Abstract
The present study deals with the development of mucoadhesive controlled release tablets of Cefpodoxime Proxetil to increase the gastric residence time and thus prolong drug release, reduce dosing frequency and improve oral bioavailability. Tablets were prepared using sodium alginate and karaya gum, a natural polymer, with a synthetic polymer hydroxypropylmethylcellulose (K100LV) and Karaya gum with HPMC K100LV in various ratios to optimize the drug release profile using D-Optimal technique. Pre- and post-compression parameters of tablets prepared with various formulations (S1–S9, C1–C9) were evaluated. The FTIR and DSC studies revealed that no physiochemical interaction between excipients and drug. The formulation S7 showed prolonged drug release, and the mechanism of drug release from the optimized formulation was confirmed using the Korsmeyer–Peppas model to be non-Fickian release transport and n value was found 0.605 indicating both diffusion and erosion mechanism from these natural gums. The optimized formulation showed mucoadhesive strength >35 g. An in vivo study was performed on rabbits using an X-ray imaging technique. The radiological evidence suggests that the tablets adheres (more than 10 hours) to a rabbit’s stomach. No significant changes were found in the physical appearance, drug content, mucoadhesive study and in vitro dissolution pattern after storage at 40 °C/75% relative humidity for 3 months.
Introduction
Gastroretentive drug delivery systems can improve the oral bioavailability of various drugs by controlled delivery (Iannuccelli et al., Citation1998; Garg & Sharma, Citation2003). Rate-controlled oral drug delivery systems overcome physiological obstacles such as a short gastric residence time or gastric emptying time (Lehr, Citation1994; Singh & Kim, Citation2000; Kakumanu et al., Citation2008). Such systems are particularly important for drugs that have higher absorption rates in the gastrointestinal tract. Prolonged gastric retention improves oral bioavailability extensive first-pass metabolism of drugs that have an absorption window in a particular region of the gastrointestinal tract (Caldwell et al.,Citation1988a,Citationb; Dollery, Citation1999). Natural gums are among the most popular hydrophilic polymers because of their cost effectiveness and regulatory acceptance. Various gastroretentive techniques have been used to increase the gastric retention of dosage forms including floating (Arora et al., Citation2005; Chavanpatil et al., Citation2006), swelling (Todd, Citation1994), high densities (Saathoff et al., Citation1992) and using bioadhesive systems (Santus et al., Citation1997; Chary & Rao, Citation2000). Cefpodoxime Proxetil is an orally administered third generation, extended spectrum, semi-synthetic antibiotics of the cephalosporin class. The bioavailability of Cefpodoxime Proxetil is only 50% when it is administered in the conventional dosage form. About half of the administered drug is available for therapeutic use. However, it is stable and well absorbed within a pH range of 1–4 (Deshpande & Rhodes, Citation1996; Rouge et al., Citation1998; Klausener et al., Citation2003). The physicochemical properties of Cefpodoxime Proxetil and its short half-life make it suitable for preparation of oral mucoadhesive tablets. Sodium alginate is extracted from Macrocystis pyrifera and karaya gum is a natural polymer obtained as gum exudates of plant species Sterculia (Gershon et al., Citation1972). Gums of natural sources are biodegradable and non-toxic, which hydrates and swells on contact with aqueous media (Nakano & Ogata, Citation1984). Karaya gum is water-soluble thickening agents, which have not been much studied for their pharmaceutical applications. The recent regulatory guidelines issued by the key federal agencies to practice (quality by design (QbD) during drug product development). Design of experiment is a statistical tool used in quality by design approach for formulation optimization, which is used for determining the relationship between different manufacturing parameters and understanding their impact on final product. Thus, design of experiment methods can provide the necessary depth of product and process understanding, which will improve the quality, safety and efficacy of drug product.
In the present study, D-Optimal design was selected as an optimization tool to statistically optimize the mucoadhesive formulations and evaluation of the effects of formulation on the physicochemical and release characteristics. The objective of the present research work was to prepare and evaluate mucoadhesive controlled-release tablets of Cefpodoxime Proxetil that can be adhered in the stomach, so that the gastric residence time is increased, thus prolonging the drug release and improve oral bioavailability by using gelling polymers such as hydroxypropylmethylcellulose and novel natural polymer Karaya gum, Sodium alginate. An optimized formulation was selected for in vivo evaluation through an X-ray study conducted on rabbits over a period of more than 10 hours (Dyer, Citation1997). Different studies have been conducted on using D-Optimal design in optimization of various formulations, but no published work was found regarding the formulation and optimization of Cefpodoxime proxetil mucoadhesive tablets.
