Abstract
Present investigation deals with intranasal delivery of ropinirole hydrochloride (ROPI HCl), loaded in solid lipid nanoparticles (SLNs). Prime objectives of this experiment are avoidance of hepatic first pass metabolism and to improve therapeutic efficacy in the treatment of Parkinson’s disease. SLNs were fabricated by emulsification-solvent diffusion technique. A 32-factorial design approach has been employed to assess the influence of two independent variables, namely Pluronic F-68 and stearylamine concentration on particle size, ζ-potential and entrapment efficiency of prepared SLNs. Prepared samples were further evaluated for in vitro drug diffusion, ex vivo drug permeation, histopathological and stability studies. Differential scanning calorimetry analysis revealed the encapsulation of amorphous form of drug into lipid matrix, while scanning electron microscopy studies indicated the spherical shape. Fabricated SLNs had shown no severe signs of damage on integrity of nasal mucosa. Release pattern of prepared drug-loaded sample was best fitted to zero-order kinetic model with non-Fickian super case II diffusion mechanism. In vivo pharmacodynamic studies were carried out to compare therapeutic efficacy of prepared nasal formulation against marketed oral formulation. Results of analysis of variance demonstrated the significance of suggested model. Three-dimensional response surface plots and regression equations confirmed the corresponding influence of selected independent variables on measured responses. Our findings suggested the feasibility of investigated system for intranasal administration.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder, the prevalence of which is mainly due to death of dopaminergic neurons of substantia nigra pars compacta, a condition being characterized, in major, by resting tremors, muscle rigidity and bradykinesia, with the presence of intracellular proteinaceous inclusions (called Lewy bodies) throughout the brain. This results in reduction of dopamine levels in the striatum. Deficiency of dopamine creates imbalance in normal dopamine: acetylcholine levels, exasperating the symptoms of PD (Hurley & Jenner, Citation2006; Rudra et al., Citation2007; Salawu et al., Citation2010).
Ropinirole, a new non-ergoline dopamine receptor agonist, binds specifically to D2-receptors in striatum and substantia nigra, with selectivity similar to that of dopamine. It is thought to draw its antiparkinsonian action through enhancing striatal neuronal firing rates via selective activation of D2-dopamine receptors. ROPI HCl is a highly hydrophilic, low molecular weight bioactive. Its mean plasma half-life is 5–6 h. The bioavailability of ROPI HCl after oral administration is ∼50% due to extensive hepatic first pass metabolism (Fukuzaki et al., Citation2000). Presently, ROPI HCl conventional tablet dosage forms are available in market, which are unable to achieve sufficient oral bioavailability. IV administration is very irritating and is not advised. Therefore, alternative routes and drug delivery systems are needed to improve its therapeutic efficacy.
Diseases of central nervous system require delivery of drug to the brain for treatment. Hydrophilic and large molecular weight drugs usually face problems while being transported to the brain because of permeability issues relevant to blood–brain barrier (BBB). However, animal and human investigations proved that transport of exogenous materials directly from nose-to-brain is a potential route for bypassing the BBB (Illum, Citation2000; Mistry et al., Citation2009).
The past 20 years have witnessed an unheralded explosion in research on the development of novel colloidal carriers as drug delivery systems. Most attractive area of research in drug delivery, now-a-days, is the design of nanosystems, which are able to deliver drugs to right place, at appropriate times and at right dosage (Motwani et al., Citation2007).
A new class of colloidal drug carriers, lipid-based nanoparticles, has emerged in early 1990s and is now being looked up by the research frontiers around the world due to exploitation of their potential applications in the core areas of pharmaceutical sciences such as drug delivery, clinical and therapeutic medicine and other such varied fields (Mukherjee et al., Citation2009). Patel et al. (Citation2011) reported the formulation and evaluation of risperidone-loaded solid lipid nanoparticles (SLNs) for brain targeting and their findings substantiates the existence of direct nose-to-brain route for nanoparticles administered to the nasal cavity.
Design Expert® (Stat-Ease Inc, Minneapolis, MN) is an effective, proficient and systematic tool to estimate the effects of individual variables, studied in all possible combinations, using minimum experimental efforts. Application of factorial design has played a predominant role in understanding the relationship between independent variables and the responses obtained, for design and development of pharmaceutical formulations (Pardeshi et al., Citation2011).
Sustained drug release, enhanced therapeutic efficacy, reduced dosing frequency and prevention of hepatic first pass metabolism, which was extensive in the case of model drug, are the foremost objectives of the present research. Emphasis was also given to kinetic modeling, stability and histopathological studies to see whether nose-to-brain drug delivery could be viewed as an efficient form of delivering hydrophilic antiparkinsonian agent, ROPI HCl.
