Abstract
The present study was aimed to evaluate the nanostrucured lipid carriers (NLC) containing duloxetine (DLX-NLC) for intranasal infusion through the nasal cavity of rat. The in vivo nasal infusion studies were performed using Wistar rats and the amount of DLX permeated and its amount in brain and blood was estimated. The effects on absorption rate and type of drug delivery systems (nanocarriers and drug solution) for nose to brain/blood permeation were assessed. DLX was found to be permeated from the nasal cavity into the body of rat and the permeated amount was found to be more in case of DLX-NLC. Approximately 2.5-times better permeation was exhibited by DLX-NLC than DLX-solution. Appreciable amount of DLX was estimated in blood and brain and the estimated amount was higher in case of DLX-NLC. Thus the administration of NLC containing DLX through intranasal route was found to be potential method for the delivery of DLX for the treatment of depression.
Introduction
The blood brain barrier (BBB) restricts the access of drugs to the brain on oral as well as intravenous administration. Intranasal administration offers a non-invasive method to deliver the therapeutic agents to the brain (Alam et al., Citation2010). The major advantages of this administration route are the avoidance of blood-brain barrier and first pass metabolism and rapid onset of action compared to oral administration (Bjerre et al., Citation1996). Moreover the intranasal administration provides effortless, efficient administration that is apposite for self-medication, and does not necessitate any sterile equipment (Tas et al., Citation2006). The neuronal and extracellular pathways constitute a direct pathway for nose to brain delivery of therapeutic agents. The olfactory and trigeminal neural pathways are considered to be responsible for drug distribution to the brain (Alam et al., Citation2012). Furthermore, the highly permeable nasal epithelium allows rapid drug absorption directly to the systemic blood circulation. Nasal administration can, therefore, be used as an alternative to oral administration for the drug which is extensively degraded in the gut or liver. The drug can reach the brain also, by systemic to brain pathways by crossing the BBB. However the use of this pathway is highly dependent on the characteristics of the drug (Illum, Citation2000). A number of drugs administered intranasally for systemic effects include analgesics, cardiovascular drugs, hormones, anti-inflammatory agents and antiviral drugs (Pires et al., Citation2009). Among the several strategies tested to improve nasal absorption, one of them was the use of lipid nanocarriers (e.g. NLC) (Haque et al., Citation2011).
Nanostructured lipid carriers (NLC) are considered to be a promising approach of drug delivery without any modification to the drug molecule. The small diameter of nanocarriers makes it versatile to be transported transcellularly through the neuronal pathway to the brain. This could be achieved by way of the various endocytic pathways of sustentacular or neuronal cells in the olfactory membrane (Mistry et al., Citation2009). The lipid nanocarriers are considered to be more effective to be absorbed after intranasal administration (Alam et al., Citation2011). These are interesting candidates because of their rapid uptake by the brain, bioacceptability and biodegradability. The incorporated drug into NLC is protected from degradation and/or efflux back into the nasal cavity may further enhance the availability in brain and blood. Moreover, the feasibility in scale-up and absence of burst effect make them promising carriers for drug delivery (Alam et al., Citation2012).
It is rare to find in vivo intranasal infusion studies using lipid nanocarriers in literature. NLC containing duloxetine (DLX-NLC) was prepared for intranasal administration and evaluated for in vitro characteristics and pharmacodynamic studies (Alam et al., Citation2011; Alam et al., Citation2012). The aim of present study was to evaluate the DLX-NLC to be infused through the nasal cavity of rat. It was further evaluated for the estimation of permeated amount of DLX in brain and blood.
Materials and methods
Drugs and reagents
DLX was provided by Dr. Reddy’s Laboratories (Hyderabad, India). Glyceryl monostearate (solid lipid) (Loba chemie Pvt. Ltd., Mumbai, India), Pluronic F-68 (surfactant) and capryol PGMC (liquid lipid) (Sigma Chemical Company, MO), Bile salt (sodium taurocholate) (co-surfactant) (Thomas Baker, chemicals, Ltd., Mumbai, India), and Mannitol (cryoprotectant) (S.D. fine-chem Ltd., Mumbai, India) were used as received from suppliers. All other chemicals and solvents were of analytical reagent grade and were used without further purification.
Animals
The study was performed on Swiss albino Wistar rats of either sex (200–250 g). All animals were given free access to water and food and kept at a temperature of 23–30 °C with a natural light-dark cycle. Three animals (n = 3) for each formulation per time point were used in the studies. Approval to carry out the study was obtained from the Institute Ethical Committee, Jamia Hamdard, New Delhi (India) and their guidelines were followed throughout the study.