Materials and methods
Hydroxypropylmethylcellulose (HPMC K100LV) was supplied by Colorcon Asia Pvt Ltd (Goa, India), Cefpodoxime Proxetil was obtained as a gift sample from Emcure Pharmaceuticals (Pune, India), sodium alginate and MCC KG-100 (microcrystalline cellulose) were obtained from Lubrizol (Mumbai, India). Karaya gum and magnesium stearate were obtained from S.D. Fine Chemicals (Mumbai, India). All other chemicals were of analytical grade.
Drug–excipients compatibility study
Fourier transformation-infrared spectroscopy
Fourier transformation-infrared spectroscopy (FTIR) is used to identify the drug–excipients interaction. FTIR studies were performed on the drug, polymer and a statistically optimized formulation at 40 °C/75% RH over a period of 4 weeks. Sample preparation involved mixing the samples with potassium bromide (KBr), triturating them in a glass mortar and placing them in a sample holder. Samples were analyzed using the potassium bromide pellet method in an IR spectrophotometer (FTIR 8001, Shimadzu, Kyoto, Japan) in the region between 4200 cm−1 and 400 cm−1 (Streobel et al., Citation2006).
Differential scanning calorimetry
Differential scanning calorimetry (DSC) analysis of the drug, polymer (Sodium alginate with HPMC K100LV) and the statistically optimized formulation was carried out using a differential scanning calorimeter (Perkin Elmer Pyris-DSC, Waltham, MA). Samples (8–10 mg) were weighed and placed on aluminum pans, which were hermetically sealed with aluminum lids. Thermograms were obtained at a scanning rate of 10 °C/minute over the temperature range from 25 °C to 250 °C in a nitrogen environment (Streobel et al., Citation2006).
Experimental design
In the present study, statistical experimental design was used in order to get more information about the effect of formulation components on drug release and to obtain the optimum formulation through minimum time and expenses. In this design, two independent variables (concentration of Sodium alginate (X1) and HPMC K100LV (X2)) were selected at three different levels and drug release at 4, 8 and 12 h being dependent variables. The levels of the two factors were selected on the basis of preliminary studies carried out before implementing the experimental design. The experimental design was generated using state-of-art computerized optimization software Design Expert Version 8.0.3.1 (Design Expert, Citation2010). Total nine possible formulations were generated at three different levels of selected polymers X1 (5.0%, 6.5%, 8.0%) and X2 (8%, 10%, 12%) respectively.
Preparation of mucoadhesive tablets of Cefpodoxime Proxetil
Tablets of Cefpodoxime Proxetil were prepared using the direct compression method. Each tablet contained about 250 mg of the drug. All the ingredients were sifted through sieve no. 40, and magnesium stearate was passed through sieve no 60. The required quantities of the materials were mixed thoroughly for 15 min in an octagonal blender and lubricated with magnesium stearate for 3 min. The bulk density, tapped density, flow properties and compressibility index of the power blend were evaluated. The blends were compressed using a single-punch tablet compression machine (Cadmach, Ahmedabad, India) using 8.0 mm standard concave punch, and their physical parameters, such as the average weight, thickness, hardness and friability, were evaluated (Lachman & Hebert et al., Citation1987).
In vitro dissolution studies
The drug release of various formulations (S1–S9, C1–C9) was studied in vitro using USP type II apparatus (Electrolab) set at 100 rpm. A buffer medium with pH 3.0 (900 mL) at 37.5 ± 0.5 °C was used. A 10-mL sample was withdrawn at 1, 2, 4, 6, 8, 10 h time intervals over a period of 12 h and replaced with the same dissolution media (Atyabi et al., Citation1996; Kiortsis et al., Citation2005). The withdrawn samples were analyzed using HPLC at 236 nm.
Kinetic modeling of drug release
The dissolution profile of all the batches was analyzed using the zero order, first order (Gibadi & Feldman, Citation1967; Wagner, Citation1969), Higuchi (Higuchi Citation1963, Citation1967; Cobby & Mayersohn, Citation1974), Hixon–Crowell (Hixson & Crowell, Citation1931), Korsmeyer–Peppas model (Korsmeyer et al., Citation1983; Peppas, Citation1985). PCP Dissolution version 2.08 was selected to perform kinetic modeling of the drug release.
