Abstract
The glutathione-conjugated bovine serum albumin (BSA) nanoparticles were constructed in the present exploration as a novel biodegradable carrier for brain-specific drug delivery with evaluation of its in vitro and in vivo delivery properties. BSA nanocarriers were activated and conjugated to the distal amine functions of the glutathione via carbodiimide chemistry using EDAC as a mediator. These nanoparticles were characterized for particle shape, average size, SPAN value, drug entrapment and in vitro drug release. Further, presence of glutathione on the surface of BSA nanoparticles was confirmed by Ellman’s assay, which has suggested that approximately 750 units of glutathione were conjugated per BSA nanoparticle. To evaluate the brain delivery properties of the glutathione-conjugated BSA nanoparticles fluorescein sodium was used as a model hydrophilic compound. Permeability and neuronal uptake properties of developed formulations were evaluated against the MDCK-MDR1 endothelial and neuro-glial cells, respectively. The permeability of glutathione-conjugated BSA nanoparticles across the monolayer of MDCK-MDR1 endothelial tight junction was shown significantly higher than that of unconjugated nanoparticles and fluorescein sodium solution. Similarly, glutathione-conjugated nanoparticles exhibited considerably higher uptake by neuro-glial cells which was inferred by high fluorescence intensity under microscope in comparison to unconjugated nanoparticles and fluorescein sodium solution. Following an intravenous administration, nearly three folds higher fluorescein sodium was carried to the rat brain by glutathione-conjugated nanoparticles as compared to unconjugated nanoparticles. The significant in vitro and in vivo results suggest that glutathione-conjugated BSA nanoparticles is a promising brain drug delivery system with low toxicity.
Introduction
The brain is the most delicate organ of the human body; hence evolution of perplexed human anatomy established very efficient ways to protect it. These protective mechanisms also restrict the access of majority of the active pharmaceutical agents to the brain (Catuogno et al., Citation2012), which hinder the therapeutic interventions (Banks, Citation2012; Benarroch, Citation2012) and make management of brain disorders very refractory. Permeability of majority active pharmaceuticals to the brain is altered principally due to blood brain barrier (BBB) (McDannold et al., Citation2012). The BBB is a complex architecture of capillary endothelial cells, astrocytes and pericytes lining the brain microvessels, which are coupled by tight junction proteins, i.e. occludin and claudin and thus acts as a major exchange interface for para-cellular as well as trans-cellular transport of neurotherapeutics between the blood and the brain (Bowman et al., Citation2007). Additionally presence of various efflux transport systems, degrading enzymes and a reduced level of pinocytosis limit the passage of molecules between blood and brain. Hence, targeting active pharmaceutical drugs to the brain is crucial for the effective management of brain diseases (Muro et al., Citation2006; Patel et al., Citation2011; Sunitha et al., Citation2011).
To ameliorate delivery of drugs to the brain, various techniques have been explored amongst them targeting of drug to the brain by the use of nanocarriers is found to be most promising approach. (Liu & Chen, Citation2005; Pardridge, Citation2005). Hence, current research is planned with the objective to develop a novel polymeric colloidal carriers conjugated with endogenous tripeptide, i.e. glutathione to improve delivery of drugs to the brain. In the present work, we have explored bovine serum albumin as a matrix forming material which is approved by the USFDA and thus it endows good safety to our system as well as easy to fabricate with relatively simple process (Kumari et al., Citation2010; Acharya & Sahoo, Citation2011). The prepared BSA nanoparticles were decorated with glutathione which acts as an endogenous ligand for the glutamate receptors that are abundantly expressed in the brain and at the BBB (Krizbai et al., Citation1998; Chin et al., Citation2006; Benarroch, Citation2010). Further, glutathione also reported to be endogenous peptide for active uptake transporters expressed at the BBB (Kannan et al., Citation1990, Citation1992; Zlokovic et al., Citation1994), which can potentially target the drug-loaded nanoparticles to the brain. Additionally glutathione also regulates the permeability of BBB through its action on BBB tight junction proteins, i.e. occludin and claudin (Martin & Teismann, Citation2009; Reijerkerk et al., Citation2010). An improved efficacy using glutathione PEGylated (GSH-PEG) liposomes has already been shown for opioid peptide and methylprednisolone in animal models (Gaillard et al., Citation2012; Lindqvist et al., Citation2012), which further confirms the potential of glutathione as a transport vector for drug delivery to the brain. Further a clinical trial for determining the safety, tolerability and pharmacokinetics of glutathione-conjugated PEGylated liposomes containing doxorubicin in patients with solid tumors and brain metastases or recurrent malignant glioma is currently ongoing [clinicaltrials.gov identifier NCT01386580], which clearly demonstrated clinical significance of glutathione-conjugated nanocarriers for brain-specific drug delivery (Lindqvist et al., Citation2012).
In the present investigation, glutathione was coupled to the surface of BSA nanoparticles via carbodiimide conjugation chemistry. To evaluate the brain targeting efficacy of glutathione-conjugated BSA nanocarriers, a hydrophilic fluorescent marker, i.e. fluorescein sodium was chosen as a model compound. Fluorescein sodium is pharmacologically inactive, freely soluble salt with a molecular weight of 330 Da of the negatively charged species which proves its lack of ability to easily cross the tightly fenestrated endothelial cells in the BBB (Bagger & Bechgaard, Citation2004; Fluorescein Sodium Solution for Injection, Novartis). The uptake and transport of fluorescein sodium solution, unconjugated and glutathione-conjugated nanoparticles were determined in primary neuro-glial cell culture and MDCK cells with P-gp over expressing multi-drug resistant MDR1-MDCK cell lines, respectively. The CNS bioavailability and biodistribution of the fluorescein sodium following intravenous injection of different formulations loaded with fluorescein sodium into male Wistar rats was determined. Distribution of the fluorescein sodium in brain and other organs were detected using a spectrofluorimetric technique. Results of the in-vivo studies demonstrated that brain uptake of fluorescein sodium were significantly increased by entrapment in glutathione-conjugated nanoparticles as compared to the unconjugated nanoparticles and fluorescein sodium solution.
Materials and methods
Materials
Bovine Serum Albumin (BSA), Fraction V (Molecular Weight 66 kDa) was procured from Himedia (Mumbai, India). Fluorescein sodium and glutathione were purchased from Sigma Aldrich (Bangalore, India). Pluronic ®P85 and Brij 35 were obtained as gift samples from Torrent Research Centre (Ahmedabad, India). EDAC was purchased from MP Biomedicals (Bangalore, India). Glutaraldehyde, acetone, dichloromethane and ethanol were purchased from S.D. Fine-Chem (Ahmedabad, India). All other reagents used were of analytical grade. Double distilled water used during studies was filtered through 0.22-μm filter (Millipore, Mumbai, India).
Animals
Male Wistar rats weighing 250 ± 50 g were utilized for the biodistribution study and procured from the animal house of Institute of Pharmacy, Nirma University after approval from the Institutional Animal Ethics Committee (Project No. IPS/PCOG/PhD10-11/2002/25). All the experimental work was done as per the CPCSEA guidelines of India.
