Abstract
The present study aims to prepare and evaluate carbon nanotubes (CNTs)-based colon-specific microspheres using irinotecan as a model of drug. The synthesis of CNTs-based microspheres including attachment of folate–chitosan conjugate and irinotecan to CNTs via non-covalent interaction, followed by microencapsulation with Eudragit S-100 by an oil-in-oil solvent evaporation technique. The obtained samples were characterized in case of surface morphology, drug loading efficiency and particle size. In vitro drug release behavior was studied in different pH medium and the obtained data were subjected to kinetic equations. It was found that the Eudragit-coated microparticles were spherical with smooth surface, and the particle size varied with the core/coat ratio. In vitro drug release shows that the irinotecan released in a slow and sustained fashion from the CNTs-based carriers without coating with Eudragit. No drug release was observed from Eudragit-coated microspheres when the medium pH below 7, while when the pH reached 7.4, the coating layer of Eudragit began to dissolve and a controlled release of irinotecan was observed. The cell viability test indicates that the drug free FA-CS decorated CNTs had no influence on the cell proliferation rates of HT-29 cells, while the irinotecan-loaded CNTs drug system proved to be the most cytotoxic.
Introduction
Carcinomas of the colorectal region, including colon and rectal cancer, account for around 8%–9% of malignant tumor in man among the three leading causes of cancer mortality rate, which is ranked just after lung and bronchus cancers (Siegel et al., Citation2011). Complete cure of this disease is a challenge for oncologists because the malignancy of tumors is usually detected at advanced stage. Anticancer drug is toxic to normal cells after administration since the therapeutic agent can also be uptaken by healthy cells. Treatment will be more effective if anticancer drug is targeted directly into the cancerous cells, which can enhance both the patient compliance and the treatment efficiency.
Different approaches for colon specific drug delivery systems (CDDS) have been reported over the last few decades, including prodrugs, pH sensitive polymer-coated drug delivery, time controlled release system, microbially triggered system, pressure controlled colonic delivery capsules and osmotic controlled drug delivery (Yang et al., 2002; Jose et al., 2009; Philip & Philip, 2010). Most techniques used to delivery drugs to the colon have certain limitations due to the complex physiological environment of the gastrointestinal tract. The pH-sensitive polymer-coated drug delivery system, for example, may start to release in the lower small intestine or even not release after reaching target areas because of the difference between subjects, the pH values of digestive tract and the pathological state of patients. Therefore, an ideal colon-targeted drug delivery system could provide protection to the drug molecules at pH value below 7, avoiding the release of drug before reaching the destination. To facilitate the delivery of anticancer drug molecules across the gastrointestinal tract, the approach of applying enteric-coating materials has been proposed (Lorenzo-Lamosa et al., Citation1998). The studies indicated that no drug was released below pH 7. Despite this promising characteristics, a limitation of such systems is its poor structure stability and rapid degradation because the carrier materials of those drug delivery systems were biodegradable, which cause the therapeutic effect of drug limited (Thakral et al., Citation2010; Shivani et al., Citation2011).
Carbon nanotubes (CNTs) have been widely investigated as an excellent candidate for drug delivery carrier due to their quasi-one-dimensional nanostructure, ultra-high cargo loading capacity, unique intrinsic stability and structure flexibility (Liu et al., Citation2008). Moreover, it has been proved that CNTs can penetrate cell membrane (Kam et al., Citation2004) and effectively delivery drug molecules into cytoplasm without causing a side toxic effect (Bianco et al., Citation2005). Nevertheless, the pristine CNTs proved to be unsuitable as drug delivery carriers because they tend to form bundles. Therefore, they are not suitable for pharmacological application. This problem can be solved by functionalization CNTs with synthetic polymer or natural polysaccharides. CS, the only natural alkaline polysaccharide with two hydroxyl groups and one amino group, is attractive for functionalizing CNTs because it can improve the stability of CNTs in aqueous solution. Furthermore, CS has some remarkable characteristics such as non-toxicity, biocompatibility and it can be degraded by colonic bacteria (Zhang et al., Citation2002; Patel et al., Citation2008); cationic chitosan-based carriers can adhere to negatively charged phospholipids biolayer of cellular membrane.