Mucoadhesive study
These were measured by modified balance method, a balance left pan was replaced with a weight to the bottom of which a tablet was attached and both sides were balanced with weight. Porcine gastric mucosa having a thick layer of mucus was fixed to a rubber cork, which was previously attached to the bottom of the beaker containing related medium with a level slightly above the mucosa. The weight was attached to the tablet brought into contact with the porcine mucosa, kept undisturbed for 5 min and then the pan was raised. Weights were continuously added on the right side pan in small increments and the weight at which the tablet detached from the mucosa was recorded as the mucoadhesive strength (Chien, Citation1992; Nur & Zhang, Citation2000), the force of adhesion was calculated using the following formula:
Swelling study
The tablets were weighed individually (weight designated as W1), placed separately in glass beaker containing 200 mL of 0.1 N HCl and incubated at 37 ± 1 °C. The mucoadhesive tablets were removed from the beakers at 1-hour intervals (over a total of 12 h), and the liquid was removed carefully from the surface using paper. The swollen tablets were then weighed (W2). The swelling index (SI) was calculated using the following formula (Dorozynski et al., Citation2004).
where W1 – Initial weight of tablet, W2 – Weight of the swollen tablet.
In vivo study
The protocol for the in vivo study was approved by the Institutional Animal Ethics Committee of R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur (RCPIPER/IPEC/2011-12/01). Permission was obtained from animal ethical committee prior to the experiment. The final formulation (S7) was studied in vivo on New Zealand white rabbits using an X-ray imaging technique (Desai & Balton, 1993; Whitehead et al., Citation1998). In each experiment, three animals were made to fast overnight, but they were given free access to water. Tablets containing 25% barium sulphate was administered to the rabbits, after which they were administered 50 mL of water. A radiograph was taken just before administration of a tablet to verify that there was no radio-opaque material in the stomach. The rabbits were placed in a supine posture and radiographic images taken at 2-hour intervals over a total period of 12 h using an X-ray machine to check the position of the tablet in the gastric region. The distance between the X-ray source and each animal was maintained at a constant value. The X-ray images were taken at Indira Memorial Hospital, Shirpur.
Stability studies
Stability studies were performed to check the effects of environmental conditions and storage conditions on the formulation. The optimized batch, S7, was maintained at 40 °C/75 ± 5% relative humidity over a period of 3 months to study the stability according to the ICH guidelines (International Conference on Harmonization Steering Committee, Citation1999; Mathews, Citation1999). Samples were withdrawn after 1, 2 and 3 months, and the drug content, mucoadhesive study and in vitro dissolution pattern were evaluated.
Results and discussion
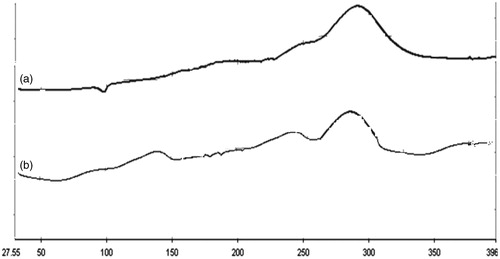
The drug–excipients studies reveal that there were no physical changes in the mixture of drug and excipients. The principal IR absorption peaks observed in the spectrum of the drug are close to those in the spectrum of the drug–excipients mixture indicating that there was no significant interaction between the drug and excipients showed in .
Figure 1. (a) FTIR spectrum of Cefpodoxime Proxetil; (b) FTIR spectrum of drug–excipients mixture; (c) FTIR spectrum of excipients mixture.
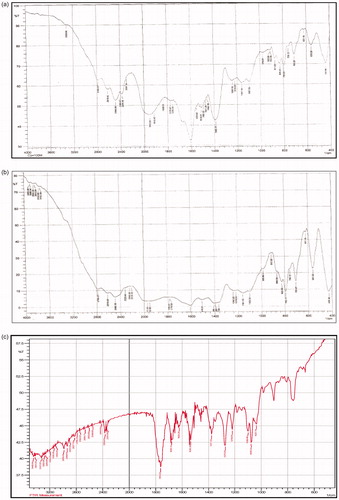
The major IR peaks observed in the spectrum of Cefpodoxime Proxetil are 3066.92 and 2868.60 cm−1 (C–H stretching, aliphatic), 2975 (C–H stretching, aromatic), 3410.26 (N–H stretching), 1623.25 (N–H bending), 1676.20 and 1536.20 (C=N stretching), 1060.17 (C–O stretching), 1753.52 (C=O stretching), 669.32 (C–S–C stretching), 1642 (C=C stretching), 1276.92 and 1211.34 (C–N stretching) and 1357.58 (C–H bending).