Preparation of fluorescein sodium-loaded BSA nanoparticles
Fluorescein sodium-loaded BSA nanoparticles were prepared by a modified coacervation technique described by Jun & coworkers (Citation2011). One hundred milligrams of BSA was dissolved in purified water containing stabilizer, i.e. Pluronic ®P85 (1.5%w/v) and Brij 35 (0.25% w/v). Twenty milligrams of fluorescein sodium was suspended in a BSA solution containing stabilizer and incubated for 30 min. Subsequently anhydrous ethyl alcohol was carefully injected to the above mixture till the solution became turbid. The mixture was stirred using blade homogenizer at 2000 rpm for 15 min (Remi Equipments, Mumbai, India) followed by sonication for 5 min at a pulse of 10 s (Trans-o-sonic, Mumbai, India) to precipitate BSA as nanoparticles containing entrapped fluorescein sodium. The prepared BSA nanoparticles were rigidized by an addition of 0.6 mL of 25% v/v glutaraldehyde, which promotes cross-linking reaction. The resulting nanoparticle dispersion was stirred for 8 h at room temperature till the completion of cross-linking reaction. Nanoparticles were recovered by dual centrifugation process at 15 000 g for 30 min (Biolab, Mumbai, India) from the dispersion followed by washing with double distilled water to remove excess of stabilizer, glutaraldehyde and free fluorescein sodium. Finally the nanoparticle formulation was lyophilized for 24 h (EIE Instruments Pvt. Ltd., Ahmedabad, India) and stored at 4 °C in a sealed vial till further use.
Glutathione conjugation to BSA nanoparticles
Glutathione was conjugated to the surface of fluorescein sodium-loaded BSA nanoparticles by two step carbodiimide chemistry reactions. The carboxylic groups on the surface of fluorescein sodium-loaded BSA nanoparticles (60 mg) were activated in 2.4 mL phosphate buffer (pH 4.0, 100 mM) by drop-wise addition of an equal volume of EDAC solution (50 mg/mL). The mixture was incubated at room temperature for 45 min. The unreacted EDAC was removed by centrifugation and the nanoparticles were resuspended in 2.4 mL phosphate buffer (pH 7.4) (Nobs et al., Citation2003; Jin et al., Citation2011). The same volume of glutathione solution (50 mg/mL) was added to the dispersion containing the activated nanoparticles and the mixture was stirred gently at room temperature for 6 h. The conjugated nanoparticles were centrifuged for 30 min at 15 000 g (Biolab, Mumbai, India), to remove excess of glutathione. Finally, glutathione-conjugated BSA nanoparticles were lyophilized for 24 h (EIE Instruments Pvt. Ltd., India) and stored at 4 °C in a sealed vial till further use.
Characterization of glutathione-conjugated and unconjugated nanoparticles
Determination of process yield
The process yield of glutathione-conjugated and unconjugated BSA nanoparticles was determined as weight of the final product after drying, with respect to the initial total amount of drug, polymer and other solid materials used for the preparation of nanoparticles.
Determination of fluorescein sodium loading capacity
The content of fluorescein sodium in the BSA nanoparticles was determined using the procedure described by Kashi & coworkers (Citation2012). Briefly 20 mg of glutathione-conjugated and unconjugated nanoparticles containing fluorescein sodium were sonicated with a 50 mL phosphate buffer of pH 7.4 for 12 h and then samples were centrifuged at 15 000 g for 30 min and content of fluorescein sodium in the supernatant layer was analyzed by a spectrofluorimeter (Jasco Ltd., Tokyo, Japan) (494 nm/516 nm: Excitation wavelength/Emission wavelength) against dummy nanoparticles as reagent blanks and treated similarly to the fluorescein sodium-loaded nanoparticles.
Particle size analysis
The mean particle diameter of the BSA nanoparticles was determined by a dynamic light scattering technique using a particle size analyzer (Nanotrac NPA 252, Meerbusch, Germany) at a fixed angle of 180° at 25 °C. The dried nanoparticle samples were dispersed in deionized water and sonicated gently for 3 min and the resultant dispersion was utilized for the measurement of mean particle diameter. Values of particle diameter were represented as mean ± standard deviation (SD) from three replicate samples. The width of the size distributions were characterized by the SPAN value using following formula, where D90%, D50% and D10% are the mean diameter at which 90%, 50% and 10% (Cumulative %) of the nanoparticles were counted and calculated.
Particle shape and morphology analysis
The surface topology and morphology of plain BSA nanoparticles, fluorescein sodium-loaded and glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles were observed using a scanning electron (SEM) microscope. Briefly, dry nanoparticles were dispersed in methanol and dried onto a metal grid followed by gold sputtering using argon plasma to coat the surface of nanoparticles for better observation of its surface. The stub was fixed into a sample holder and placed in the vacuum chamber and observed under low vacuum (10−3 torr) (Jayant & Srivastava, Citation2007).
Zeta potential analysis
Zeta potentials of glutathione-conjugated and unconjugated BSA nanoparticles loaded with fluorescein sodium were measured in deionized distilled water using Zetasizer 3000 HSA (Malvern Instruments, Malvern, UK).
Quantitative estimation of glutathione in conjugated BSA nanoparticles with Ellman’s reagent
Approximate number of glutathione units conjugated to the surface of each nanoparticle unit was determined using Ellman’s assay. Because glutathione unit contains thiol group which specifically reacts with Ellman’s reagent and gives yellow color solution, intensity of which is proportional to the concentration of thiol groups. Hence, degree of glutathione conjugation on BSA nanoparticles was measured using Ellman’s assay against plain BSA nanoparticles treated similarly as glutathione-conjugated nanoparticles which served as reagent blank. Briefly, 30 mg of glutathione-conjugated and unconjugated BSA nanoparticles were dispersed in 2.5 mL of phosphate buffer pH 7.2 containing 1 mM EDTA. Ellman’s reagent (0.25 mL, 0.04% w/v) (5,5′-Dithio-bis (2-nitrobenzoic acid)) was added to the nanoparticle dispersion and incubated for 15–30 min at room temperature under mild stirring. After incubation, the mixture was centrifuged at 15 000 g for 15 min to collect supernatant layer for estimation of thiol functionality. To the supernatant layer, 2 mL of 0.3 M sodium hydrogen phosphate solution was added and concentration of glutathione in the sample was determined spectrophotometrically at λmax of 412 nm. The difference in the amount of thiol functions present in the glutathione-conjugated and unconjugated nanoparticle samples revealed the approximate number of glutathione unit anchored to the surface of BSA nanoparticles and calculated using the formula described by Nobs (Nobs et al., Citation2003). According to the formula, density of the BSA nanoparticles was determined using the mercury porosimeter analysis and found to be 1.2 g/cm3, whereas shape of synthesized particles was found to be spherical from the SEM imaging and hence mean radius of particles calculated using mean diameter obtained from particle size analysis.
where, n = amount of thiol functionality, i.e. glutathione unit per nanoparticle; a = mol of –SH per gram of BSA; d = density of nanoparticles; r = mean radius of nanoparticles; N = Avogadro number (6.011 × 1023).
In-vitro release studies
Release pattern of fluorescein sodium from the optimized nanoparticle formulations was studied using the dialysis bag diffusion technique in phosphate buffer with pH 4 and 7.4, which represents the pH of endo-lysosomal compartment and physiological compartment, respectively. Nanoparticles equivalent to 1 mg of fluorescein sodium was placed in a dialysis bag, which was sealed at both ends with clips. The dialysis bag was immersed in the receptor compartment containing 20 mL of either of the aforementioned phosphate buffer and stirred continuously at 75 rpm with controlled temperature (37.0 ± 0.5 °C). The receptor compartment was closed with lid to prevent evaporative loss of the dissolution medium. Samples were withdrawn at predetermined time intervals and the same volume was replenished with fresh dissolution medium. The samples were centrifuged for 8 min. at 15 000 g and supernatant was analyzed by spectroflourimetry against dummy nanoparticles as reagent blanks (Lu et al., Citation2005; Jayant & Srivastava, Citation2007).