Experiments
Preparation of DLX-NLC and lyophilization
DLX-NLC was prepared by homogenization (Heidolph, Diax 900, Schwabach, Germany) followed by ultrasonication (Altrasonics India, Mumbai, India). Optimization of the DLX-NLC formulations was carried out using the response surface methodology (RSM) and the Box–Behnken design model as reported earlier by the authors (Alam et al., Citation2011). Briefly, DLX was dissolved in melted solid lipid (glyceryl monostearate) and liquid lipid (capryol PGMC) was mixed with it. The DLX-containing lipid mixture was homogenized with hot aqueous solution of surfactants (pluronic F-68 and bile salt in water) for 20 min. DLX-NLC dispersion was obtained after ultrasonication for 10 min (). It was followed by lyophilization using mannitol as cryoprotectant at a concentration of 3% (w/w). After 72 h in a freeze-dryer (Heto Drywinner, Denmark) at −70 °C, the lyophilized DLX-NLC powder was collected for further studies.
Table 1. Amount of ingredients required for the preparation of DLX-NLC.
In vivo nasal infusion studies
The animal models employed for nasal infusion studies consisting of two types, (1) in vivo nasal absorption studies (whole animal or in vivo model), (2) ex vivo nasal perfusion studies (isolated organ perfusion or ex vivo model) (Chien, Citation1985).
Healthy Wistar rats were selected for the study and randomly divided into two groups for intranasal administration of formulations. One group of rat received intranasal administration of DLX solution, and another group received DLX-NLC suspension (DLX-NLC powder suspended in double distilled water). The surgical preparation of rat for in vivo nasal infusion studies was carried out as follows:
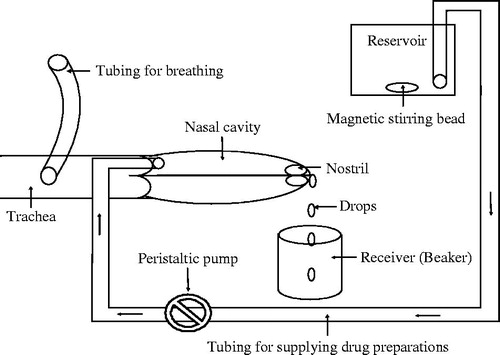
The rats were anesthetized by intraperitoneal (i.p.) injection of chloral hydrate (3.5 mg/kg of body weight). An incision was made in the neck and the trachea was cannulated with a polyethylene tube for breathing. Another tube was inserted through the esophagus towards the posterior region of the nasal cavity to supply the drug preparations (DLX solution or DLX-NLC suspension). During the study, a funnel (if needed) was placed between the nose and receiver (beaker) to minimize the loss of drug preparations coming out of the nostrils. The drug preparation was placed in a reservoir (Marriott bottle) maintained at 37 °C and was circulated through the nasal cavity of the rat with the help of a peristaltic pump after maintaining the flow rate at 0.5 ml/min. A schematic presentation for in vivo nasal infusion studies is shown in . The drug preparation in the reservoir was continuously stirred. The DLX solution/DLX-NLC suspension passing out from the nostrils was collected at 5, 10, 15, 30, 45 and 60 min of the study in the beaker. The amount of drug absorbed was estimated by measuring the residual drug concentration in the collected samples. The passage of nasopalatine was sealed to prevent the drainage of the drug preparation from the nasal cavity through the mouth. Since all the possible outlets in the rat model were blocked, the only possible passage for the drug to be absorbed and transported into the body was permeation and/or diffusion through the nasal mucosa. For nasal absorption studies (in vivo model), the blood samples and brain (ex vivo nasal perfusion) were collected at the end of the study.
Extraction of DLX from blood and brain
The collection of blood and brain and extraction of DLX was done as per the method reported earlier (Alam et al., Citation2012). Briefly, the blood (2 ml) was centrifuged for 15 min for separation of plasma. Liquid-liquid extraction technique was used to separate DLX from plasma. The plasma (0.5 ml) was taken in a clean test tube and mixed with internal standard (venlafaxine – 1 ml) and chloroform (5 ml). It was then vortexed and centrifuged at 5000 rpm for 10 min to bring the drug in chloroform. Upper layer was discarded and the chloroform layer was transferred to a clean test tube and evaporated to dryness. The residue was reconstituted in 100 μl of methanol, mixed well, filtered through 0.2 µm filter. The final clear solution was analyzed for the estimation of DLX by HPLC (Alam et al., 2012).
