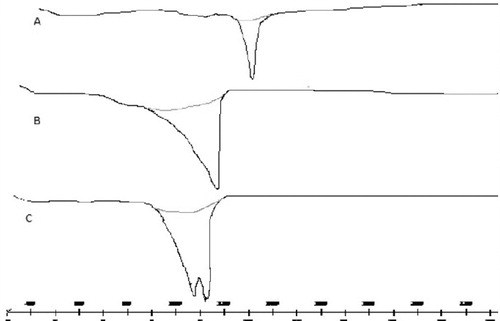
DSC study () was carried out to study the thermal behavior of RPG-loaded ethosomes. The DSC thermogram of RPG displayed an endothermic peak at 139 °C corresponding to its melting point. However this peak was shifted to lower temperature (100.17 °C) in RPG-loaded ethosomes. The thermogram of blank ethosomes was different from that of the drug-loaded one, thus giving evidence of incorporation of RPG within the ethosomes. These observations, like the FTIR study, highlight the interaction of RPG with ethosomal constituents.
Statistical analysis ensures accurate and convincing interpretation of results of a study. In the present study, ethosomal composition was optimized utilizing the approach of factorial design. To study the effects of the ethosomal constituents (DPPC and ethanol) on its attributes and performance, a 32 full factorial design was employed. The independent variables, i.e. concentration of DPPC and ethanol at three levels were evaluated for their effect on vesicle size, entrapment efficiency and ex-vivo permeation. The responses obtained are given in . All the responses studied were largely affected by the variables chosen as reflected from the results of regression anlysis ().
Table 2. Results of responses of ethosomes as per 32 full factorial design.
Table 3. Summary of regression analysis and ANOVA.
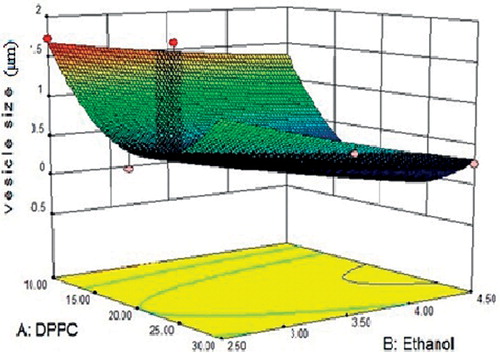
Vesicle size of RPG ethosomes () was found in the range 0.171–1.727 µm. The result of regression analysis tabulated in indicated that the vesicle size was significantly affected by the variables of the study (r2 = 0.9454). A comparison of the magnitudes of the regression coefficient brings forth the predominant effect of the phospholipid, DPPC. The response surface curve generated out of the results () pointed out the curvilinear effect of DPPC. Increasing amounts of DPPC in a particular range reduced the vesicle size probably by an interaction with ethanol or RPG. Amounts in excess of this added to the hydrophobic domain of the bilayer, thus increasing bilayer thickness. The increase in ethanol concentration causes decrease in the vesicle size of ethosomes.
Figure 3. Response surface curve depicting the effect of DPPC and ethanol on the vesicle size of ethosomes.
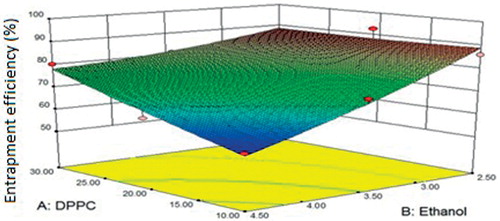
indicated a significant influence (r2 = 0.9541) of the variables on the entrapment efficiency, which varied from 56.75 ± 0.71 to 92.28 ± 0.27%. Ethanol did not favor entrapment () as its increasing levels induced excessive fluidity in the vesicles which caused leakage of the drug out of the vesicles (Godin & Touitou, Citation2005).
Figure 4. Response surface curve depicting the effect of DPPC and ethanol on the entrapment efficiency of ethosomes.
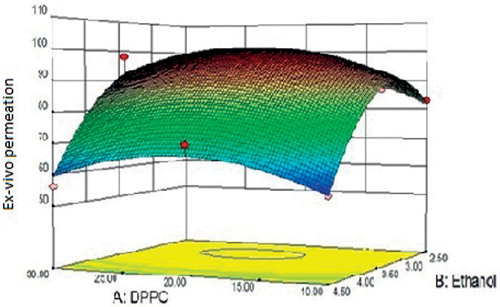
Ex-vivo skin permeation of ethosomal RPG was observed to be in the range of 63.64 ± 0.49% to 96.96 ± 0.18% (). revealed significant favorable effect of ethanol on the permeation characteristic of RPG ethosomes. This could be related to the fluidizing effect of ethanol on the layers of stratum corneum which eventually reduces the barrier property of the stratum corneum. Also, the presence of ethanol in ethosomal architecture contributes to its flexibility which allows it to squeeze through the pores of the skin. The data obtained from ex-vivo release study of different ethosomal formulations was fitted in various kinetic models in order to elucidate the mechanism of drug release from formulations. Calculation of regression coefficient of different kinetic models (data not shown) led to conclude that the release occurred in accordance with the kinetics of the Korsmeyer–Peppas model. The release exponent (n) indicated that drug release from formulation occurred by the non-Fickian mechanism, i.e. by erosion mechanism. Aqueous and hydroethanolic dispersion of RPG showed 53.01 ± 2.11% and 69.96 ± 3.08% release up to 24 h, respectively. In comparison, batch F8 showed a significantly higher release of 96.96 ± 0.18% and an average flux of 2.16 μg/cm2/h. The ex-vivo permeation study concluded that ethosomes effectively carried RPG across the skin over the aqueous and hydroethanolic solution of free RPG. Similar benefits of ethosomal encapsulation were observed with molecules such as propranolol and trihexyphenidyl (Touitoua et al., Citation2001).