Development of cell-culture
MDCK-MDR1 cell line
Madine darby canine kidney cells over-expressing P-gp (MDCK-MDR1) cells were obtained from the national center for cell science (NCCS, Pune). MDCK-MDR1 cells are capable of polarized growth with the formation of tight junctions, which along with the expression of P-gp, serve as a suitable model for BBB transport and permeability studies (Garberg et al., Citation2005). The MDCK-MDR1 cells were cultured in DMEM media containing 15% fetal calf serum, 1% streptomycin and supplemented with 80 ng/mL of colchicine in order to maintain high levels of P-gp expression. The transfected cells were incubated in 5% CO2 at 37 °C for 72 h wherein medium was replaced every other day (Rao et al., Citation2008).
Primary neuro-glial co-culture
Primary cortical neuro-glial co-cultures were obtained from embryos of 16–18 days-old Wistar rats. Briefly 16–18 d after gestation period animal was anaesthetized and fetuses are removed from the uterine horns and collected in petri dish containing cold HBSS on ice. Cortices were isolated from the embryonic brains and tissues were pooled separately while meninges and blood vessels were cleared away. Isolated cortices were stored in calcium-free HBSS and tissues were dissociated using 0.1% w/v trypsin solution for about 20 min at 37 °C and then suspended in DMEM media supplemented with 10% v/v fetal bovine serum and 10% v/v horse serum. Dissociated cells were then placed on the poly-L-lysine coated cover slips in 24-well plates at a density of 5 × 105 cells per cover slip. After 48 h of plating, cytosine arabinocyde was added at a concentration of 10 μM and the medium was changed to DMEM with 10% v/v horse serum 24 h later and subsequently at every 3 to 4 d. All the uptake studies were carried out on the confluent cells, which were achieved after 10 d of incubation period.
In-vitro cytotoxicity of nanoparticle formulation on MDCK-MDR1 cell line and primary neuro-glial co-culture
To evaluate in-vitro cytotoxicity of fluorescein sodium, fluorescein sodium-loaded glutathione-conjugated and unconjugated BSA nanoparticles; cell viability assay was performed by methylthiazoletetrazolium (MTT) hydrolysis using MDCK-MDR1 and primary neuro-glial cell line. Both types of cells were seeded separately on 96-well plates at a density of 10 000 cells/well and cultured in 150 μL of cell growth medium for 24 h at 37 °C in 5% CO2 incubator. After pre-incubation with Hanks’ balanced salt solution (HBSS) at pH 7.4, the medium was replaced with fluorescein sodium solution, fluorescein sodium-loaded BSA nanoparticles and glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles samples to obtain a concentration of 0.01 to 2.0 mg/mL in HBSS and was incubated further for 72 h at 37 °C and 5% CO2 environment. The incubated nanoparticles were treated with 100 μL of (MTT) (0.5 mg/mL in RPMI medium without phenol red) solution, for 3 h at 37 °C. The test solution was decanted and 100 μL of DMSO was added to solubilize the blue colored formazan crystals formed by viable cells during incubation process. The absorbance of the formazan was measured at 570 nm using an ELISA plate reader and the cell survival was calculated as percentage of absorbance in comparison with that of the control, which comprised of the cells without exposure to the samples. The IC50 and IC20 represented the concentrations at which 50% and 20% of cell growth were inhibited by test samples (Lu et al., Citation2005; Rao et al., Citation2008; Mittnacht et al., Citation2010).
Fluorescein sodium permeability studies across monolayer of MDCK-MDR1 cells
The transport of fluorescein sodium, glutathione-conjugated and unconjugated fluorescein-loaded BSA nanoparticles was studied using MDCK-MDR1 cells. The cells were cultured on trans-well cell culture inserts (0.4 μm pore size, 0.33 cm2 growing area per well) using MDCK-MDR1 at a cell density of 1 × 105 cells/cm2 in 24-well plates. Cell culture medium was added to the apical and basolateral chambers and medium was changed every other day. The experiments were carried out after the cell layer reached the confluence stage. During the culture period, the cells grown on insert membranes were tested for monolayer integrity by measuring their transepithelial electrical resistance (TEER) using a Millicell ERS (Millipore, Mumbai, India). The experiments were conducted only with cells that had a TEER of >200 Ω cm2 after correcting for the resistance obtained in blank insert membranes. Prior to the permeability assay, existing growth medium was removed and the cells were washed with HBSS. To perform the apical-basolateral transport studies, the donor side of the chamber was filled with 100 μL of test compound solution/dispersion in assay buffer. The receiver side of the chamber was filled with 500 μL of assay buffer containing 150 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 10 mM HEPES, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2 and 0.4 mM K2HPO4 adjusted to pH 7.4. The sandwich assembly was incubated at 37 °C and 100 μL of the sample was withdrawn from the receiver compartments at predetermined time interval and replenished with the same volume of fresh assay buffer to maintain sink condition. The control experiments were carried out by following the same procedure for all the formulations using trans-well inserts without the cells. The collected luminal samples from each well were analyzed using spectroflurimetry. The experiment was performed in triplicate and permeability of fluorescein sodium from the different formulation was calculated using the following formula for apparent permeability co-efficient (Lu et al., Citation2005; Kratzer et al., Citation2007; Rao et al., Citation2008).
where, Papp = Apparent permeability co-efficient; V = Volume of donor compartment; A = Surface area of insert membrane; MR = Concentration of compound in receiver chamber; MD = Concentration of compound in donor chamber; t = Time (sec).
Uptake of nanoparticle formulation by primary neuro-glial co-culture
The primary neuro-glial cells with 80% confluency were used for uptake studies of fluorescein sodium-loaded nanoparticles. Briefly, all the formulations were suspended in double distilled water with the aid of mild sonication followed by dilution with RPMI medium to attain final concentration of 100 μg/mL. The growth medium from the primary neuro-glial co-culture plated well was aspirated and 10 μL of either of the suspended formulations, i.e. fluorescein sodium solution, glutathione-conjugated and unconjugated nanoparticle suspension was added to the well. One group was used as a negative control containing only 10 μL of RPMI media. All the plates were then incubated in a humidified atmosphere with 5% CO2 environment at 37 °C for 4 h. After the exposure, the formulation medium was aspirated and the cells were washed twice with RPMI medium. After washing, the cells were visualized under inverted microscope (Olympus, NY) in phase contrast and fluorescence mode to determine internalization of different formulations loaded with fluorescein sodium in primary neuro-glial co-culture cell model.
In-vivo study
The brain targeting efficiency of glutathione-conjugated nanoparticles was evaluated in rats. Healthy adult Wistar rats weighing 180 to 220 g were used for the study. The animals were maintained on a 12:12 h light/dark cycle in large spacious cages throughout the experimental period. The animals were provided with food and water ad libitum. The animals were divided into three groups, each group contained six rats. The group 1 received the fluorescein sodium solution, group 2 administered with fluorescein sodium-loaded BSA nanoparticles and group 3 received glutathione-conjugated BSA nanoparticles loaded with fluorescein sodium. For the in-vivo experiments, formulations were dispersed in sterile phosphate buffer saline. Formulations were injected intravenously in a dose equivalent to 1 mg/kg body weight of fluorescein sodium (Bagger & Bechgaard, Citation2004). One hour post-injection all the rats were sacrificed and different organs like brain, liver, lungs, spleen and kidneys were removed and washed thoroughly with phosphate buffer saline (pH 7.4) for removal of surface adsorbed drug followed by weighing and storage at −20 °C until further analysis. The collected organs were homogenized in ice-cold phosphate buffer saline with pH 7.4 to extract the fluorescein sodium. The samples were centrifuged to remove organ debris and supernatants were collected for analysis of fluorescein sodium using spectrofluorimeter. The content of fluorescein sodium in each organ was calculated by comparing fluorescent intensity of the test sample with the standard against blank organ homogenate (Umnarat, Citation2006; Wilson et al., Citation2008).