After the collection of brain from the treated rats (DLX solution or DLX-NLC), it was washed twice using normal saline, made free from adhering tissue/fluid, dried and weighed. The whole brain was homogenized for 5 min using a Tissue Homogenizer (Fisher Scientific, Germany) after adding saline at a ratio of 1:4 (w/v). The homogenate (500 µl) was mixed with venlafaxine (1 ml) as internal standard and n-hexane (2 ml) as organic solvent and vortexed for 1 min followed by centrifugation at 5000 rpm for 5 min. Upper layer was transferred to a clean test tube and evaporated to dryness. The residue was reconstituted in 100 μl of methanol, mixed well, filtered through 0.2 µm filter. The final clear solution was analyzed for the estimation of DLX by HPLC (Alam et al., 2012).
Statistical analysis
All the data were expressed as the mean standard deviation (±SD). The data was analyzed using Kruskal–Wallis test (non-parametric test). A Dunn's multiple comparison test were used to compare different formulations and a p value of less than 0.10 (p < 0.10) was considered to be significant.
Results
The profiles of absorption of DLX through nasal epithelium are shown in and . The results clearly indicated the absorption of sufficient amount of DLX through nasal epithelia during 1 h period of the study. A maximum of 1.3 mg of DLX was absorbed after continuous infusion of DLX-NLC. This amount was found to be 0.52 mg in case of DLX solution.
Figure 2. Amount of DLX absorbed into the body of rat after intranasal infusion of DLX solution and DLX-NLC dispersed in water.
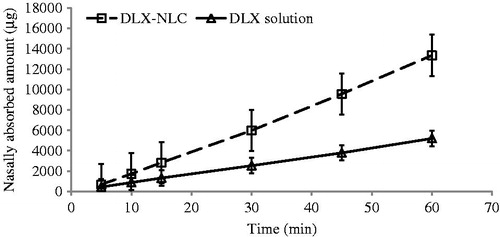
Figure 3. Rate of absorption of DLX in rats after intranasal infusion of DLX solution and DLX-NLC dispersed in water.
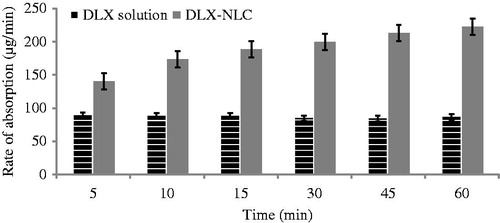
The absorption rate was found to be smooth and continuous through the nasal cavity of rat. The rate of absorption of DLX-NLC was found to be higher than DLX solution (p < 0.10). Moreover, a continuous increase in absorption rate with time was observed in case of DLX-NLC. The effect of DLX solution on absorption rate with time was found to be constant ().
The brain and blood samples were analyzed for the estimation of absorbed amount of DLX. The concentration of DLX in brain and blood after intranasal infusion studies is shown in . The estimated amount of DLX was found to be higher in case of DLX-NLC (228.8874 ± 83.72 µg/g) when compared with DLX solution (95.75 ± 24.28 µg/ml) in brain. Similarly DLX-NLC (1869.247 ± 112.61 µg/g) exhibited higher concentration than DLX-solution (728.34 ±63.52 µg/ml) in plasma. Thus the higher amount of DLX was estimated in brain as well as blood when administered as DLX-NLC (p < 0.10) than DLX solution. Furthermore, the permeation enhancement was calculated as a ratio of amount of DLX in brain and plasma after intranasal administration of DLX-NLC and DLX solution. NLC exhibited approximately 2.5-times better permeation than DLX solution.
Table 2. Concentration of DLX in brain and blood after intranasal infusion studies.
Discussion
In vivo nasal infusion studies were performed by infusing aqueous preparations through the nasal cavity. The study was performed on live rat to evaluate the potential of absorption through nasal cavity into the body. Bain and blood samples were evaluated to estimate the permeated amount of DLX through the nasal route of drug administration.
The infusion containing DLX solution and DLX-NLC dispersed in water were given intranasally for a period of 1 h. The infused sample was collected in a beaker after passing through the nasal cavity of the rat. The samples were collected at specified time and the amount of DLX absorbed into the body was estimated. The absorbed amount of DLX was estimated from the amount of DLX present in the infusion before and after passing through the nasal cavity of the rat.