There were no significant changes in the position of the peak in the thermogram of the drug–excipients mixture compared with the drug. The thermogram of the drug exhibited a single sharp endothermic peak at 98.79 °C, related to its melting transition temperature (), and the thermogram of the drug–excipients mixture shows a melting endothermic peak at 100.92 °C (). On the basis of the DSC study, it may be concluded that the drug is compatible with all the excipients, as found by the compatibility study.
Figure 2. (a) DSC thermogram of Cefpodoxime Proxetil; (b) DSC thermogram of drug–excipients mixture.
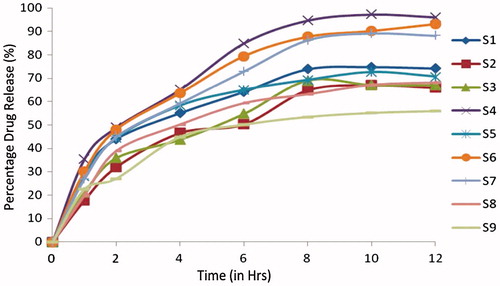
All the physicochemical parameters of the formulations S1–S9 and C1–C9 ( and ) were within the acceptance limit ( and ). The drug content was found to be within a narrow range as specified in pharmacopoeia 2010 (90.0%–110.0%) in all the formulations. The tablets with formulations S1 to S9, containing combinations of sodium alginate with HPMC K100LV in different ratios were evaluated. The formulations S1, S2, S3 S4, S5 and S6 burst within 4 h with cumulative drug release of 99.20 ±0.22%, 99.80 ± 2.89%, 99.50 ± 3.87%, 98.78 ± 3.87%, 98.00 ± 3.20% and 96.000.52%, respectively. Formulations S8, S9 could not maintain its matrix integrity for more than 8 h with release of 98.90 ± 1.23% and 99.10 ± 3.20% of drug, respectively. The mucoadhesive tablets containing sodium alginate with HPMC K100LV (S7) showed a constant drug release up to 12 h (97.22 ± 0.57%). It was observed that type of polymer influences the drug release pattern as shown in and .
Table 1. Composition of Cefpodoxime Proxetil mucoadhesive tablets (S1–S9).
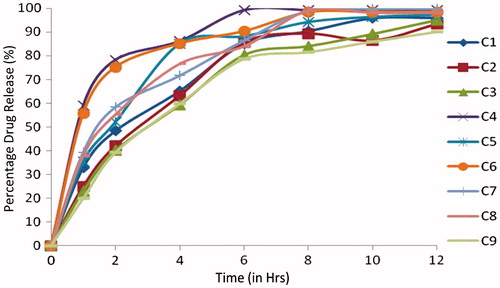
Table 2. Composition of Cefpodoxime Proxetil mucoadhesive tablets (C1–C9).
Table 3. Evaluation of powder blend of Cefpodoxime Proxetil mucoadhesive tablets (S1–S9).
Table 4. Evaluation of powder blend of Cefpodoxime Proxetil mucoadhesive tablets (C1–C9).
The mucoadhesive tablets of formulations C1 to C9 containing combinations with various ratio of karaya gum with HPMC K100LV exhibited cumulative % drug release 96.2 ± 1.35, 95.8 ± 0.63, 93.12 ± 0.56, 98.1 ± 0.24, 96.7 ±0.35, 98.1 ± 1.25, 58.23 ± 0.63, 68.1 ± 0.36 and 79.91 ± 0.45 respectively. Formulations C1, C2 and C3, could not maintain their matrix integrity for more than 4 h and formulations C4 and C6 release within 8 h, formulations C5 and C7 maintain their integrity for 10 h but release is not to the extent of formulation S7. Formulations C8 and C9 forms a very thick gel structure that delays drug release. In presence of karaya gum with HPMC K100LV produces a firm gel that entraps the gas for longer time and delays the release of drug. This controlled release of the drug from formulation C9 could be attributed to the formation of a thick gel structure that delay drug release up to 85.0% at the end of 12 h. Sodium alginate with HPMC K100LV which has a high rate of hydration, thus in vitro dissolution was more rapid compared with karaya gum with HPMC K100LV. The data obtained from the in vitro dissolution studies were fitted to zero-order; first-order, Higuchi, Hixon and Korsmeyer–Peppas equations and the data were analyzed. Kinetic values obtained from different plots of all formulations were, the first-order release rate (R2: 0.895 to 0.992), zero-order release rate (R2: 0.834 to 0.879), Hixon–Crowel release rate (R2: 0.888 to 0.965). Higuchi’s release rate (R2 = 0.970 to 0.978) and Korsmeyer–Peppas release rate (R2: 0.892 to 0.990). From Korsmeyer–Peppas equation the r2 value of the optimized formulation was found to be 0.992 and for n value of 0.605. Korsmeyer’s plot proved that the n values of all the formulation was found between 0.456 and 0.725, therefore all formulations follows non-Fickian release mechanism. Mathematical modeling of in vitro dissolution data indicated the best-fit release kinetics was achieved with first-order release kinetics, which evidenced that the formulations are useful for a controlled release of Cefpodoxime Proxetil.