Materials and methods
Materials
ROPI HCl was received as generous gift from Wockhardt Pharmaceuticals Pvt. Ltd., Aurangabad, India. Dynasan 114 (Trimyristine), Pluronic F-68 (Poloxamer 188), glucose, sucrose, lactose and mannitol were procured from Hi-Media Laboratories Pvt. Ltd., Mumbai, India. Stearylamine was purchased from Sigma Aldrich GmbH (St. Louis, MO). Phospholipon® 90 G (P90G) was a kind gift from Nattermann PHOSPHOLIPID GmbH, Lipoid, Koln, Germany. Benzyl alcohol was procured from Loba Chemie, Mumbai, India. All other reagents used were of analytical grade and used as received.
Experimental design
A 2-factor 3-level factorial experimental design technique was used herein, to evaluate the influence of two selected factors each at three levels and experimental trials were performed at all possible nine combinations. In this investigation, concentration of Pluronic F-68 (X1) and concentration of stearylamine (X2) were selected as independent variables. Three responses, particle size (Y1), ζ-potential (Y2) and entrapment efficiency (EE, Y3), were measured for each sample and taken as dependent variables. Design parameters, formulation composition and physico-chemical characterization are depicted in .
Table 1. 32-Factorial design parameters, formulation composition and characterization of SLNs.
Preparation method for SLNs
ROPI-loaded SLNs were prepared using emulsification-solvent diffusion technique (Trotta et al., Citation2003), with slight modification. Benzyl alcohol and water were mutually saturated at 47 ± 2 °C for 15 min in order to ensure initial thermodynamic equilibrium of both solvents. Typically, Dynasan 114 and stearylamine were dissolved in 2.0 ml of water-saturated organic solvent and 25 mg of drug in 8.0 ml of solvent-saturated aqueous solution containing surfactant (Pluronic F-68) and co-surfactant (soy lecithin). Organic phase was added dropwise into aqueous phase under high mechanical stirring (REMI Instruments Ltd., Mumbai, India) at 600 rpm and 61 °C for 30 min to give primary emulsion. Lipid nanoparticles were then precipitated by quickly adding 50.0 ml cold water into initially formed primary emulsion to extract organic solvent into continuous phase under magnetic stirring and stirred further for next 10 min. The suspension so formed was passed through high pressure homogenizer (Panda 2 K, Niro Soavi Ltd., Segrate, Italy) at 600 bar pressure for nine consecutive cycles. The obtained nanodispersion was then lyophilized using suitable cryoprotectant, after preliminary evaluation. Lyophilization was carried out according to following protocol: vials were first frozen at −25 °C for 1 h. Samples were then lyophilized at 0.25 mbar for 24 h with increasing shelf temperature from −15 °C to 0 °C and final drying at +15 °C, 0.01 mbar for 2 h. Obtained lyophilized samples were stored at −4 °C until further investigation.
Optimization data analysis and model validation
Statistical validation of polynomial equations generated by Design Expert® software version 8.0.1 was established by analysis of variance (ANOVA). Fitting a multiple linear regression statistical model to 32-factorial design gives a predictor equation incorporating interactive and polynomial terms to evaluate the responses:
where Y is measured response (dependent variable) associated with each factor-level combination; β0 is an intercept representing arithmetic average of all quantitative outcomes of nine runs; βi (β1, β2, β11, β12, β22) are regression coefficients computed from the observed experimental values of Y; X1 and X2 are coded levels of independent variables. X1X2 represents the interaction term. The main effects (X1and X2) represent the average result of changing one factor at a time from its low to high value. The interaction terms show how the response changes when two factors are changed simultaneously. Polynomial terms (
and
) are included to investigate non-linearity. The polynomial equation was used to draw conclusions after considering magnitude of coefficients and the mathematical sign it carries, i.e. positive or negative. Positive sign indicates synergistic effect, whereas negative sign stands for antagonistic effect.
In model fitting analysis, the responses, namely particle size, ζ-potential and EE of all formulation samples were treated by Design Expert® software. Best fitting mathematical model was selected on the basis of comparison of several statistical parameters like coefficient of variation (CV), multiple correlation coefficient (R2), adjusted multiple correlation coefficient (adjusted R2) and predicted residual sum of square, provided by Design Expert® software. The level of significance was considered at p < 0.05. Three-dimensional response surface plots resulting from equations were obtained by Design Expert® software.
Physico-chemical characterization of SLN dispersion
Particle size, polydispersity index (PDI) and ζ-potential
Particle size, PDI, as a measure of width of particle size distribution and ζ-potential (ζ) as a measure of surface electric charge on SLN were determined by photon correlation spectroscopy using Zetasizer (Nano ZS 90, Malvern Instruments Ltd., Malvern, UK). All samples were diluted with filtrated bidistilled water (1:10) to get optimum 100–200 kilo-counts/s (kcps) for measurement. All measurements were performed at 25 °C, at scattering angle of 90°. ζ-potential measurements were run at an electric field strength of 25 V/m. Results are presented as mean ± standard deviation (SD) from three replicate determinations (Shegokar et al., Citation2010; Tan et al., Citation2010; Zhang et al., Citation2010).