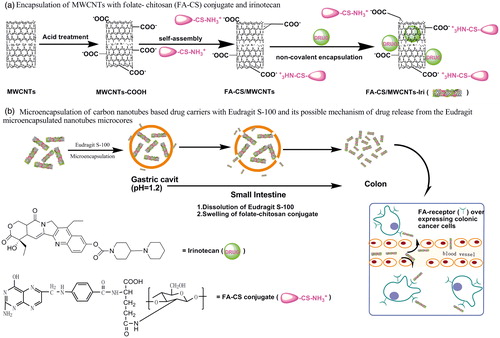
Despite excellent progress in using carbon nanotubes as drug delivery carriers, there are only a few publications reporting surface appearance and inner structure of CNTs-based microspheres encapsulated with enteric materials. In this article, we present a novel multiparticulate system, such a system aims to develop folate–chitosan conjugate decorated CNTs (folate-chitosan (FA-CS)/multi-walled carbon nanotubes (MWCNTs)) for targeted delivery of irinotecan to colon. Drug-loaded FA-CS/MWCNTs were then coated by Eudragit S-100 (ES-100) to protect the drug from releasing in the upper gastrointestinal tract (GIT), and finally to target irinotecan to the colon only. The morphological features of the system, cell viability, and in vitro drug release profiles of the prepared MWCNTs-based drug carriers have also been evaluated.
Materials and methods
Materials
Folate-chitosan (FA-CS) conjugate was self-made in our lab according to the method reported in our previous work (Li et al., Citation2011). MWCNTs were purchased from Shenzhen Nanotech Port Co. Ltd. (Shenzhen, China). Eudragit® S-100 was kindly supplied by Shenzhen Youpuhui Pharmaceutical Co. Ltd. (Shenzhen, China). Liquid paraffin oil, irinotecan and span 85 were purchased from Sigma-Aldrich Company (St. Louis, MO). All other chemicals and solvents were of analytical grade.
Preparation of MWCNTs
The preparation of MWCNTs was carried out as follows using the procedure documented in literature (Peng et al., Citation2010). MWCNTs (500 mg) were added into a solution containing 65% HNO3 and 98% H2SO4 (V: V = 1:3) and treated with ultrasonic for 1 h. This solution was kept at 150 °C for 1.5 h and diluted with 2000 mL distilled water. Finally, this solution was filtered through a micro-porous filtration membrane (Φ = 0.1 um) after it was cooled to room temperature, and then washed with distilled water till the pH became neutral. The resultant samples were dried at −40 °C for 48 h.
Preparation of FA-CS/MWCNTs composites
MWCNTs treated by acid (50 mg) and FA-CS conjugate (100 mg) were dissolved in a PB buffer solution (pH = 5, 100 mL) under rapid stirring condition. After stirring for 16 h at room temperature, the FA-CS/MWCNTs were collected and washed with PB buffer solution. Finally, the obtained samples were dried at −40 °C for 48 h.
Preparation of irinotecan-loaded FA-CS/MWCNTs
As shown in , FA-CS/MWCNTs (20 mg) which were dispersed in pH 7.4 phosphate-buffered saline (PBS) (40 mL) and treated with irinotecan (40 mg) under sonic irradiation, and the mixture solution was kept stirring at room temperature for 24 h. The products (denoted as MWCNTs-Iri, CS/MWCNTs-Iri and FA-CS/MWCNTs-Iri) were collected, rinsed with PBS and dried (−40 °C, 48 h). The amount of unbound irinotecan (Wfree Iri) was determined by measuring the UV absorbance at 370 nm (the characteristic absorbance of irinotecan). The drug loading efficiency was obtained by the following expression:
All experiments were run in triplicate.
Microencapsulation of FA-CS/MWCNTs-Iri
Microspheres were coated with Eudragit S-100 using an oil-in-oil solvent evaporation technique (Lorenzo-Lamosa et al., Citation1998; Rodríguez et al., 1998; Chourasia & Jain, 2004; Paharia et al., 2007). As shown in , FA-CS/MWCNTs-Iri (50 or 100 mg) were dispersed into 5 mL organic solvent (acetone: methanol, ratio 2:1) in which Eudragit S-100 was previously dissolved to give either 1:5 (sample CIS-1) or 1:10 (sample CIS-2) core/coat ratio, This organic phase was slowly dripped into 70 mL liquid paraffin containing span 85 (1% w/w) to emulsify. The emulsified solution was then maintained stirring at 1000 rpm for 3 h to allow the evaporation of the solvent. Finally, the microspheres were collected, washed with n-hexane and dried at −40 °C for 48 h.