The optimized formulation showed mucoadhesive strength of 35.0 g and force of adhesion was 3.43 N. The in vitro mucoadhesive study of formulations (S1–S9 and C1–C9) was recorded in and . These results for the mucoadhesive strength are encouraging keeping in view the dynamic conditions under which the tablet has to adhere to the gastric mucosa thereby increasing the gastric residence time thus prolonging the drug release and improve oral bioavailability.
Table 5. Evaluation parameters of Cefpodoxime Proxetil mucoadhesive tablets (S1–S9).
Table 6. Evaluation parameters of Cefpodoxime Proxetil mucoadhesive tablets (C1–C9).
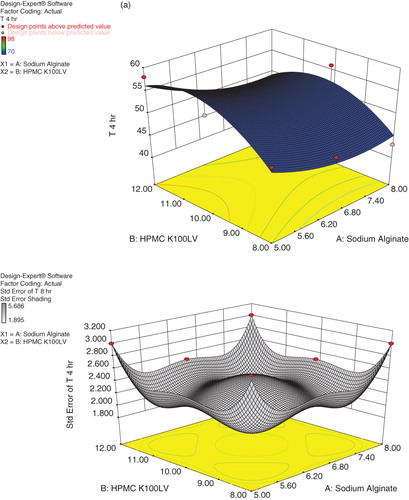
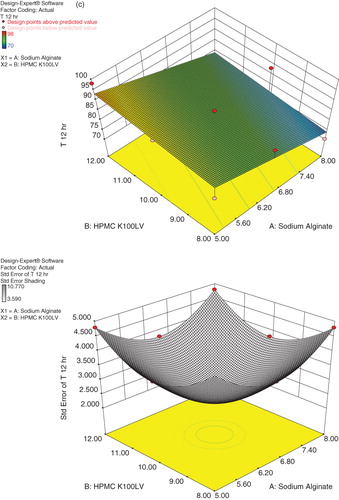
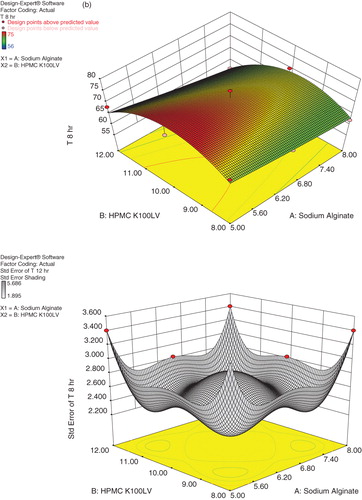
Present study deals with the optimization of formulation variables using mathematical equations and response surface plots to prepare desired Cefpodoxime Proxetil mucoadhesive tablets with suitable physicochemical characteristics. Response surface methodology and multiple response optimizations utilizing the polynomial equation were used to search for the optimal formulation with release rate at different time intervals. A D-Optimal design was constructed to study the effect of amount of sodium alginate and HPMC K100LV on drug release from tablets. The dependent variables chosen were drug release at 4, 8 and 12 h. On the basis of data obtained from formulations subjected to optimization, a statistical model incorporating interactive, polynomial terms as shown in Equation (2) and response surface plots were used to evaluate the responses.
where Y represents the estimated response, b0 is arithmetic mean response of nine runs, and b1 is the estimated coefficient for factor X1; sodium alginate and X2; HPMC K100LV. The main effect represents (X1 and X2) average result of changing one factor at a time from its low to high value. The interaction (X1X2) term shows how the response changes when two factors changed simultaneously. The polynomial terms
are included to investigate nonlinearity. All the responses observed for nine formulations were simultaneously fitted to linear, quadratic and special cubic model by Design-Expert software. The fitted equations relating the response T4, T8 and T12 to the transformed factors are shown in Equations (3), (4) and (5), respectively.