Drug loading and entrapment efficiency
Drug loading (DL, %) and EE (%) were determined by measuring the concentration of unentrapped free drug in aqueous medium of SLN dispersion. A known dilution of each drug-loaded SLN dispersion sample was prepared in bidistilled water, placed in Eppendorf tubes and centrifuged at 8000 rpm for 1 h at 10 °C to separate lipid and aqueous phase (Cooling Centrifuge, C24, REMI Instruments Ltd., Mumbai, India). 300 µl of supernatant was then diluted to 3.0 ml with methanol and amount of free ROPI HCl in supernatant was determined spectrophotometrically at 250 nm using UV-spectrophotometer (1700, Shimadzu®, Tokyo, Japan). DL and EE were calculated by using following Equations (2) and (3), respectively (Varshosaz et al., Citation2010):
where Wt is the total weight of drug used, Ws is the drug remaining in the supernatant and Wl is the weight of lipid used in preparing SLN formulations.
Screening and optimization of cryoprotectant for lyophilization
The most popular cryoprotectants encouraged in the literature for lyophilization of nanoparticles are: glucose, sucrose, lactose, mannitol and trehalose (Abdelwahed et al., Citation2006). Here, glucose, sucrose, lactose and mannitol were screened at 5, 7, 5 and 10 wt% concentration levels to evaluate their cryoprotectant efficiency for lyophilization of prepared samples.
Characterization of lyophilized SLNs
Scanning electron micrographic studies
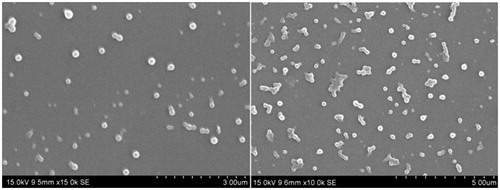
The shape and morphology of lyophilized SLN was studied using scanning electron microscopy (SEM, JSM 6390®, JEOL DATUM Ltd., Tokyo, Japan). Lyophilized powder of formulation was dusted onto double-sided tape on an aluminum stub and coated with gold using a cold sputter coater in SEM chamber to a thickness of 400 Å, and then photomicrographs were captured by operating at an accelerating voltage of 15 kV electron beam.
Histopathological examination
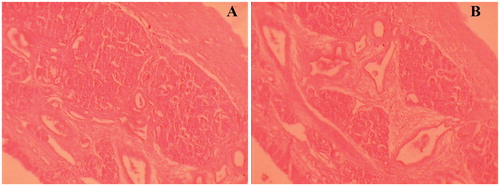
Histopathological studies were concluded on sheep nasal mucosa to assess any damage on integrity of nasal mucosal tissues, under study. Sheep nasal mucosa, obtained from local slaughterhouse (Shirpur, India) within 1 h of sacrificing the animal, was cleaned with isotonic saline solution. After applying drug-loaded lyophilized SLN formulation sample, nasal mucosa was fixed in 10% neutral carbonate buffered formalin solution. To assure optimum viability of tissues, an experiment was carried out in cell culture incubator (MCO-5AC, Sanyo Incubator, Tokyo, Japan). Paraffin sections (7 µm), stained with hematoxylin–eosin (HE), were examined under light microscope, to detect any damage to the tissues (Mahajan et al., Citation2012). The mucosa, fixated directly after isolation at slaughterhouse, was used as a control.
Differential scanning calorimetry
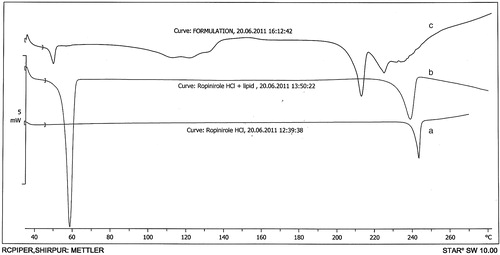
Differential scanning calorimetry (DSC) analysis was performed on pure drug, drug–lipid physical mixture and lyophilized drug-loaded SLN formulation sample. Thermograms were obtained by Differential Scanning Calorimeter (DSC 1, Mettler-Toledo®, Zurich, Switzerland) at a heating rate of 10 °C/min from 30 °C to 300 °C under nitrogen purge of 40 ml/min.
In vitro drug diffusion studies
An in vitro drug release from lyophilized SLN formulation was performed using Franz diffusion cell across dialysis membrane (Mw cut-off 12 000–14 000). The membrane was equilibrated overnight before carefully dispersing the formulation, equivalent to 5 mg of drug into donor compartment, already containing 3 ml of SNES. Receptor compartment was filled with phosphate buffer solution (pH 6.6), within the pH range in nasal cavity. Temperature was maintained at 37 ± 1 °C using circulating water bath. Samples were periodically withdrawn from receptor compartment, replaced with same quantity of fresh pre-warmed buffer solution, and assayed using UV-Vis spectrophotometer (1700, Shimadzu®, Tokyo, Japan) at 250 nm.