The enhanced absorption of DLX-NLC from the nasal cavity may be elucidated on the basis of different explanations. It is likely that the formulation was covering a large area of nasal mucosa in the nasal cavity of rat for a longer period of time (longer residence time) resulting in greater nasal absorption. Another reason may be the volume of the formulations (DLX-NLC, DLX solution) applied to unit nasal epithelial area (Hirai et al., Citation1981). A greater volume of the formulations were administered per unit area of rat nasal mucosa. One more reason may be attributed was the application to anesthesia that could decrease the nasal mucociliary clearance in rats (Al-Ghananeem et al., Citation2011), thereby increasing the residence time and nasal absorption of DLX from the nasal mucosa.
The absorption rate was found to be smooth and continuous may be attributed to the continuous infusion of the formulations with constant flow rate (0.5 ml/min). The increase in absorbed amount of DLX in case of DLX-NLC may be attributed to the nanopaticulate as well as lipidic nature of NLC and protection from metabolic enzymes localized in the nasal mucosal cavity and epithelial cells lining the cavity (Hussain & Aungst, Citation1994; Chung & Donovan, Citation1996). The continuous increase in absorption rate may be explained on the basis of lipidic nature of NLC and permeation enhancing effects by bile salt (Maggio, Citation2011) and poloxamer (a non-ionic surfactant). Bile salts and poloxamer may improve the availability by both solubilization of drug and direct effect on the cell membrane by disrupting the lipid bilayer thereby creating pathways for drug penetration (Aungst & Rogers, Citation1988).
A number of strategies have been surveyed to improve the bioavailability by enhancing the absorption of drugs through nasal mucosa. It includes the use of penetration enhancers, designing suitable dosage formulations, proteolytic enzyme inhibitors and bioadhesive polymers. Enhancement in absorption of drugs through nasal mucosa using bioadhesive polymers may be achieved by prolonging the residence time in the nasal cavity. Therapeutic effect was improved by increasing the residence time of a number of drugs using bioadhesive polymers including apomorphine (Ugwoke et al., Citation2000), insulin (Nagai et al., Citation1984), Oxprenolol (Preda & Leucuta, Citation2003), tetrahydrocannabinol (Al-Ghananeem et al., Citation2011), and vaccine (Jabbal-Gill, Citation2010). An alternative way (other than using bioadhesive polymers) of increasing the residence time was used in this study. It was done by continuous supply of drug (DLX solution)/formulation (DLX-NLC suspension) at constant rate (infusion) through the nasal cavity of rat. Similar method of intranasal administration was used for nose-to-systemic (Hussain, Citation1998; Chen et al., Citation2010) and nose-to-brain (Hussain, Citation1998) delivery of therapeutic substances. This single pass in-situ nasal perfusion technique was applied to examine the rate and extent of nasal absorption of paeonol by rats. The results demonstrated prompt and thorough absorption and exhibited enhanced bioavailability systemically of paeonol by intranasal administration in rats (Chen et al., Citation2010). Similarly, the nasal administration of two prodrugs resulted in higher CSF and olfactory bulbs levels of .-dopa than that observed after intravenous administration (Kao et al., Citation2000). Moreover, the drug concentration in CSF after nasal perfusion was increased with increasing the lipophilicity of the drugs (Sakane et al., Citation1991).
Nasal absorption of drug is dependent on drug concentration, dose and volume of administration. The rate of nasal absorption of aminopyrine was found to be constant as a function of concentration. On the contrary, salicylic acid exhibited decreased nasal absorption with concentration (Hirai et al., Citation1981). However, .-Tyrosine was shown to increase in nasal absorption with drug concentration in nasal perfusion experiments (Huang et al., Citation1985).
Conclusion
Appreciable amount of DLX was estimated in blood and brain after intranasal infusion indicating its absorption through the nasal epithelium. The estimated amount of DLX was found to be enhanced in case of DLX-NLC. Thus, the rate of nasal absorption can be enhanced using lipid nanocarriers, suitable surfactants and increasing the nasal residence time. Consequently, the combination of NLC and intranasal route of drug administration can be considered as a better option for the delivery of DLX for the treatment of depression.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
The authors are thankful to Dr. Reddy’s Laboratories, Hyderabad, (India) for providing gift sample of the drug and CSIR, New Delhi, for providing a Senior Research Fellowship to M. Intakhab Alam.