The three dimensional response surface plot presented in shows the plot of concentrations of Sodium alginate and HPMC K100LV verses release at 4, 8 and 12 h, respectively. It was found that as the concentration of sodium alginate increases percentage release was decreased, while increase in concentration of HPMC K100LV percentage drug release was found to be increased. But the X1 coefficient has more pronounced effect on percentage release as compared to X2 coefficient. It may be concluded that higher level of X2 (HPMC K100LV) and moderate levels of X1 (Sodium alginate) favor the preparation of mucoadhesive tablets. The data demonstrate that amount of both X1 and X2 that affects the drug release. The values of correlation coefficient indicate good fit.
It was revealed that as the amount of polymer concentration increases bioadhesive strength also increases by providing more adhesive sites and polymer chains for construal with mucin. Formulation with karaya gum with HPMC K100LV showed stronger mucoadhesion than sodium alginate with HPMC K100LV this may be due to capability of karaya gum to form bioadhesion bond with mucin and construal of polymer chain in the interfacial region, while sodium alginate with HPMC K100LV undergoes only superficial bioadhesion.
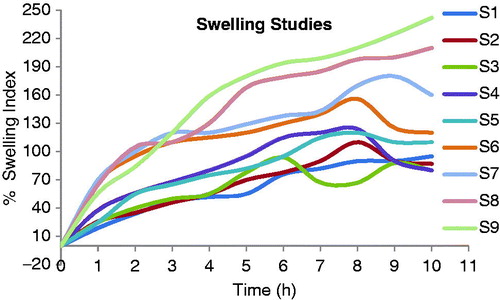
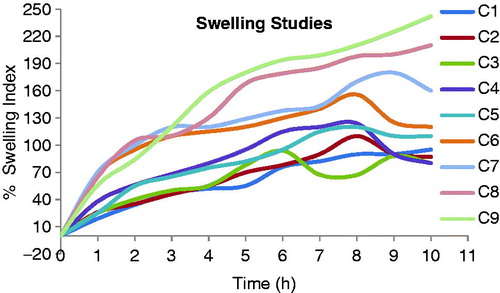
Swelling is also a very important factor that ensures dissolution of the drug in a mucoadhesive tablet. A gel layer is formed around the core of an mucoadhesive tablet with a polymeric matrix when the tablet comes in contact with water. This gel layer governs the release of the drug from the matrix of the tablet. The mucoadhesive tablets containing sodium alginate with HPMC K100 LV (S7) showed a constant increase in swelling, since sodium alginate swell to a great extent in presence of HPMC L100LV and maintain the integrity of their matrix for more than 7 to 8 h. This erosion of the polymer matrix dominates over water sorption after 7 h. Hence a reduction in tablet weight occurs after 8 h. The mucoadhesive tablets containing karaya gum with HPMC K100LV (C5) showed less swelling index at the beginning but thick gel was formed at the end of 8 h. The integrity of matrix of these tablets was also maintained for up to 6–7 h, but the release rate was slightly less due to the formation of a thicker gel matrix compared with the formulation containing sodium alginate with HPMC K100LV (S7). The swelling index of mucoadhesive controlled release tablets of formulations S7 and C5 were 125 ± 10% and 155 ± 10% at 10 h. The swelling index profiles of all the formulations (S1–S9 and C1–C9) are shown in and .
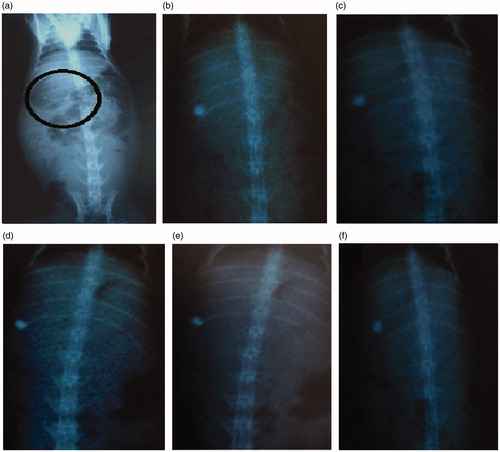
In-vivo study of final formulation S7 was performed using New Zealand Albino rabbits by X-ray imaging technique. Prepared tablets of various concentration of barium sulphate were evaluated for in-vitro mucoadhesion study and it was observed that tablets containing 25% of barium sulphate (relative density 4.477 g/cm3) showed good mucoadhesion property and sufficient integrity. Therefore, tablet containing 25% barium sulphate was selected for in-vivo study administered to rabbit followed by 30 mL water. Rabbit was placed in supine posture for checking the position of tablet in gastric region by using an X-ray machine. The in vivo buoyancy of mucoadhesive controlled release tablets were confirmed by an X-ray imaging at 2-h regular time interval after ingestion of tablet containing BaSO4 to a group of rabbits.