Drug release kinetics
To study drug release kinetics of SLN formulation, data obtained from in vitro drug release studies were plotted in various kinetic models: zero order (see Equation (5)) as cumulative percentage of drug released versus time, first order (see Equation (6)) as log cumulative percentage of drug remaining versus time and Higuchi’s model (see Equation (7)) as cumulative percentage of drug released versus square root of time.
Zero order
where K0 is the zero-order rate constant (concentration/time) and t is time (min). A graph of concentration versus time would yield a straight line with slope equal to K0 and intercept the origin of axes.
First order
where C0 is the initial concentration of drug, K is the first-order rate constant and t is time (min).
Higuchi
where Qt is the amount of drug released in time t, K is kinetic constant and t is time (min).
Mechanism of drug release
Mechanism of drug release from drug-loaded SLN was evaluated by subjecting the data obtained from in vitro drug diffusion studies to Korsmeyer–Peppa’s model (see Equation (8)) as log cumulative percentage drug released versus log time. Release exponent (n) and kinetic constant (k) were calculated from slope of straight line:
where Mt represents the amount of released drug at time t, M∞ is the total amount of drug released after an infinite time, K is the diffusional (kinetic) constant of drug–lipid system and n is the release exponent that determines the mechanism of drug release from drug delivery system. If n = 0.5 then, the drug release mechanism is Fickian diffusion, if n < 0.5, the mechanism is quasi-Fickian diffusion, if n = 0.5–1.0 then, it is non-Fickian or anomalous diffusion, if n = 1.0, the mechanism is non-Fickian case II diffusion, and if n > 1.0 then, it is non-Fickian super case II diffusion (Jain et al., Citation2009).
Ex vivo permeation studies
Ex vivo drug permeation studies were performed using Franz diffusion cell across sheep nasal mucosa as permeation barrier, obtained from local slaughterhouse within 1 h of sacrificing the animal. Nasal mucosa was carefully cut with a scalpel and mounted on the diffusion chamber with mucosal and serosal surfaces facing donor and receptor compartments, respectively. Other experimental and sample collection procedures were performed in same fashion as in vitro diffusion studies (Tas et al., Citation2006).
Stability studies
Lyophilized SLN formulation sample was packed in glass vials. Vials were sealed with rubber caps and kept at accelerated temperature and humidity conditions (40 ± 2 °C and 75 ± 5% RH) as per ICH [Q1A (R2)] guidelines for a period of three months in stability chamber (CHM-10S, REMI Instruments Ltd., Mumbai, India). Every month, stability samples were re-dispersed in bidistilled water and stability of SLN formulation was evaluated on the basis of measurement of particle size and EE, using same procedures as mentioned earlier (Belgamwar et al., Citation2011; Chalikwar et al., Citation2012).
In vivo pharmacodynamic studies
Non-invasive in vivo pharmacodynamic studies were performed according to guidelines approved by the committee for the purpose of control and supervision of experiments on animals, Ministry of Social Justice and Empowerment, Government of India. Study protocol was duly approved by Institutional Animal Ethics Committee of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India (Reg. No. 651/02/C/CPCSEA).
The below-mentioned experiment was designed to study non-invasive in vivo pharmacodynamic parameters.
Anti-tremor activity in mice
The rationale behind this examination was to compare performance in terms of anti-tremor activity, in mice, produced by lyophilized nasal SLN formulation against marketed tablet formulation of same drug. Tranquilizing agents including phenothiazines like chlorpromazine (CPZ) induce Parkinsonism-like signs such as tremors, salivation and lacrimation. These signs can be relieved by ROPI HCl.
Male albino mice with mean body weight of 25 ± 2 g were used for this study. These mice were divided into four groups containing six animals each. Toxicant group was dosed subcutaneously with CPZ solution (5 mg/kg of body weight). Control group was administered with vehicle (0.9 % saline solution) orally. Standard group was given an oral dose equivalent to 10 mg/kg of ROPI HCl from marketed oral formulation (ROPARK®, Sun Pharma Ltd., India) using an oral gavage needle while Formulation group was dosed nasally with lyophilized SLN formulation reconstituted with bidistilled water, (3 mg/kg of body weight, divided evenly between both nostrils) using a polyethylene canula (0.28 mm I.D., 0.56 mm E.D.) inserted approximately 5–7 mm into nasal cavity. Test and Standard samples were given 1 h prior the s. c. administration of CPZ solution. Dose calculations were based on body weight of selected animal models (Vogel, Citation2002).
Tremor scores were observed visually and recorded, by expert personnel, in individual animal models in 10 s observation periods at 10 min intervals for 1 h, using a rating scale of 0–3, as (Fukuzaki et al., Citation2000);
0 = No tremors,
1 = Slight twitches,
2 = Moderate to intermittent tremors,
3 = Pronounced continuous tremors.
Data treatment and statistical analysis
Data were expressed as mean ± SEM for three animals in each group. Statistical comparisons were performed by one-way ANOVA followed by Dunnett’s test using GraphPad Prism ver. 4.0, (San Diego, CA). Differences between formulations were considered to be statistically significant at p < 0.05.