References
- Alam MI, Baboota S, Ahuja A, et al. (2011). Nanostructured Lipid Carrier Containing CNS Acting Drug: Formulation, Optimization and Evaluation. Cur Nanosci 7:1014–27
- Alam MI, Baboota S, Ahuja A, et al. (2012). Intranasal administration of nanostructured lipid carriers containing CNS acting drug: pharmacodynamic studies and estimation in blood and brain. J Psychiatr Res 46:1133–8
- Alam MI, Beg S, Samad A, et al. (2010). Strategy for effective brain drug delivery. Eur J Pharm Sci 40:385–403
- Al-Ghananeem AM, Malkawi AH, Crooks PA. (2011). Bioavailability of Δ9-tetrahydrocannabinol following intranasal administration of a mucoadhesive gel spraydelivery system in conscious rabbits. Drug Dev Ind Pharm 37:329–34
- Aungst BJ, Rogers NJ. (1988). Site dependence of absorption-promoting actions of laureth-9, Na salicylate, Na2EDTA, and aprotinin on rectal, nasal, and buccal insulin delivery. Pharm Res 5:305–8
- Bjerre C, Bjork E, Camber O. (1996). Bioavailability of the sedative propiomazine after nasal administration in rats. Int J Pharm 144:217–24
- Chen X, Lu Y, Du S, et al. (2010). In Situ and in vivo study of nasal absorption of Paeonol in rats. Int J Mol Sci 11:4882–90
- Chien YW. (1985). Transnasal systemic medications: fundamentals, developmental concepts, and biomedical assessments. Amsterdam, New York: Elsevier
- Chung FY, Donovan MD. (1996). Nasal pre-systemic metabolism of peptide drugs: substance P metabolism in the sheep nasal cavity. Int J Pharm 128:229–37
- Haque S, Shadab M, Alam MI, et al. (2011). Nanomedicines for Brain Targeting: a patent review. Recent Patent Nanomed 1:149–61
- Hirai S, Yashika T, Matsuzawa T, Mima H. (1981). Absorption of drugs from the nasal mucosa of rat. Int J Pharm 7:317–25
- Huang C, Kimura R, Nassar A, Hussain A. (1985). Mechanism of nasal drug absorption of drug II: absorption of .-Tyrosine and the effect of structural modification on its absorption. J Pharm Res 74:1298–301
- Hussain AA. (1998). Intranasal drug delivery. Adv Drug Deliv Rev 29:39–49
- Hussain MA, Aungst BJ. (1994). Nasal mucosal metabolism of an LH-RH fragment and inhibition with boroleucine. Int J Pharm 105:7–10
- Illum L. (2000). Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 11:1–18
- Jabbal-Gill I. (2010). Nasal vaccine innovation. J Drug Targeting 18:771–86
- Kao HD, Traboulsi A, Itoh S, et al. (2000). Enhancement of the systemic and CNS specific delivery of L-dopa by the nasal administration of its water soluble prodrugs. Pharm Res 17:978–84
- Maggio ET. (2011). Intranasal administration of active agents to the central nervous system. US Patent 20110129462A1
- Mistry A, Stolnik S, Illum L. (2009). Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm 379:146–57
- Nagai T, Nishimoto Y, Nambu N, et al. (1984). Powder dosage form of insulin for nasal administration. J Control Rel 1:15–22
- Pires A, Fortuna A, Alves G, Falcao A. (2009). Intranasal Drug Delivery: How, Why and What for? J Pharm Pharm Sci 12:288–311
- Preda M, Leucuta SE. (2003). Oxprenolol-loaded bioadhesive microspheres: preparation and in vitro.in vivo characterization. J Microencapsul 20:777–89
- Sakane T, Akizuki M, Yamashita S, et al. (1991). The transport of a drug to the cerebrospinal fluid directly from the nasal cavity: the relation to the lipophilicity of the drug. Chem Pharm Bull 39:2456–8
- Tas C, Ozkan CK, Savaser A, et al. (2006). Nasal absorption of metoclopramide from different Carbopol-981 based formulations: in vitro. ex vivo and in vivo evaluation. Eur J Pharm Biopharm 64:246–54
- Ugwoke MI, Kaufmann G, Verbeke N, Kinget R. (2000). Intranasal bioavailability of apomorphine from carboxymethylcellulose-based drug delivery systems. Int J Pharm 202:125–31