Surface morphology and particle size
The surface morphology and inner structure of the microspheres were examined by scanning electron microscopy (SEM) (PHILIPS XL30, Philips-FEI, the Netherlands), and the surface appearance of functionalized MWCNTs was characterized by transmission electron microscopy (TEM) (JEM 2100, JOEL, Tokyo, Japan). The particle size of the microspheres was measured using CAMSIZER® XT (CAMSIZER XT, Retsch, Haan, Germany).
Evaluation of irinotecan release from the CNTs-based drug vectors
The drug release was carried out in simulated gastric fluid (pH = 1.2) and simulated intestinal fluid (pH = 7.4) at 37 °C. Drug-loaded samples were sealed in a dialysis bag and submerged in 100 mL release medium. The release medium was stirred at 100 ± 2 rpm. Samples were withdrawn at pre-determined time intervals, and the amount of irinotecan in the buffer solution was quantified using UV-visible absorption spectra (L-35, PerkinElmer, Waltham, MA) at 370 nm. All experiments were performed in triplicate.
Evaluation of irinotecan release from the Eudragit-coated CNTs microspheres
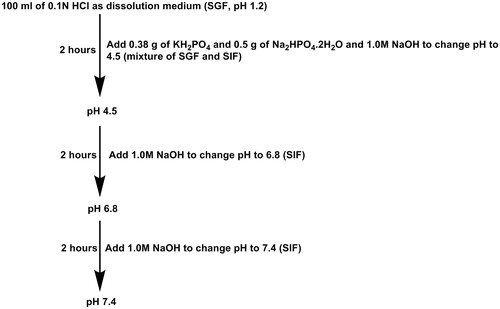
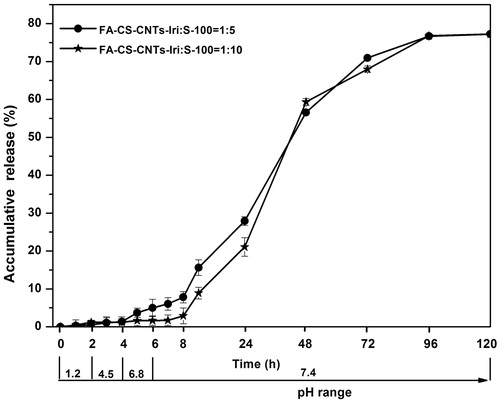
In vitro drug release response from Eudragit-coated CNTs microspheres were studied at 37 ± 0.5 °C, simulating GIT conditions. The studies were carried out in 100 mL release medium which was stirred at 100 ± 2 rpm. The pH of the release medium was gradually increased: pH 1.2 during the first 2 h, 4.5 during the third and fourth hours, then 6.8 during the next 2 h, and finally pH 7.4 was maintained until the end of the experiment (). Samples were withdrawn at specific time intervals, and the amount of irinotecan in the buffer solution was quantified using UV-visible absorption spectra (L-35, PerkinElmer, Waltham, MA) at 370 nm. All experiments were run in triplicate.
Cell viability test
MTT assay was used to measure the cell viability (Belyanskaya et al., Citation2007; Lay et al., Citation2010). HT-29 cells were seeded into a 96-well microplate at a density of 1 × 105 cells/mL in 100 μL RPMI1640 medium. After 24 h culture, the cells were washed with 100 μL/well PBS and treated by MWCNTs with different concentration in culture medium for 24 h and 48 h. In order to evaluate the role of FA in the cellular uptake of FA-CS/MWCNTs-Iri, the cells were pretreated with free FA (0.5 mg/mL) for 1 h to block the FA receptor on cell surface before incubated with FA-CS/MWCNTs-Iri. Meanwhile, wells containing only cell culture medium were employed as controls. The cells were then washed with PBS to remove non-internalized MWCNTs. Then 180 μL of DMEM and 20 μL of MTT solution were added into each well. After incubation for 4 h at 37 °C, the solution was removed and the formazan precipitate was dissolved in 200 μL of dimethyl sulfoxide (DMSO). The absorbance was measured at 492 nm using a microplate reader. The cell viability was calculated by the following expression:
where AbsSample was the absorbance of the cells treated with the sample, while the AbsControl was the absorbance of control cells. All the tests were performed in triplicate.