Response surface analysis
Three-dimensional response surface plots were generated by Design Expert® software using central composite design to illustrate the influence of two independent variables, namely Pluronic F-68 concentration and stearylamine concentration on measured responses, namely particle size, ζ-potential and EE of SLNs.
Results and discussion
Formulation of SLN
Nine formulation samples of ROPI HCl-loaded SLNs were fabricated by emulsification-solvent diffusion technique, using 32-factorial design, in which independent variables were Pluronic F-68 concentration (X1) and stearylamine concentration (X2) whereas particle size (Y1), ζ-potential (Y2) and EE (Y3) were taken as responses (dependent variables).
Optimization data analysis and model validation
Fitting of data to the model
The two factors with upper, middle and lower design points in actual and coded values are shown in . The responses, Y1, Y2, and Y3 were found to be in the range of 66.22 ± 6.22 to 271.61 ± 4.26 nm, −23.60 ± 3.32 to 47.13 ± 1.19 mV, and 55.23 ± 0.45 to 64.73 ± 0.34% respectively. All the responses observed for nine formulations prepared were fitted to various models using Design-Expert® software. It was revealed that the best fitted model was Quadratic for Y1 (particle size), and linear for Y2 (ζ-potential), and Y3 (EE). The values of R2, adjusted R2, predicted R2, SD and %CV are given in . The results of ANOVA in , for dependent variables demonstrate that the models were significant for all measured responses.
Table 2. Summary of results of regression analysis for measured responses.
Table 3. Summary of results of ANOVA for measured responses.
It was observed that, both independent variables X1 (Pluronic F-68 concentration) and X2 (stearylamine concentration) had a positive effect on particle size (Y1), means particle size increases with increase in X1 and X2, as confirmed by Equation (8). X1 had negative effect while X2 had a positive influence on ζ-potential (Y2) (see Equation (9)). Both X1 and X2 had a positive effect on EE (Y3) of SLNs (see Equation (10)).
Optimization and validation
A numerical optimization technique by desirability approach was used to generate optimum settings for the formulation. The process was optimized for dependent variables such as particle size (Y1), ζ-potential (Y2) and EE (Y3). Optimized formulation was selected on the basis of criteria of attaining the minimum value of particle size, optimum ζ-potential and considerable acceptable value of EE of fabricated SLNs. Formulation sample L5 containing 1.0 wt% Pluronic F-68 and 0.05 wt% stearylamine fulfilled all the criteria set by desirability search. For all the responses obtained with sample L5, desirability was found to be close to 1 (0.911 for Y1, 0.951 for Y2 and 0.802 for Y3), suggesting that the selected sample was optimized one among all formulation samples. A new optimized formulation was prepared according to predicted values and evaluated for responses, to estimate the reliability of response surface model. Then comparison was made between observed and predicted values of each measured response and prediction error was obtained in the range of 0.17–2.53 (results not shown). It was found that there was a reasonable agreement between predicted and experimental values. Furthermore, it can be concluded that the regression equations describe the influence of selected independent variables on measured responses adequately. Also, low magnitude of prediction error (0.17–2.57) in the present investigation justifies high prognostic ability of optimization technique by the 32-factorial design approach.
Characterization of SLN dispersion
Particle size, PDI and ζ-potential
The z-average particle diameter of the prepared SLNs ranged from 66.22 ± 6.22 to 271.61 ± 4.26 nm and the polydispersity varied from 0.016 ± 0.06 to 0.460 ± 0.16, implying narrow particle size distribution in all samples, whereas the ζ-potential of SLN formulations were found to be in the range of |−16.76 ± 2.17| to |47.13 ± 1.19| mV (). Initially, SLNs were found to possess negative surface charge due to the negatively charged functional groups in Dynasan 114 (Trimyristine).
Regression equation (9) shows that both factors, X1 and X2, have positive effect on particle size of SLNs. The correlation coefficient was found to be 0.8405. illustrates the influence of factors Pluronic F-68 concentration (X1) and stearylamine concentration (X2) on particle size (Y1) of SLNs. Particle size decreased with increase in Pluronic concentration at constant lipid concentration, but this reduction in particle size was observed only up to certain percentage of concentration (1% w/v), beyond which particle size increased. This may be attributed to its tendency to get adsorbed onto the surface of already formed particles forming a thicker layer, leading to coalescence of droplets with subsequent agglomeration and particle growth. This results in increased particle diameter. Here, Pluronic F-68 acts as a steric stabilizer (Heurtault et al., Citation2003).
Figure 1. Response surface plots showing influence of Pluronic F-68 concentration (X1) and stearylamine concentration (X2) on particle size (Y1), zeta potential (Y2) and entrapment efficiency (Y3).