The radiological evidence suggests that formulation S7 was hydrodynamically well balanced for more than 10 h in the stomach of the rabbit, and so we can conclude that the tablets adhere satisfactorily in a rabbit’s stomach as showed in .
Figure 8. X-ray photographs of Cefpodoxime Proxetil mucoadhesive tablets at different time intervals. (a) at 0 h; (b) after 2 h; (c) after 4 h; (d) after 6 h; (e) after 8 h; (f) after 10 h.
On the basis of the in vitro drug dissolution studies, it may be concluded that formulation S7 is most stable. The mucoadhesive tablets were maintained at 40 °C/75% relative humidity in closed high-density polyethylene bottles for 3 months. There were no significant changes in the physicochemical parameters and drug contents as shown in .
Table 7. Evaluation of Cefpodoxime Proxetil mucoadhesive tablets (S7) kept for stability at 40 °C/75% relative humidity.
Conclusion
The observed independent variables were found to be very close to predicted values of most satisfactory formulation, which demonstrates the feasibility of the optimization procedure in successful development of Cefpodoxime Proxetil mucoadhesive tablets. The system with karaya gum with HPMC K100LV cannot delivery the drug over a prolonged period because of its slowly swelling and thick gel formation of the polymers resulting in a lack of hydrogel formation, thus these polymers were found to be unsuitable for the Cefpodoxime Proxetil tablets. It may possibly concluded that increasing percentage of polymer in formulation the decreased drug release pattern, which was dependent on type of polymer used in the formulation. The mucoadhesive tablets containing sodium alginate with HPMC K100 LV (S7) showed controlled drug release up to 12 h and stable at 40 °C/75% relative humidity for a period of 3 months may possibly be a better delivery system for drug like Cefpodoxime Proxetil.
Declaration of interest
All co-authors have seen and agree with the contents of the manuscript and there are no conflicts of interest.
Acknowledgements
The authors are thankful to Emcure Pharmaceuticals for providing a gift sample of the drug, to Colorcon Asia Pvt. Ltd. for providing gift samples of polymers, to Torrent Laboratory for providing gift samples of the excipients and to Indira Memorial Hospital, Shirpur for performing the radio-imaging.
References
- Arora S, Javed A, Khar RK, Baboota S. (2005). Floating drug delivery system: a review. AAPS PharmSciTech 6:372–90
- Atyabi F, Sharma H, Mohammad H, Fell J. (1996). In vitro evaluation of a novel gastroretentive formulation based on ion exchange resins. J Control Release 42:105–13
- Caldwell L, Gardner R, Cargill R. (1988a). Drug delivery device which can be retained in the stomach for a controlled period of times. US patents, 30th Aug 1988; 4,767,627
- Caldwell L, Gardner R, Cargill R, et al. (1988b). Drug delivery device which can be retained in the stomach for a controlled period of times. US patents, 5th April 1988; 4,735--804
- Chary R, Rao Y. (2000). Formulation evaluation of methocel K15 M bioadhesive matrix tablet. Drug Dev Ind Pharm 26:901–6
- Chavanpatil MD, Jain P, Chaudhari S, et al. (2006). Novel sustained release, swellable bioadhesive gastroretentive drug delivery system for ofloxacin. Int J Pharm 316:89–92
- Chien Y. (1992). Novel drug delivery systems. 2nd ed. New York (NY): Marcel Dekker Inc
- Cobby J, Mayersohn M. (1974). Walker GC influence of shape factors on kinetics of drug release from matrix tablets. J Pharm Sci 63:732–7
- Desai S, Balton S. (1983). A floating controlled-release drug delivery systems: in vitro--in vivo evaluation. Pharm Res 10:1321--5
- Deshpande A, Rhodes C. (1996). Controlled released drug delivery systems for prolonged gastric residence: an over view. Drug Dev Ind Pharm 22:531–9
- Design Expert@ software version 8.0.3.1 Operation Manual (2010)