Statistical analysis
The results were presented as mean ± SD calculated over at least three data points. Statistical significance of differences was processed by an one-way analysis of variance and a subsequent post hoc Tukey comparison. A value of p < 0.05 was considered to be statistically significant. All the statistical calculations were performed using the software GraphPad Prism 5.0 (La Jolla, CA).
Results
Physico-chemical properties of fluorescein sodium-loaded BSA nanoparticles
The mean particle diameter of optimized fluorescein sodium-loaded BSA nanoparticles was found to be 235.7 ± 8.6 nm (SPAN = 0.46) as shown in suggesting the uniformity in particle size distribution with percentage entrapment of fluorescein by 60.69 ± 6.03 (). Formulation parameters such as drug to polymer ratio, electrolyte addition and pH alteration were screened to evaluate their influence on different properties of nanoparticles. BSA nanoparticles were prepared at different pH, i.e. 2–3 and 6.5–7 to evaluate its effect on encapsulation of fluorescein sodium in BSA nanoparticles. Maximum entrapment efficiency of fluorescein sodium (67.73 ± 5.12%) was observed at acidic pH (2–3) but processing at acidic pH tend to form aggregated particles and hence alteration of pH was restricted to improve entrapment of fluorescein sodium in BSA nanoparticles and further experimentation was conducted at pH 6.5–7. The effect of fluorescein sodium to polymer ratio (1:1 and 1:5) on the process yield, entrapment efficiency and on mean particle diameter was studied. The entrapment efficiency, process yield and particles size were found to be satisfactory at fluorescein sodium to polymer ratio 1:5; while it was found to be non-satisfactory with ratio 1:1. Hence subsequent experiments were carried out with fluorescein sodium to polymer ratio 1:5. BSA nanoparticles were also prepared by altering the osmolarity of external phase by adding different concentrations of electrolyte, i.e. NaCl. Results of the experiments revealed that formulations prepared with external phase containing 0.05 M and 0.1 M of NaCl yielded satisfactory BSA nanoparticles with entrapment efficiency of fluorescein sodium by 52.80 ± 5.71 and 60.69 ± 6.03, respectively. With reference to above observations further formulation were prepared using external phase containing 0.1 M NaCl. It was further concluded from SEM images of glutathione-conjugated and unconjugated nanoparticles loaded with fluorescein sodium (), which showed smooth surface with spherical shape and slight aggregation that can be easily redispersed in suitable solvent.
Figure 1. Particle size distribution of (a) fluorescein sodium-loaded BSA nanoparticles; (b) glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles.
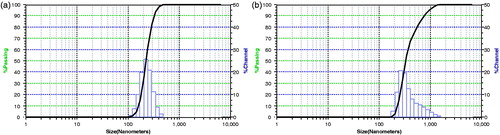
Figure 2. SEM image of (a) fluorescein sodium-loaded BSA nanoparticles; (b) glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles.
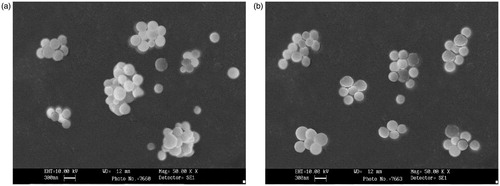
Table 1. Optimization of formulation variables to improve entrapment of fluorescein sodium inside the BSA nanoparticles.
Conjugation of fluorescein sodium-loaded BSA nanoparticles with glutathione
Glutathione was conjugated on the surface of BSA nanoparticles via carbodiimide chemistry using EDAC as a mediator. Conjugation reaction was optimized using different concentrations of EDAC and varied reaction time. and depict the effect of concentration of EDAC and reaction time on conjugation of glutathione with BSA nanoparticles. Conjugation reaction was optimized at EDAC concentration (60 mg) and reaction time (45 min), which yielded BSA nanoparticles with approximately 755 ± 187 units of anchored glutathione per nanoparticle. The optimized glutathione-conjugated nanoparticles demonstrated the fluorescein sodium entrapment efficiency of 53.77 ± 2.2% and mean particle diameter of 270.9 ± 3.78 nm (SPAN = 0.58) as shown in .
Table 2. Effect of amount of EDAC on conjugation of glutathione with BSA nanoparticle.
Table 3. Effect of EDAC reaction time on conjugation of glutathione with BSA nanoparticles.
In-vitro release studies
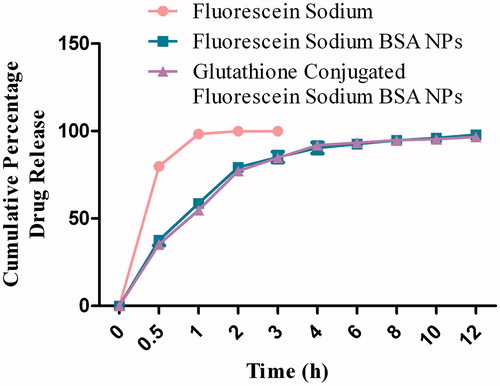
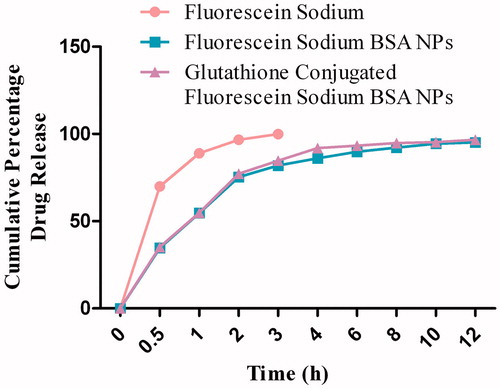
The release profile of fluorescein sodium from BSA nanoparticles was observed with initial rapid drug release followed by a slower exponential release of the remaining drug over the following 12 h. The control profile (fluorescein sodium) showed that almost 99% of the drug was released within first hour in phosphate buffer media with pH 7.4 (), while 88% of the drug was released within first hour using phosphate buffer with pH 4 (). The cumulative percentage release of fluorescein sodium from different formulations was found to be from 95% to 98% within 12 h. The release kinetics for fluorescein sodium (control) and for optimized formulation was determined by applying different drug release models. Fluorescein sodium was dissolved as per the first-order kinetic model, while the glutathione-conjugated and unconjugated nanoparticles showed best fit for the Weibull model.
In-vitro cytotoxicity of nanoparticle formulation on MDCK-MDR1 cell line/primary neuro-glial co-culture
MTT assay on MDCK-MDR1 cells demonstrated the influence of fluorescein sodium, glutathione-conjugated and unconjugated fluorescein-loaded BSA nanoparticles on cell viability depending on their concentrations ranging from 0.01 to 1 mg/mL (). It was observed from the cell viability–concentration graph that the IC20 of fluorescein sodium solution, unconjugated and glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles was found to be 250, 750 and 1000 μg/mL, respectively. IC50 of fluorescein sodium solution was found 1000 μg/mL and more than 2000 μg/mL for glutathione-conjugated and unconjugated fluorescein sodium-loaded BSA nanoparticles. Results of these studies revealed that glutathione-conjugated BSA nanoparticles presented no toxicity in the concentration range of 250–1000 μg/mL as indicated by more than 80% cell viability. Hence, in vitro permeability studies were carried out with formulations containing fluorescein sodium equivalent to concentration 100–250 μg/mL, at which it has not presented any significant toxicity.