From a stability standpoint, sufficient electrical potential (>±30 mV) must be retained by nanoparticles in order to induce static repulsion on reproach (Tan et al., Citation2010). Herein stearylamine, a surface charge modifier, has been utilized to assess its influence on ζ-potential of SLNs. As it is a cationic lipid, it pulls the ζ-potential values toward positive side. Stearylamine induces positive charge on the surface of nanoparticles and also contributes to increased particle size (Venkateswarlu & Manjunath, Citation2004). Stearylamine serves to be an electrostatic stabilizer by maintaining the ζ-potential of fabricated system to optimum. Regression equation (9) shows that only factor X2 (stearylamine concentration) has positive effect while factor X1 (Pluronic F-68 concentration) has negative effect on ζ-potential of SLNs. The correlation coefficient was found to be 0.8595. illustrates the influence of factors Pluronic F-68 concentration (X1) and stearylamine concentration (X2) on ζ-potential (Y2) of SLNs.
Homogenization process had given optimum particle size and maximum dispersivity with nine homogenization cycles at 600 bar pressure. Increasing the number of homogenization cycles or operating pressure had given, in trial samples, a pronounced increase in particle diameter. The additional energy input, due to increased homogenization cycles, higher operating pressure and rise in temperature during high pressure homogenization process led to higher kinetic energy of the droplets, leading to particle coalescence with subsequent agglomeration and increased particle diameter (Mehnert & Mader, Citation2001).
Drug loading and entrapment efficiency
DL of all samples of SLN was found in the range of 9.20 ± 0.34%–10.68 ± 0.29% while EE was obtained between 55.23 ± 0.45% and 64.73 ± 0.34%, determined immediately after production was achieved (). Such low loading and encapsulation may be because of poor solubility of drug in lipid phase which tends to pull the drug out of lipid phase.
Regression equation (10) shows that both factors, X1 and X2, have positive effect on EE of SLNs. The correlation coefficient was found to be 0.8939. illustrates the influence of factors Pluronic F-68 concentration (X1) and stearylamine concentration (X2) on EE (Y3) of SLNs. Drug encapsulation was increased with increasing concentration of surfactant (Pluronic F-68) due to its tendency to reduce surface tension between aqueous and lipid phase. Also, increasing concentration of charge modifier (stearylamine) tends to increase drug encapsulation which may be due to hydrophobic nature of stearylamine and availability of more amount of lipid to encapsulate the drug (Paliwal et al., Citation2009).
Screening and optimization of cryoprotectant for lyophilization
Cryoprotectant concentration was optimized on the basis of macroscopic observation of each formulation sample for aggregation, reconstitution time and particle size distribution upon reconstitution (Abdelwahed et al., Citation2006). Among the various cryoprotectants screened for lyophilization, lactose at 5 wt% concentrations had given the acceptable product upon reconstitution with short reconstitution time, no much difference in particle size distribution (PDI) (data not shown) and no macroscopic aggregation, Thus, all formulation samples were lyophilized using 5 wt% of lactose as a cryoprotectant.
Characterization of lyophilized SLN formulation
Based on the results of particle size, ζ-potential and EE, formulation sample L5 was considered as optimized sample and subjected to make further evaluation including non-invasive in vivo pharmacodynamic studies.
Scanning electron micrographic studies
Shape and surface morphology of lyophilized SLN sample (L5) was examined by SEM analysis. SLNs appeared to be well spherical in shape with smooth surfaces, which would result in slow clearance and good deposition pattern in nasal cavity () (Wang et al., Citation2011).
Histopathological examination
Histopathological studies showed control (untreated) mucosa stained with HE () and effect of lyophilized SLN formulation (L5), 1 h after application, on sheep nasal mucosa (treated) (). Tissue examination showed appearance of ciliated respiratory epithelium and normal goblet cells. On comparison of treated nasal mucosa with control, no severe signs of damage such as appearance of epithelial necrosis or sloughing of epithelial cells were detected on the integrity of nasal mucosa.
Differential scanning calorimetry
reports thermal profiles of pure drug, drug–lipid physical mixture and lyophilized sample of drug-loaded SLNs. DSC thermogram of bulk ROPI HCl exhibited a sharp endothermic peak at 243.43 °C (curve a), indicating its melting point which comply with the literature. In curve b, representing thermogram of drug–lipid physical mixture, no significant thermal transitions were observed over entire range of thermoanalytical measurement (30–300 °C). First peak was obtained at 58.48 °C explaining lipid melting and second slightly broad peak at 238.90 °C explaining drug melting in mixture. Reduced sharpness of ROPI HCl endothermic peak in DSC thermogram (curve c) of lyophilized drug-loaded SLN formulation (L5) may be due to partial conversion of drug from crystalline to amorphous form concluding its partial adsorption on the surface of nanoparticles and partial encapsulation and/or accommodation inside the cavities of lipid matrix (Jain et al., Citation2009).