Figure 5. Response surface curve depicting the effect of DPPC and ethanol on ex-vivo skin permeation of ethosomes.
The optimized batch F8 was incorporated into a 1.5% w/w Carbopol 940 gel base, which is a widely used vehicle for topical use. This ethosomal gel (viscosity of 2507 ± 4.5 cps and pH of 7.36 ± 0.05) was studied for ex-vivo permeation and was found to release 88.76 ± 2.75% of the administered dose. It was further used for in-vivo study.
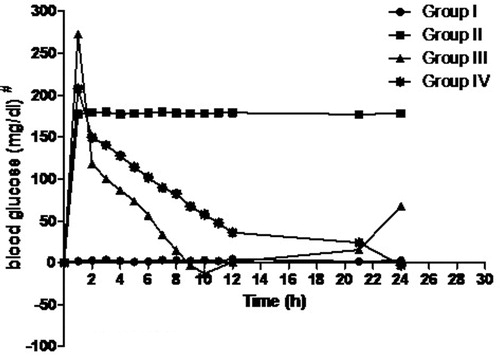
In-vivo antidiabetic activity in rats was undertaken to assess the antidiabetic potential of ethosomal RPG. The time course profile of blood glucose levels in rats is shown in . The blood glucose level in rats that received oral RPG drastically (p < 0.01) reduced to 98.7 ± 11.7 mg/dl over 11 h. Thereafter RPG’s hypoglycemic activity was not noticed as blood glucose levels raised up to 209 ± 7 mg/dl by the end of 24 h probably owing to its short half-life. However, a prolonged antidiabetic action was displayed by the ethosomal RPG suggesting a sustained release from the transdermal formulation. Also, the RPG ethosomal gel-treated rats elicited significantly lower (p < 0.01) blood glucose level (97.3 ± 3.5 mg/dl) at the end of 24 h. The in-vivo study concluded that ethosomal drug delivery is a potential approach to deliver RPG transdermally that can overcome the shortcomings related to its oral use.
Conclusion
The present study demonstrated that with the help of transdermal drug delivery of ethosomal system, RPG can be successfully delivered through skin for the treatment of type II diabetes mellitus. Ethosomal RPG delivery is capable of prolonging drug release that might reduce the dosage frequency.
Acknowledgements
S. S. Bodade is thankful to All India Council of Technical Education, New Delhi, India, for providing GPAT scholarship.
Declaration of interest
The authors report no declarations of interest.
References
- Ambavane V, Patil R, Ainapure S. (2002). RPG: a short acting insulin secretagogue for postprandial hyperglycaemia. Drug Rev 48:246–8
- Ashoniya S, Chauhan M. (2001). Ethosomes as vesicular carrier for enhanced transdermal delivery of Ketoconazole-formulation and evaluation. J Pharm Cosmeto 3:1–14
- Elisabetta E, Enea M, Rita C. (2004). Ethosomes and liposomes as topical vehicles for azelaic acid: a preformulation study. J Cosmetic 55:253–64
- Elsayed M, Abdallah OY, Naggar VF, Khalafallah NM. (2007). Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm 332:1–16
- Godin B, Touitou E. (2005). A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: an in vivo study. J Antmicrob Chemo 55:989–94
- Jadhav SM, Morey P, Karpe M, Kadam V. (2012). Novel vesicular system: an overview. J App Pharm Sci 2:193–202
- Jain S, Saraf S. (2009). Influence of processing variables and in vitro characterization of glipizide loaded biodegradable nanoparticles. Diabetes Metabolic Syndrome: Clin Res Rev 3:113–17
- Kumar K, Radhika P, Sivakumar T. (2010). Ethosomes – a priority in transdermal drug delivery. Int J Adv Pharm Sci 1:111–21
- Kundlik G, Ranju P, Hitesh G. (2010). Ethosomes: a novel vesicular carrier for enhanced dermal delivery of Ciclopirox olamine. Der Pharmacia Lett 2:360–7
- Misra AN, Sachin N. (2009). Ethosome – a novel delivery system of antifungal drugs in treatment of topical fungal diseases. Ind J Exp Bio 47:368–75
- Sarath Chandran C, Arun S. (2011). Development and evaluation of ethosomes for transdermal delivery of fluconazole. J Chem Bio Phy Sci B2:254–60
- Sheo D, Ramchand D. (2011). Enhancement of transdermal permeation of indinavir sulfate via ethosome vesicles. Afr J Pharm Sci 2:33–47
- Singh A, Rathore P, Shukla M, Nayak S. (2010). Comparative studies on skin permeation of miconazole using different novel carriers. Int J Pharm Sci Res 1:61–6
- Tiwari RK, Chauhan NS, Yogesh HS. (2010). Ethosomes – a potential carries for transdermal drug delivery. Int J Drug Dev Res 2:448–52
- Touitou E, Dayana N. (2000). Ethosomes – novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Rel 65:403–18
- Touitoua E, Godina B, Dayana N. (2001). Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2:3053–9
- Verma P. (2011). Transdermal penetration efficacy of ethosomal systems with and without penetration enhancer: a comparative study. Int J Pharm Sci Res 2:2472–4
- Verma P, Ram A. (2011). Effect of different penetration enhancers on skin permeation of drug using ethosomal carrier systems. J Curr Pharm Res 5:42–4