Results and discussion
The aim of this work was to develop a new multiparticulate colon-specific drug delivery system which combines pH-dependent and ligand targeted delivery. Drug-loaded CNT cores were functionalized by FA-CS conjugate, and then they were microencapsulated by enteric polymers. Irinotecan which is mainly used in the treatment of colon cancer (Vanhoefer et al., Citation2001) was chosen as drug model. The drug release system was prepared in two steps: (1) irinotecan was entrapped into functionalized CNTs by non-covalent encapsulation; (2) irinotecan-loaded CNTs were microencapsulated by enteric polymers through oil-in-oil solvent evaporation technique.
Morphological property and drug loading efficiency of MWCNTs
The drug loading efficiency of MWCNTs are summarized in . The drug loading efficiency are 61.74 ± 0.85, 67.38 ± 1.02 and 65.56 ± 0.97% for MWCNTs-Iri, CS/MWCNTs-Iri and FA-CS/MWCNTs-Iri, respectively. We attribute this sequence to the properties of materials that were used to encapsulate MWCNTs. Because the Un-decorated MWCNTs tend to form bundles, the interaction between MWCNTs and drug molecules is limited. After decorated with CS, the water dispersity of MWCNTs is increased, and the interaction between MWCNTs and drug molecules become stronger, thus the drug loading efficiency of CS/MWCNTs-Iri is higher than that of MWCNTs-Iri. In contrast, with FA-CS decorated MWCNTs the irinotecan loading is in between due to its high dispersity and steric hindrance. To endow the MWCNTs with cancer targeting properties, we choose FA-CS/MWCNTs-Iri as further research objective.
Table 1. Drug loading efficiency of modified MWCNTs.
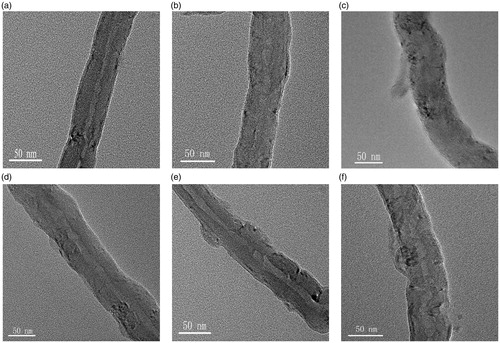
The structure of the decorated MWCNTs was characterized by TEM (). As shown in , the surface morphology of acid-treated MWCNTs is smooth. After decorating with chitosan or FA-CS conjugate, the surface of MWCNTs becomes rough and uneven, and chitosan can be observed on the sidewall of MWCNTs. Moreover, the nanotubes decorated with chitosan was thicker than that of un-decorated MWCNTs, and the clarity of the sidewall of MWCNTs was decreased (). Previous research proved that irinotecan can be encapsulated onto the sidewalls of MWCNTs through non-covalent interaction. It will obtain nanotubes with very heterogeneous surface () by simply mixing the MWCNTs with irinotecan. In contrast, when irinotecan was loaded onto the chitosan decorated MWCNTs, uniformly coated nanotubes were obtained ().
In-vitro drug release profile
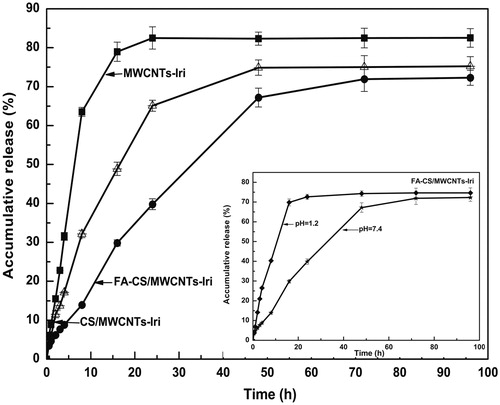
Sustained-release behavior is an important indicator for drug delivery systems. The sustained-release of anticancer drug into tumor tissue can achieve high local concentration with reducing systematic toxicity and enhanced the drug bioavailability. The drug release was affected by a number of factors including pH, surface properties, the interaction force between drug and carriers, particle size (Zhang & Misra, Citation2007). In vitro release behavior of irinotecan-loaded MWCNTs shown in indicate the differences between MWCNTs formulations caused by the properties of materials decorated on the MWCNTs. The release of drug from MWCNTs-Iri and CS/MWCNTs-Iri showed a biphasic release pattern with an initial higher release followed by a slow drug release. FA-CS/MWCNTs-Iri released the drug in a more progressive manner, the release rate being nearly constant throughout the study, and the burst effect was avoided. However, the FA-CS/MWCNTs-Iri released rapidly at pH 1.2, which indicated that the CNTs-based drug delivery system was not suitable for acidic releasing.