Figure 5. Effect of fluorescein sodium-loaded formulations on (a) MDCK-MDR1 cell viability; (b) primary neuro-glial cell viability.
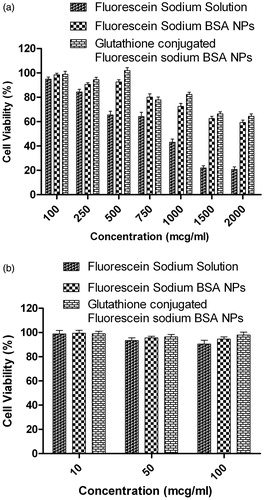
Similar type of viability assays were also carried out on primary neuro-glial co-culture cells using all three formulations at concentration 10, 50 and 100 μg/mL. The cells exhibited more than 80% cell viability () even at highest test study concentration for all three formulations. Hence subsequent uptake assays were carried out at formulation concentration of 100 μg/mL.
Fluorescein sodium permeability studies across monolayer of MDCK-MDR1 cells
The permeability results of fluorescein sodium across the monolayer of MDCK-MDR1 are represented in and . revealed the transport pattern of different formulations equivalent to 100 μg/mL of fluorescein sodium at different time intervals, i.e. 1, 2, 3 and 4 h. The Papp value calculated from the transport studies demonstrated greater permeability of fluorescein sodium when entrapped in glutathione-conjugated BSA nanoparticles compared to fluorescein sodium in either solution or in unconjugated BSA nanoparticles. The high TEER value (310 ± 11 Ω cm2) indicated that the monolayer integrity was maintained for the entire duration of the experiment. Glutathione-conjugated BSA nanoparticles showed almost 2.4 folds higher transport of fluorescein sodium across the MDCK-MDR1 monolayer in comparison to the unconjugated BSA nanoparticles.
Figure 6. Apparent permeability of fluorescein sodium-loaded formulations through MDCK-MDR1 cell monolayer at different time interval.
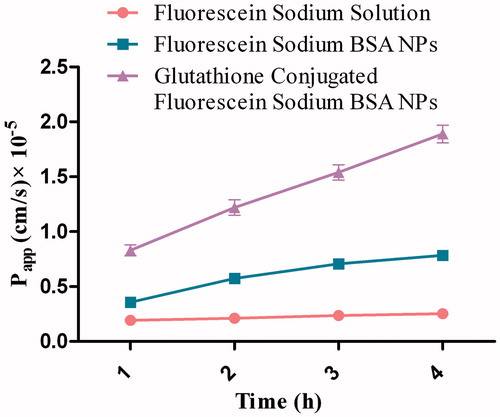
Table 4. The apparent permeability (Papp) of fluorescein sodium solution and glutathione-conjugated and unconjugated BSA nanoparticles loaded with fluorescein sodium across in vitro MDCK-MDR1 cell monolayer (n = 3).
Uptake of nanoparticle formulation by primary neuro-glial co-culture
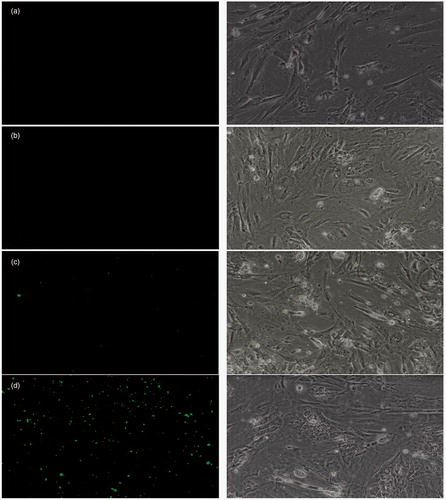
Primary rat cortical neurons were incubated with 100 μg/mL BSA nanoparticles for 4 h at 37 °C under 5% CO2 environment. After 4 h cells, were washed and examined under microscope, where intense green fluorescence was observed in the cells as dotted pattern with glutathione-conjugated nanoparticles. Such fluorescence pattern was not observed in the cells treated with fluorescein sodium solution as well as controlled cells and cells incubated with unconjugated nanoparticles exhibited very less fluorescence intensity in the cells (). Overall uptake of fluorescein sodium-loaded glutathione-conjugated BSA nanoparticles by the primary neuro-glial cultures revealed the specific role of glutathione as a vector to improve internalization of nanoparticles within the neuro-glial cell culture.
Figure 7. Uptake of fluorescein sodium-loaded formulations by primary neuro-glial cells under fluorescent (left) and phase contrast (right) microscopy. (a) Blank control, (b) Fluorescein sodium solution, (c) Fluorescein sodium-loaded BSA nanoparticles, (d) Glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles.
In vivo study
The concentration (ng/g organ weight) of fluorescein sodium in different organs 1 h post-intravenous administration in Wistar rats were presented in and . Localization of fluorescein sodium in different organ was found varied depending on the type of formulation administered. The glutathione-conjugated nanoparticles significantly increased the uptake of fluorescein sodium into the brain in comparison with the free fluorescein sodium alone and fluorescein sodium-loaded BSA nanoparticles. The concentration of fluorescein sodium achieved in the brain after administration of fluorescein sodium solution, fluorescein sodium-loaded BSA nanoparticles and glutathione-conjugated fluorescein sodium-loaded BSA nanoparticles was found to be 159.90 ± 24.40, 476.41 ± 30.88 and 1439.20 ± 35.25 ng/g of brain, respectively. The BSA nanoparticles conjugated with glutathione increased the concentrations of fluorescein sodium in the brain substantially when compared to the fluorescein sodium solution. Further, it was observed that nanoparticulate formulations exhibited higher uptake by reticulo endothelial system (RES) containing organs like liver, spleen, lungs etc. that might be due to the mean particle diameter of formulation less than 5 μm and these observations were found to be in accordance with previously published report (He et al., Citation2010; Elsabahy & Wooley, Citation2012; Yu et al., Citation2012). The maximum accumulation of fluorescein sodium following intravenous administration of fluorescein sodium-loaded BSA nanoparticles was observed in liver (2446.70 ± 110.67 ng/g of liver), but conjugation of fluorescein sodium-loaded BSA nanoparticles with glutathione significantly reduced (1986.05 ± 70.94 ng/g of liver) (p < 0.001) the uptake of fluorescein sodium by liver.
Figure 8. Fluorescein sodium concentrations (ng/mL) in different organs after intravenous injection of nanoparticles formulations.
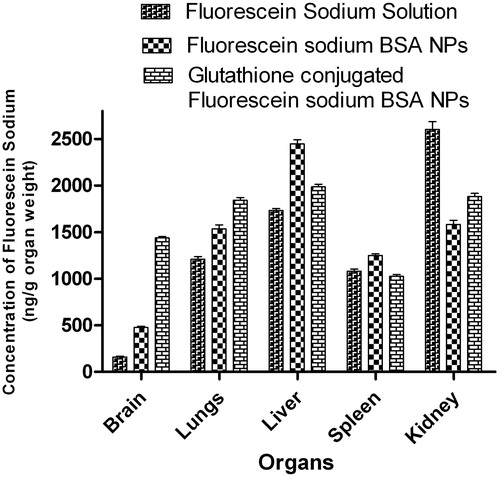
Table 5. Fluorescein sodium concentrations (ng/g organ weight) in different organs after intravenous injection of BSA nanoparticles.