In vitro drug diffusion studies
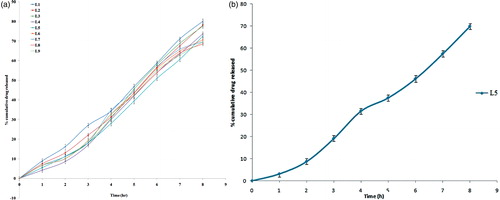
shows drug release profile of SLN formulation samples, after 8 h in phosphate buffer (pH 6.6). Release pattern was appeared to be sustained release, which may be due to loading and deposition of drug inside the cavities of Dynasan 114, which was thought to provide more space for accommodation of drug in SLN formulation. Slight difference in drug release among the various formulation samples (L1–L9) may be due to the presence of stearylamine, increased concentration of which again contributed to the sustained drug release because of its lipidic nature.
Drug release kinetics
Release data were fitted to kinetic models in order to investigate the drug release kinetics. It was found that, in vitro drug release was best fitted to zero-order kinetic model, as the plot showed highest linearity regression coefficient (R2) of 0.985 compared to first order (0.956) and the Higuchi model (0.959).
Drug release mechanism
Release data were also fitted to Peppa’s exponential model to investigate the mechanism of drug release from SLNs. The corresponding plot of Korsmeyer–Peppa’s equation indicated a good linearity of regression coefficients (R2 = 0.995). Release exponent (n) was found to be 1.385 while kinetic constant (k value) was found to be 1.231. The n value indicated that SLN formulation followed the non-Fickian super case II diffusion mechanism of drug release (Jain et al., Citation2009) that represents a linear, time-dependent (zero order) drug release governed by a combined mechanism of diffusion and erosion of glassy (non-swellable) systems (Lee et al., Citation1985).
Ex vivo drug permeation studies
SLN formulation sample (L5) was further subjected to ex vivo drug permeation studies across sheep nasal mucosa, as permeation barrier. The percentage of drug permeated after 8 h was found to be 69.88 ± 1.48%, after triplicate determinations. Permeation profile is shown in .
Stability studies
Formulation sample (L5) was subjected to stability studies (ICH [Q1A (R2)] guidelines) for a period of three months. Samples were evaluated for particle size and EE (%) at 1 month intervals. Particle size was increased from 66.22 ± 6.22 nm to 96.62 ± 6.70 nm while the EE (%) was reduced from 61.90 ± 0.18% to 41.37 ± 0.17% (). The obtained results revealed that there was no significant change in mean particle size after three month storage period at accelerated conditions. A slight reduction in EE may be due to expulsion of drug from lipid matrix during storage. This reduction in EE may be attributed to transition or modification of dispersed lipid from the metastable form to the stable form that may occur on storage due to smaller particle size and the presence of emulsifier (Venkateswarlu & Manjunath, Citation2004).
Table 4. Stability characteristics of lyophilized SLN sample (L5).
In vivo pharmacodynamic studies
The in vivo pharmacodynamic studies were performed in order to compare therapeutic activity (in terms of ability to reduce tremors) of lyophilized nasal SLN formulation (L5) against marketed oral formulation of ROPI HCl. Records suggested that therapeutic activity obtained with SLN formulation (L5) was surprisingly comparable to that with marketed oral formulation. SLN containing Formulation group showed significant reduction in tremors () when compared with marketed formulation containing Standard group against Toxicant group. Formulation group had shown better therapeutic activity in low dosage as compared to that given by Standard group with higher dose. This would help in decreasing the dose and dosing frequency, thereby maximizing the therapeutic index of selected drug candidate.
Table 5. Evaluation of anti-tremor activity in mice.
Data treatment and statistical analysis
Records suggested that the reduction in signs of Parkinsonism-like symptoms such as tremors, rigidity and immobility by lyophilized SLN nasal formulation was comparable to that of results obtained with marketed oral formulation of the same drug and found to be significant (p < 0.01) with marketed oral formulation.
Response surface analysis
demonstrate that increase in concentration of Pluronic F-68 (X1) and stearylamine (X2) increases the particle size (Y1) of SLNs. shows that the ζ-potential (Y2) increases with increase in concentration of stearylamine while decreases with increased concentration of Pluronic F-68. From , it is clear that the increased concentration of both X1 and X2 results in increased EE (Y3).
Conclusion
In the present investigation, we have fabricated triglyceride-based SLNs loaded with hydrophilic antiparkinsonian agent, ROPI HCl, using the emulsification-solvent diffusion technique. For long-term treatment, pharmaceutical formulations should provide extended drug permeation across nasal mucosa, so as to reduce the number of required daily doses, thus improving compliance, removing peak-to-valley fluctuations and reducing the risk of adverse effects. Pharmacodynamic studies have given surprising results. Records suggested that the therapeutic activity obtained with lyophilized lipid-based SLN was comparable to that with marketed oral formulation, which would help in decreasing the dose and dosing frequency, thereby maximizing the therapeutic index. Also, the drug releases from the solid-lipid matrix was found to be sustained release. The release data were best fitted to zero-order kinetics following the non-Fickian super case II diffusion of drug release, which is governed by combined diffusion and erosion of matrix.