Figure 2. In vitro release of irinotecan from the MWCNTs-Iri, CS/MWCNTs-Iri and FA-CS/MWCNTs-Iri drug vectors at 37°Cin PBS (pH = 7.4 and 1.2).
In order to evaluate the drug release kinetics and mechanism, the drug release data obtained from in vitro release experiments were subjected to various kinetic equations. The kinetic models used in this paper were first-order dynamic model, Higuchi distribution model, Korsmeyer & Peppas (K–P) equation, Logistic distribution model and Hixson–Crowell model (Cube root law). The release constants and regression coefficients for all the MWCNTs drug carriers using different kinetic equations are listed in . As shown in , the best fit for in vitro drug release from the different MWCNTs-based drug carrier was found to be the Logistic distribution model, and the first-order dynamic model fitting results also show high consistency. For MWCNTs-Iri and CS/MWCNTs-Iri, value of “n” as per K–P model was found to be lower than 0.45, which means that the release was mainly depended on the Fick diffusion. For FA-CS/MWCNTs-Iri, value of “n” as per K–P model was found to be between 0.45 and 0.89, which is indicative of anomalous behavior of irinotecan release, where diffusion and erosion play an important role (Peppas, Citation1985).
Table 2. Fit for various drug-carbon nanotube carriers using different kinetic equations for describing release kinetics.
Cell viability studies
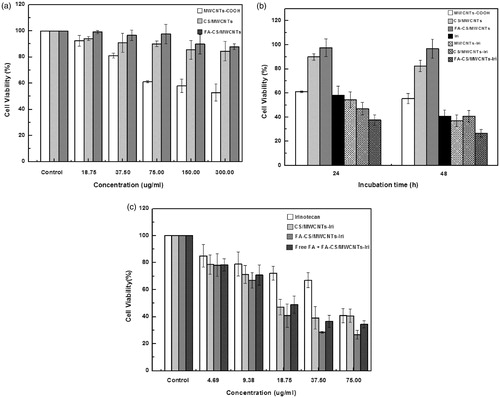
The cytotoxicity of free irinotecan, various carbon nanotubes and irinotecan-loaded MWCNTs was performed on HT-29 cells. Cell viability was assessed by the standard thiazolyl blue 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (Sayes et al., 2006; Ali-Boucetta et al., 2008). As shown in , the cytocompatibility of MWCNTs was greatly affected by the functional groups, and the cell viability of MWCNTs cultured HT-29 decreased when increasing the concentrations of MWCNTs. CS/MWCNTs and FA-CS/MWCNTs, however, impose little effects on the cell proliferation rates of HT-29 cells in 24 h incubation (). As shown in , the FA-CS/MWCNTs-Iri shows the highest cytotoxic, the cell viability lower than 40% after incubation for 24 h, and the cell viability decreased when increasing the incubation time. While incubations with free irinotecan or the un-loaded MWCNTs did not lead to appreciable cytotoxicity at the same concentration. The enhanced cell toxicity of irinotecan after been loaded onto CS/MWCNTs or FA-CS/MWCNTs should be attributed to the unique cell membrane permeability of CNTs. shows the dependency between the cytotoxicity and the concentration of free irinotecan, irinotecan-loaded CS/MWCNTs and irinotecan-loaded FA-CS/MWCNTs. The results indicate that the cell viability increased with the decreasing of sample concentrations, and the toxicity was only observed when the concentration increases beyond a given value. To further evaluate the role of FA in the cellular uptake of nanotubes, the FA receptor was blocked on the surface of HT-29 cells by pretreating HT-29 cells with free FA for 1 h. As shown in , following such treatment a much higher cell viability is observed, demonstrating that the uptake of FA-CS/MWCNTs-Iri and FA is competitive.