Discussion
Physico-chemical properties of fluorescein sodium-loaded BSA nanoparticles
The experimental results clearly indicated that increased entrapment efficiency of fluorescein sodium in BSA nanoparticles was observed, when available in unionized form in the system. Maximum entrapment efficiency of fluorescein sodium was observed at acidic pH (2–3), because of its least solubility at this pH (Govender et al., Citation1999). However, lowering of pH lead to formation of aggregated nanoparticles and hence this approach was excluded for further formulation development (Peltonen et al., Citation2004; Patel & Acharya, Citation2012). Further, the entrapment efficiency of fluorescein sodium was also modulated by varying drug to polymer ratio and satisfactory entrapment of fluorescein sodium in BSA nanoparticles was observed at a drug to polymer ratio 1:5; while it was found unsatisfactory with ratio 1:1. The lower entrapment efficiency observed at fluorescein sodium to polymer ratio (1:1) might be due to formation of more porous polymer matrix, as penetration of water in the BSA matrix caused solubilization and subsequent leaching of the drug solution, which might have resulted in porous BSA nanoparticles with lesser drug content (Witschi & Doelker, Citation1998; Lamprecht et al., Citation2000). Entrapment efficiency of fluorescein sodium was also influenced upon addition of electrolyte, i.e. NaCl as a modulator of osmotic gradient between the inner and outer phases (Uchida et al., Citation1996; Freytag et al., Citation2000). Addition of NaCl to the external phase with concentrations 0.05 and 0.1 M resulted in considerable improvement in the entrapment efficiency inside the BSA nanocarriers by 52.80 ± 5.71% and 60.69 ± 6.03%, respectively, indicating that electrolyte addition decreased solubility of fluorescein sodium in outer phase. By considering the above results, concentration of sodium chloride was optimized at 0.1 M to achieve increased entrapment efficiency of fluorescein sodium with desirable particle diameter.
Conjugation of fluorescein sodium-loaded BSA nanoparticles with glutathione
In the present research, glutathione was selected as a targeting vector and conjugated to the BSA nanoparticles by two-step carbodiimide chemistry. In the first step of reaction, carboxylic acid groups of the BSA nanoparticles were activated using EDAC which resulted in the formation of active O-isoacylurea complex. Later step of reaction involves nucleophilic attack of the amine groups of the glutathione on the active O-isoacylurea complex, leads to formation of nanoparticles with exposed glutathione functions on their surface attached via amide linkage. Excess glutathione and urea derivative of EDAC formed during the conjugation reaction were removed by washing with double distilled water (Bernkop-Schnurch & Steininger, Citation2000). We have also optimized the conjugation reaction by considering two variables, i.e. concentration of EDAC and reaction time. Further, glutathione conjugation on nanoparticles was confirmed using Ellman’s assay as described by Chakraborty et al. (Citation2012). The number of glutathione unit conjugated per nanoparticle at different concentration of EDAC and reaction time are displayed in and . From the experimental results, the concentration of EDAC and the reaction time was selected at 60 mg and 45 min respectively, to achieve maximum coupling of glutathione with fluorescein sodium-loaded BSA nanoparticles (Patel & Acharya, Citation2012).
In-vitro release studies
The release profile of fluorescein sodium from BSA nanoparticles was biphasic. The initial burst release was observed within first 60 min attributed to the drug molecules present near to the outer surface of the nanoparticles dissolved instantaneously when it comes in contact with the release medium. The small size of the particle and higher surface area are the major factors which influence the burst release effect. Subsequently, lateral release of the drug was in a sustained manner over a period of 12 h probably due to the slow erosion of the cross-linked polymer matrix. Drug release data revealed that glutathione-conjugated nanoparticles exhibited almost similar release pattern as with unconjugated BSA nanocarriers. In vitro drug release data of optimized fluorescein sodium-loaded BSA nanoparticles were fitted to various kinetics equations which revealed that fluorescein sodium was dissolved as per the first-order kinetic model, while the glutathione-conjugated and unconjugated nanoparticles showed best fit for the Weibull model (Kakran et al., Citation2010; Zhang et al., Citation2010).
In-vitro cytotoxicity of nanoparticle formulation on MDCK-MDR1 cell line/primary neuro-glial co-culture
In current exploration, nanoparticulate formulation for brain-specific drug delivery was prepared using materials approved by various FDAs for drug delivery and safety of these materials for clinical use was already established as individual component. However, combined influence of these components is not reported and hence it was necessary to evaluate cytotoxic profile of nanoparticles prepared using aforementioned components. BSA is well-accepted adjuvant for different drug delivery including recently introduced cancer nanomedicine as a carrier due to is biocompatible, biodegradable and low cytotoxicity potential (Zhao et al., Citation2010). Similarly, glutathione is a well-known endogenous antioxidant, found to be non-toxic at working concentration range and its safety has been already proven by clinical studies. Results of the cytotoxicity studies also confirmed the non-toxic behavior of glutathione-conjugated nanoparticles, as it was not showing any toxic effects on the MDCK-MDR1 cells and cell viability was always observed above 80% even at the highest concentration (1000 μg/mL). Similar findings were also observed in case of primary neuro-glial cell culture indicating, non-toxic nature of developed formulation. Overall results of the cytotoxicity studies indicated the in vitro safety of the prepared glutathione-conjugated BSA nanocarriers. However, its long-term in vivo toxicity and immunogenicity should be further investigated (Kratzer et al., Citation2007; Rao et al., Citation2008; Mittnacht et al., Citation2010).
Fluorescein sodium permeability studies across monolayer of MDCK-MDR1 cells
Results of the permeability studies revealed that greater transport of fluorescein sodium across the monolayer of MDCK-MDR1 cells was observed with glutathione-conjugated nanoparticles than with unconjugated nanoparticles and fluorescein sodium. The glutathione-conjugated and unconjugated nanoparticles were observed to have higher Papp values and greater primary neuronal cellular uptake than demonstrated by fluorescein sodium solution. This might have resulted from the combined effect of glutathione and the P-gp-bypassing mechanism of glutathione-conjugated nanoparticles (Geldenhuys et al., Citation2011). The higher uptake of fluorescein sodium in MDCK-MDR1 cells with glutathione-conjugated nanoparticles could be related to a greater affinity of glutathione to these cells over unconjugated nanoparticles and fluorescein sodium solution. Higher permeability of glutathione-conjugated nanoparticles across the MDCK-MDR1 cells might be mediated by NMDA receptors which are highly expressed on the said cell lines (Leung et al., Citation2002). Further, transport of fluorescein sodium across the cell monolayer was also facilitated due to the effect of glutathione on the cell tight junction proteins, i.e. occludin and claudin. Reduced form of glutathione inhibits the enzyme protein tyrosine phosphatase (PTP) and promotes phosphorylation of tyrosine residues of occludin/claudin which ultimately resulted into opening of tight junction for permeability of fluorescein sodium (Bernkop-Schnurch et al., Citation2003; Jiao et al., Citation2011).