Our observations suggest the feasibility of investigated drug delivery system for intranasal administration of ROPI HCl and triglyceride-based SLNs could be viewed as a promising alternative approach to conventional dosage forms. Existence of direct transport route from nasal cavity to brain, bypassing BBB, would offer an exciting mode of delivering antiparkinsonian drug, ROPI HCl, through safe and stable drug delivery system. However, extensive pharmacokinetic, cyto- or neurotoxicity and clinical studies need to be established so as to explore the investigated nasal drug delivery system and to boost its scale up for the market.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This research work is supported by research grants from All India Council for Technical Education (AICTE), India, under RPS (Research Promotion Scheme) (8023/BOR/RID/RPS-132/2009-10).
References
- Abdelwahed W, Degobert G, Stainmesse S, Fessi H. (2006). Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev 58:1688–713
- Belgamwar VS, Patel HS, Joshi AS, et al. (2011). Design and development of nasal mucoadhesive microspheres containing tramadol HCl for CNS targeting. Drug Deliv 18:353–60
- Chalikwar SS, Belgamwar VS, Talele VR, et al. (2012). Formulation and evaluation of Nimodipine-loaded solid lipid nanoparticles delivered via lymphatic transport system. Colloids Surf B: Biointerfaces 97:109–16
- Fukuzaki K, Kamenosono T, Nagata R. (2000). Effects of ropinirole on various Parkinsonian models in mice, rats, and cynomolgus monkeys. Pharmacol Biochem Behav 65:503–8
- Heurtault B, Saulnier P, Pech B, et al. (2003). Physico-chemical stability of colloidal lipid particles. Biomaterials 24:4283–300
- Hurley MJ, Jenner P. (2006). What has been learnt from study of dopamine receptors in Parkinson's disease? Pharmacol Ther 111:715–28
- Illum L. (2000). Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 11:1–18
- Jain SA, Chauk DS, Mahajan HS, et al. (2009). Formulation and evaluation of nasal mucoadhesive microspheres of Sumatriptan succinate. J Microencapsul 26:711–21
- Lee PI. (1985). Kinetics of drug release from hydrogel matrices. J Control Release 2:277–88
- Mahajan HS, Tatiya BV, Nerkar PP. (2012). Ondansetron loaded pectin based microspheres for nasal administration: in vitro and in vivo studies. Powder Technol 221:168–76
- Mehnert W, Mader K. (2001). Solid lipid nanoparticles Production, characterization and applications. Adv Drug Deliv Rev 47:165–96
- Mistry A, Stolnik S, Illum L. (2009). Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm 379:146–57
- Motwani SK, Chopra S, Talegaonkar S, et al. (2007). Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimization and in vitro characterisation. Eur J Pharm Biopharm 68:513–25
- Mukherjee S, Ray S, Thakur RS. (2009). Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Ind J Pharm Sci 71:349–58
- Paliwal R, Rai S, Vaidya B, et al. (2009). Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine 5:184–91
- Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR. (2011). Formulation, optimization and evaluation of spray-dried mucoadhesive microspheres as intranasal carriers for Valsartan. J Microencapsul 29:103–14
- Patel S, Chavhan S, Soni H, et al. (2011). Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target 19:468–74
- Rudra A, Rudra P, Chatterjee S, et al. (2007). Parkinson’s diseases and Anaesthesia. Ind J Anaesthesia 51:382–8
- Salawu F, Olokoba A, Danburam A. (2010). Current management of Parkinson’s disease. Ann Afr Med 9:55–61
- Shegokar R, Singh KK, Muller RH. (2010). Production & stability of stavudine solid lipid nanoparticles – from lab to industrial scale. Int J Pharm. 416:461–70
- Tan SW, Billa N, Roberts CR, Berley JC. (2010). Surfactant effects on the physical characteristics of Amphotericin B-containing nanostructured lipid carriers. Colloids Surf A: Physicochem Eng Asp 372:73–9
- Tas C, Ozkan CK, Savaser A, et al. (2006). Nasal absorption of metoclopramide from different Carbopol 981 based formulations: in vitro, ex vivo and in vivo evaluation. Eur J Pharm Biopharm 64:246–54
- Trotta M, Debernardi F, Caputo O. (2003). Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int J Pharm 257:153–60
- Varshosaz J, Tabbakhian M, Mohammadi MY. (2010). Formulation and optimization of solid lipid nanoparticles of buspirone HCl for enhancement of its oral bioavailability. J Liposome Res 20:286–96
- Venkateswarlu V, Manjunath K. (2004). Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release 95:627–38
- Vogel HG, ed. (2002). Psychotropic and neurotropic activity. In: Drug discovery and evaluation: pharmacological assays. 2nd ed. New York (NY): Springer, 577–81
- Wang Q, Gong T, Sun X, Zhang Z. (2011). Structural characterization of novel phospholipid lipid nanoparticles for controlled drug delivery. Colloids Surf B: Biointerfaces 84:406–12
- Zhang X, Liu J, Qiao H, et al. (2010). Formulation optimization of dihydroartemisinin nanostructured lipid carrier using response surface methodology. Powder Technol 197:120–8