Figure 3. Cytotoxicity of the decorated MWCNTs. (a) The cytocompatibility of MWCNTs, CS/MWCNTs, FA-CS/MWCNTs; (b) viability of HT-29 cells treated with 75.00 μg/mL irinotecan, MWCNTs-COOH, CS/MWCNTs, FA-CS/MWCNTs, MWCNTs-Iri, CS/MWCNTs-Iri and FA-CS/MWCNTs-Iri for 24 and 48 h; (c) efficacy of free irinotecan, CS/MWCNTs-Iri and FA-CS/MWCNTs-Iri to kill HT-29 cells at different concentration after incubation for 48 h.
Physiochemical properties of irinotecan-loaded MWCNTs microencapsulated in Eudragit S-100
The second part of this paper was focused on the microencapsulation of irinotecan-loaded MWCNTs cores using an oil-in-oil solvent evaporation technique. The enteric polymer selected was Eudragit S-100 which was soluble at pH values above 7. This polymer was selected to protect the MWCNTs-based cores through the gastrointestinal tract, avoiding any significant drug release before reaching the destination of colon.
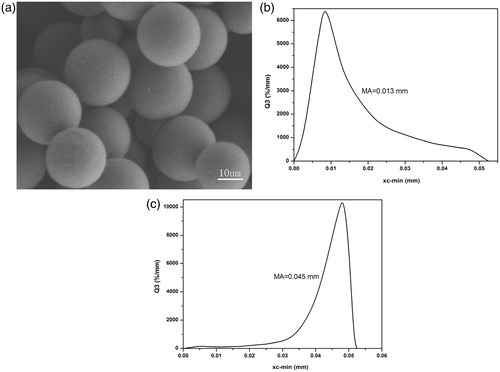
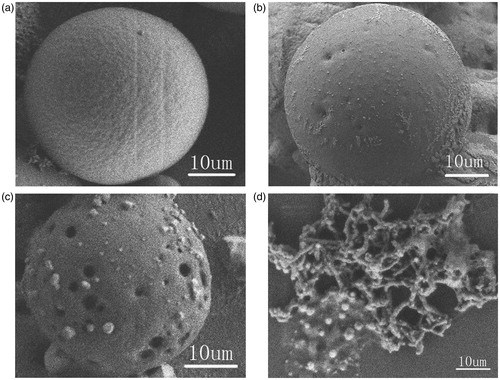
The surface appearance and particle size of Eudragit-coated MWCNTs particles is shown in . As shown in , Eudragit-coated MWCNTs particles were spherical in shape with smooth surface and non-aggregation. The size of Eudragit-coated MWCNTs microspheres are influenced by core/coat ratio. The mean particle sizes were 0.013 mm for CIS-1 and 0.045 mm for CIS-2 ().
In vitro release behavior of irinotecan-loaded MWCNTs microencapsulated by Eudragit S-100
The in vitro release behavior of Eudragit-coated FA-CS/MWCNTs-Iri microspheres was performed in simulated gastro intestinal fluids (). Results showed that nearly no irinotecan release occurred at acidic pHs, the amount of drug release from the formulations CIS-1 and CIS-2 is 4.99 ± 1.12% and 1.65 ± 0.62%, respectively. Once the Eudragit S-100 dissolves at pH values above 7, a continuous release occurred and FA-CS/MWCNTs-Iri will control the release of irinotecan.
The evaluation of the physical appearance of the microspheres during the entire release process was also followed by SEM (). The results revealed that the Eudragit-coated MWCNTs microspheres were totally intact in the release medium of pH values below 7.4 (). The slight increase in cumulative release percentage observed was due to the release of drugs from FA-CS/MWCNTs-Iri carriers which adhered to the surface of the microspheres. When the pH values of the release medium increase to 7.4, the Eudragit coating started to dissolve (), thus leaving the MWCNTs-based cores exposed to the release medium, and the loaded irinotecan started to release continuously. During the early stage of the release, the release rate of irinotecan depends on the Eudragit dissolution. As the amount of MWCNTs-based cores exposed in the medium increases, the release of irinotecan is controlled by FA-CS/MWCNTs-Iri, and the drug release from MWCNTs-based microspheres is also believed to follow the Logistic model ().
Figure 6. SEM images of: (a) Eudragit-coated MWCNTs microspheres after incubation for 6 h at pH 1.2, 4.5 and 6.8; (b), (c) and (d) Eudragit-coated MWCNTs microspheres after incubation for 0.5 h, 2 h and 16 h at pH 7.4, respectively.