Uptake of nanoparticle formulation by primary neuro-glial co-culture
The uptake behavior of different formulations containing fluorescein sodium is depicted in . Glutathione-conjugated nanoparticles showed intense dotted green fluorescence in the cells which confirms its uptake by the neuro-glial cells. On the other hand, unconjugated nanoparticles showed very little fluorescence in the cells and fluorescein sodium solution was unable to even adhere to the surface of neuro-glial cells probably due to its polar nature. Further, studies conducted by Mailander and Landfester described that the uptake of anionic nanoparticles in cells is mainly mediated by endocytosis process (Mailander & Landfester, Citation2009). Additionally, literature also reported that glutathione undergoes receptor-mediated endocytosis through glutamate receptors, which are expressed on the neuro-glial cells (Lalo et al., Citation2006). Hence, it was believed that glutathione-conjugated BSA nanoparticles is plausibly taken up by the neurons through endocytosis process due to the negative charge of the particles owing to the presence of surface-exposed carbonyl functionality of glutathione, which was confirmed by zeta potential analysis (). However, extensive cell line studies will require to prove the exact mechanism of uptake of nanoparticles by primary neuro-glial cells.
In vivo study
Results of the in-vivo biodistribution studies demonstrated higher uptake of fluorescein sodium in the visceral organs (liver, spleen, lungs) specifically upon administration of fluorescein sodium-loaded nanoparticles (). These observations might be due to higher uptake of polymeric colloidal carriers (specifically with particle size below 5 μm) by the reticuloendothelial system, particularly by the Kupffer-cells of the liver (He et al., Citation2010; Elsabahy & Wooley, Citation2012; Yu et al., Citation2012). Accumulation of fluorescein sodium in the liver and the spleen was found reduced with glutathione-conjugated fluorescein sodium-loaded nanoparticles than unconjugated nanoparticles. The higher accumulation of fluorescein sodium in the lungs with glutathione-conjugated nanoparticles was attributed to the presence of small fraction of aggregated formulation in the circulatory system which was filtered mechanically via lungs and showed higher localization in lungs (Yang et al., Citation2009). The brain distribution studies revealed that glutathione-conjugated BSA nanoparticles were able to deliver the fluorescein sodium in significantly higher concentration in the brain than the unconjugated nanoparticles and fluorescein sodium solution (). The brain distribution studies indicated almost three folds increase in accumulation of fluorescein sodium in brain, when administered as glutathione-conjugated BSA nanoparticles than the unconjugated nanoparticles, indicating potential penetration capacity of glutathione-conjugated nanoparticles across the BBB.
Despite of stringent permeability behavior of BBB, it expresses some of the potential transport system which is actually irrefutable side required to be explored for brain-specific drug delivery. Amongst the different available pathways, receptor-mediated endocytosis have distinct advantages over other pathways and hence explored extensively by conjugating receptor specific ligand to the colloidal drug carriers like nanoparticles, liposomes, solid lipid nanoparticles, etc. (Smith, Citation2005; Jain et al., Citation2006). By considering the above facts in the present study, glutathione was explored as a vector to deliver nanoparticle carriers across the BBB. Results of the in-vivo studies revealed that glutathione-conjugated BSA nanoparticles increases the transport of fluorescein sodium across the BBB. The plausible mechanism of glutathione to ferry colloidal carriers across the most stringent biomembrane, i.e. BBB are as follows. Glutathione acts as an endogenous ligand for NMDA and AMPA receptors, which are abundantly expressed on BBB as well as on neurons (Dringen & Hirrlinger, Citation2003) and this fact was proven by competitive inhibited binding of NMDA and AMPA receptor specific ligands, i.e. glycine, dizoclipine, etc, with glutathione (Dringen & Hirrlinger, Citation2003; Guo et al., Citation2003). Further, Sutariya et al., have also demonstrated that glutathione-coated PLGA nanoparticles increased the uptake of paclitaxel across the BBB by surpassing P-gp efflux system of BBB and hence glutathione also served as a vector for transport of P-gp efflux substrate drug molecules across BBB (Geldenhuys et al., Citation2011). Additionally, glutathione can also modulate the permeability of BBB by acting on the BBB tight junction proteins, occludin and claudin as per the mechanism discussed earlier by Bernkop and Jiao (Bernkop-Schnurch et al., Citation2003; Jiao et al., Citation2011).
Hence, in the present research, we have identified glutathione as a suitable vector, conjugated on BSA nanoparticles and demonstrated its role effectively for transport of fluorescein sodium across the BBB. The results of in-vivo data proved our hypothesis that glutathione-conjugated nanoparticles significantly improved the delivery of entrapped drug to the brain.
Conclusions
The present investigation has explored the role of glutathione as a vector for brain-specific drug delivery by an in-vivo and in-vitro experiments using fluorescein sodium as a fluorescent diagnostic fluorescent marker. According to the in vivo results of our experiments, fluorescein sodium nanoparticles conjugated with glutathione, effectively deliver fluorescein sodium to the brain and have shown excellent potential for brain targeting, as compared to the fluorescein sodium solution and unconjugated fluorescein sodium-loaded nanoparticles. Overall, the present investigation envisaged the role of glutathione as a safe, effective and specific ligand for brain drug delivery. Hence, the proposed technology can also be extended for the various bioactive agents specifically beneficial for the management of stringent brain disorders. The developed formulations may also reduce the dose required of bioactive agents for the therapy to various brain diseases by concurrent reduction in dose-related toxicity. However, clinical benefits to the risk ratio of the formulation developed in this investigation will decide its adoption in the clinical practice.
Declaration of interest
The authors have reported no conflict of interest.
The authors are grateful to Department of Science & Technology, Govt. of India, for providing INSPIRE Fellowship to support the present research work.
References
- Acharya S, Sahoo S.K. (2011). PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev 63:170–83
- Bagger MA, Bechgaard E. (2004). The potential of nasal application for delivery to the central brain -- a microdialysis study of fluorescein in rats. Eur J Pharm Sci 21:235–42
- Banks WA. (2012). Drug delivery to the brain in Alzheimer's disease: consideration of the blood–brain barrier. Adv Drug Deliv Rev 64:629–39
- Benarroch EE. (2010). Glutamate transporters: diversity, function, and involvement in neurologic disease. Neurology 74:259–64
- Benarroch EE. (2012). Blood–brain barrier. Neurology 78:1268–76
- Bernkop-Schnurch A, Kast CE, Guggi D. (2003). Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J Control Release 93:95–103
- Bernkop-Schnurch A, Steininger S. (2000). Synthesis and characterisation of mucoadhesive thiolated polymers. Int J Pharm 194:239–47
- Bowman GL, Kaye JA, Moore M, et al. (2007). Blood--brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology 68:1809–14
- Catuogno S, Esposito CL, Quintavalle G, et al. (2012). Nucleic Acids in human glioma treatment: innovative approaches and recent results. J Signal Transduct 11:1--11
- Chakraborty SP, Das S, Chattopadhyay S, et al. (2012). Staphylococcus aureus infection induced redox signaling and DNA fragmentation in T-lymphocytes: possible ameliorative role of nanoconjugated vancomycin. Toxicol Mech Methods 22:193–204