Based on the SEM observations above, the probable mechanism of drug release from these Eudragit-coated MWCNTs microspheres could be understood as follows (): once the Eudragit-coated MWCNTs microspheres reach the small intestine, Eudragit will continuously and slowly dissolve over time, thus leaving the MWCNT-based cores gradually exposed to the release medium. The drug loaded onto the MWCNTs will diffuse. After several hours, the MWCNTs-based vectors will reach the colonic region where the WMCNTs-based carriers will enter cancer cells via the FA receptor-mediated pathway.
Conclusions
This paper describes a novel colonic drug delivery system, consisting of FA-CS conjugate encapsulated MWCNTs nanocarrier core-coating microspheres with particle size in the range 11–45 μm. The microspheres had homogeneous surface appearance with spherical nature and uniformed texture. In vitro release behavior showed complete retardation of drug release in acidic pHs while a controlled release of drug from the MWCNTs-based carriers was observed, once the coating layer of Eudragit dissolved at pH 7.4. This double encapsulation technique employing MWCNTs matrix and Eudragit S-100 coating shows promise for targeted delivery and controlled release of irinotecan in colonic disease.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ali-Boucetta H, Al-Jamal KT, McCarthy D, et al. (2008). Multiwalled carbon nanotube--doxorubicin supramolecular complexes for cancer therapeutics. Chem Commun 4:459--61
- Belyanskaya L, Manser P, Spohn P, et al. (2007). The reliability and limits of the MTT reduction assay for carbon nanotubes–cell interaction. Carbon 45:2643–8
- Bianco A, Kostarelos K, Prato M. (2005). Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 9:674–9
- Chourasia M, Jain S. (2004). Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv 11:201--7
- Jose S, Dhanya K, Cinu T, et al. (2009). Colon targeted drug delivery: different approaches. J Young Pharm 1:13--19
- Kam NWS, Jessop TC, Wender PA, et al. (2004). Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into mammalian cells. J Am Chem Soc 126:6850–1
- Lay CL, Liu HQ, Tan HR, et al. (2010). Delivery of paclitaxel by physically loading onto poly (ethylene glycol)(PEG)-graftcarbon nanotubes for potent cancer therapeutics. Nanotechnology 21:5101--4
- Li P, Wang Y, Chen L, et al. (2011). Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29. Carbohyd Res 346:801–6
- Liu Z, Davis C, Cai W, et al. (2008). Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci USA 105:1410–15
- Lorenzo-Lamosa M, Remunan-Lopez C, Vila-Jato J, et al. (1998). Design of microencapsulated chitosan microspheres for colonic drug delivery. J Control Release 52:109–18
- Paharia A, Yadav AK, Rai G, et al. (2007). Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 8:87--93
- Patel HK, Nagle A, Murthy R. (2008). Characterization of calcium alginate beads of 5-fluorouracil for colon delivery. Asian J Pharmaceut 2:241–5
- Peng Z, Feng C, Luo Y, et al. (2010). Self-assembled natural rubber/multi-walled carbon nanotube composites using latex compounding techniques. Carbon 48:4497–503
- Peppas N. (1985). Analysis of Fickian and non-Fickian drug release from polymers. Pharmaceut Acta Helvet 60:110–11
- Philip AK, Philip B. (2010). Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J 25:79--87
- Rodríguez M, Vila-Jato JL, Torres D. (1998). Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J Control Release 55:67--77
- Sayes CM, Liang F, Hudson JL. (2006). Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 161:135--42
- Shivani N, Hetal P, Rajesh K, et al. (2011). Colon delivery of 5-fluoro uracil using cross-linked chitosan microspheres coated with eudragit S 100. Int J Drug Deliv 3:260–8
- Siegel R, Ward E, Brawley O, et al. (2011). Cancer statistics, 2011. CA: Cancer J Clinicians 61:212–36
- Thakral NK, Ray AR, Majumdar DK. (2010). Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J Mater Sci-Mater Med 21:2691–9
- Vanhoefer U, Harstrick A, Achterrath W, et al. (2001). Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol 19:1501–18
- Yang L, Chu JS, Fix JA. (2002). Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm 235:1--15
- Zhang H, Alsarra IA, Neau SH. (2002). An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int J Pharmaceut 239:197–205
- Zhang J, Misra RDK. (2007). Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: core–shell nanoparticle carrier and drug release response. Acta Biomater 3:838–50