- Chin TY, Chueh SH, Tao PL. (2006). S-Nitrosoglutathione and glutathione act as NMDA receptor agonists in cultured hippocampal neurons. Acta Pharmacol Sin 27:853–60
- Dringen R, Hirrlinger J. (2003). Glutathione pathways in the brain. Biol Chem 384:505–16
- Elsabahy M, Wooley KL. (2012). Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41:2545–61
- Fluorescein Novartis [Online]. Available from: http://www.betterhealth.vic.gov.au/bhcv2/bhcmed.nsf/pages/nvcfluin/$File/nvcfluin.pdf [last accessed 8 Jan 2013]
- Freytag T, Dashevsky A, Tillman L, et al. (2000). Improvement of the encapsulation efficiency of oligonucleotide-containing biodegradable microspheres. J Control Release 69:197–207
- Gaillard PJ, Appeldoorn CC, Rip J, et al. (2012). Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J Control Release 164:364–9
- Garberg P, Ball M, Borg N, et al. (2005). In vitro models for the blood--brain barrier. Toxicol In Vitro 19:299–334
- Geldenhuys W, Mbimba T, Bui T, et al. (2011). Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. J Drug Target 19:837–45
- Govender T, Stolnik S, Garnett MC, et al. (1999). PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release 57:171–85
- Guo P, Weinstein AM, Weinbaum S. (2003). A dual-pathway ultrastructural model for the tight junction of rat proximal tubule epithelium. Am J Physiol Renal Physiol 285:F241–57
- He C, Hu Y, Yin L, et al. (2010). Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31:3657–66
- Jain A, Chourasia MK, Soni V, et al. (2006). Brain-specific delivery of rifampin from lactyl stearate-coupled liposomes via monocarboxylic acid transporters. Adv Drug Deliv Rev 4:43–9
- Jayant RD, Srivastava R. (2007). Dexamethasone release from uniform sized nanoengineered alginate microspheres. J Biomed Nanotechnol 3:245–53
- Jiao H, Wang Z, Liu Y, et al. (2011). Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood--brain barrier in a focal cerebral ischemic insult. J Mol Neurosci 44:130–9
- Jin X, Xu Y, Shen J, et al. (2011). Chitosan–glutathione conjugate-coated poly(butyl cyanoacrylate) nanoparticles: promising carriers for oral thymopentin delivery. Carbohydrate Polymers 86:51–7
- Jun JY, Nguyen HH, Paik S-Y-R, et al. (2011). Preparation of size-controlled bovine serum albumin (BSA) nanoparticles by a modified desolvation method. Food Chem 127:1892–8
- Kakran M, Sahoo NG, Li L, Judeh Z. (2010). Dissolution of artemisinin/polymer composite nanoparticles fabricated by evaporative precipitation of nanosuspension. J Pharm Pharmacol 62:413–21
- Kannan R, Kuhlenkamp JF, Jeandidier E, et al. (1990). Evidence for carrier-mediated transport of glutathione across the blood--brain barrier in the rat. J Clin Invest 85:2009–13
- Kannan R, Kuhlenkamp JF, Ookhtens M, Kaplowitz N. (1992). Transport of glutathione at blood -- brain barrier of the rat: inhibition by glutathione analogs and age-dependence. J Pharmacol Exp Ther 263:964–70
- Kashi TS, Eskandarion S, Esfandyari-Manesh M, et al. (2012). Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomedicine 7:221–34
- Kratzer I, Wernig K, Panzenboeck U, et al. (2007). Apolipoprotein A-I coating of protamine-oligonucleotide nanoparticles increases particle uptake and transcytosis in an in vitro model of the blood--brain barrier. J Control Release 117:301–11
- Krizbai IA, Deli MA, Pestenacz A, et al. (1998). Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res 54:814–19
- Kumari A, Yadav SK, Yadav SC. (2010). Biodegradable polymeric nanoparticles based drug delivery systems. Coll Surf B: Biointerfaces 75:1–18
- Lalo U, Pankratov Y, Kirchhoff F, et al. (2006). NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26:2673–83
- Lamprecht A, Ubrich N, Hombreiro Perez M, et al. (2000). Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int J Pharm 196:177–82
- Leung JC, Travis BR, Verlander JW, et al. (2002). Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am J Physiol Regul Integr Comp Physiol 283:R964–71
- Lindqvist A, Rip J, Gaillard PJ, et al. (2012). Enhanced brain delivery of the opioid peptide DAMGO in glutathione PEGylated liposomes: a microdialysis study. Mol Pharm 14:14–24
- Liu X, Chen C. (2005). Strategies to optimize brain penetration in drug discovery. Curr Opin Drug Discov Devel 8:505–12
- Lu W, Zhang Y, Tan YZ, et al. (2005). Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J Control Release 107:428–48
- Mailander V, Landfester K. (2009). Interaction of nanoparticles with cells. Biomacromolecules 10:2379–400
- Martin HL, Teismann P. (2009). Glutathione – a review on its role and significance in Parkinson's disease. FASEB J 23:3263–72
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. (2012). Temporary disruption of the blood–brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in Rhesus Macaques. Cancer Res 72:3652–63
- Mittnacht U, Hartmann H, Hein S, et al. (2010). Chitosan/siRNA nanoparticles biofunctionalize nerve implants and enable neurite outgrowth. Nano Lett 10:3933–9
- Muro K, Das S, Raizer JJ. (2006). Convection-enhanced and local delivery of targeted cytotoxins in the treatment of malignant gliomas. Technol Cancer Res Treat 5:201–13
- Nobs L, Buchegger F, Gurny R, Allemann E. (2003). Surface modification of poly(lactic acid) nanoparticles by covalent attachment of thiol groups by means of three methods. Int J Pharm 250:327–37
- Pardridge WM. (2005). Drug and gene targeting to the brain via blood–brain barrier receptor-mediated transport systems. Int Congress Series 1277:49–62
- Patel P, Acharya S. (2012). Design and development of glutathione conjugated poly(d, l) lactide nanocarriers for delivery of hydrophilic fluorescent marker across blood brain barrier. Curr Nanosci 8:847--57
- Patel P, Acharya N, Acharya S. (2011). Potential of surface functionalized nanoparticles for improved therapy of refractory CNS disorders. J Pharmacy Res 4:1093–9
- Peltonen L, Aitta J, Hyvonen S, et al. (2004). Improved entrapment efficiency of hydrophilic drug substance during nanoprecipitation of poly(l)lactide nanoparticles. AAPS PharmSciTech 5:115--20
- Rao KS, Reddy MK, Horning JL, Labhasetwar V. (2008). TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials 29:4429–38
- Reijerkerk A, Kooij G, Van Der Pol SM, et al. (2010). The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J Neurochem 113:447–53
- Smith QR. (2005). Carrier-mediated transport to enhance drug delivery to brain. Int Congress Series 1277:63–74
- Sunitha R, Harika D, Phani A, et al. (2011). A review: nanoparticles as specified carriers in targeted brain drug delivery system. Am J Pharmaceut Technol Res 1:121--34
- Uchida T, Yoshida K, Goto S. (1996). Preparation and characterization of polylactic acid microspheres containing water-soluble dyes using a novel w/o/w emulsion solvent evaporation method. J Microencapsul 13:219–28
- Umnarat S. (2006). Brain delivery enhancement of curcumin via nanotechnology [thesis]. Mahidol University
- Wilson B, Samanta MK, Santhi K, et al. (2008). Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res 20:159–68
- Witschi C, Doelker E. (1998). Influence of the microencapsulation method and peptide loading on poly(lactic acid) and poly(lactic-co-glycolic acid) degradation during in vitro testing. J Control Release 51:327–41
- Yang R, Yang SG, Shim WS, et al. (2009). Lung-specific delivery of paclitaxel by chitosan-modified PLGA nanoparticles via transient formation of microaggregates. J Pharm Sci 98:970–84
- Yu MK, Park J, Jon S. (2012). Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2:3–44
- Zhang Y, Huo M, Zhou J, et al. (2010). DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J 12:263–71
- Zhao D, Zhao X, Zu Y, et al. (2010). Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int J Nanomedicine 5:669–77
- Zlokovic BV, Mackic JB, McComb JG, et al. (1994). Evidence for transcapillary transport of reduced glutathione in vascular perfused guinea-pig brain. Biochem Biophys Res Commun 201:402–8