- Dollery C. (1999). Therapeutic drugs. Edinburgh, Scotland: Churchill Livingstone
- Dorozynski P, Jachowicz R, Kulinowski P, et al. (2004). The polymers for the preparation of hydrodynamically balanced systems – methods of evaluation. Drug Dev Ind Pharm 30:947–57
- Dyer J, ed. (1997). Applications of absorption spectroscopy of organic compounds. 1st ed. New Delhi: Prentice-Hall of India Pvt. Ltd
- Garg S, Sharma S. (2003). Gastroretntive drug delivery system. Business Brief Pharmatech 2:160–6
- Gershon S, Pader M, Dentrifices I, et al. (1972). Cosmetics science and technology. New York: Wiley-Interscience
- Gibadi M, Feldman S. (1967). Establishment of sink condition in dissolution rate determination: theoretical consideration application to non disintegrating dosage forms. J Pharm Sci 56:1238–42
- Higuchi T. (1963). Mechanism of sustained-action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–9
- Higuchi T. (1967). Rate of released of medicament from ointment bases containing drugs in suspension. J Pharma Sci 56:1238–42
- Hixson AW, Crowell JH. (1931). Dependence of reaction velocity upon surface agitation. Ind Eng Chem 23:923–93
- Iannuccelli V, Coppi C, Bernabei M, et al. (1998). Air compartment multi unit systems for prolong gastric residence, Part I: Formulation study. Int J Pharm 174:47–54
- International Conference on Harmonization Steering Committee. (1999). Q1A – Stability testing of new drug substances and products
- Kakumanu VK, Arora VK, Bansal AK. (2008). Gastro-retentive dosage form for improving bioavailability of cefpodoxime proxetil in rats. Yakugakud Zasshi Pharm 3:439–45
- Kiortsis S, Kachrimanis K, Broussali T, Malamataris S. (2005). Drug release from tableted wet granulations comprising cellulosic (HPMC or HPC) and hydrophobic component. Eur J Pharm Biopharm 59:73–83
- Klausener EA, Lavy E, Friedman M, Hoffman A. (2003). Expandable gastro retentive dosage form. J Control Rel 90:143–62
- Korsmeyer R, Gurny R, Peppas N. (1983). Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
- Lachman L, Hebert AL, Joseph LK. (1987). Theory and practice of pharmacy. 3rd ed. Bombay: Varghese Publication House
- Lehr CM. (1994). Bioadhesion technologies for the delivery of peptide and protein drugs to the gastrointestinal tract. Crit Rev Ther Drug Carrier Syst 11:119–60
- Mathews BR. (1999). Regulatory aspects of stability testing in Europe. Drug Dev Ind Pharm 25:831–56
- Nokano M, Ogata A. (1984). Examination of natural gums as matrices for sustained release of theophylline. Chem Pharm Bul 32:782--5
- Nur A, Zhang J. (2000). Captopril floating bioadhesive tablets: designed release kinetics. Drug Dev Ind Pharm 26:965–9
- Peppas N. (1985). Analysis of Fickian non-Fickian drug release from polymer. Pharm Acta Helv 60:110–11
- Rouge N, Allemann E, Gex-Fabry M, et al. (1998). Comparative pharmacokinetic study of a floating multiple-unit capsule, a high-density multiple-unit capsule an immediate-release tablet containing 25 mg Atenolol. Pharmaceutical Acta Helv 73:81–7
- Saathoff N, Lode H, Neider K, et al. (1992). Pharmacokinetics of Cefpodoxime Proxetil interactions with an antacid an H receptor antagonist. Antimicrob Agents Chemother 36:796--800
- Santus G, Lazzarini G, Bottoni G, et al. (1997). An in vitro in vivo investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm 44:39–52
- Singh B, Kim K. (2000). Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Rel 63:235–59
- Streobel A, Siepmann J, Bodmeier R. (2006). Gastroretentive drug delivery systems. Expert Opin Drug Deliv 3:217–33
- Todd WM. (1994). Cefpodoxime proxetil: a comprehensive review. Int J Antimicrob Agents 4:37–62
- Wagner J. (1969). Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets, capsules. J Pharm Sci 58:1253–7
- Whitehead L, Fell J, Collet T, et al. (1998). Floating dosage forms: an in vivo study demonstrating prolonged gastric retention. J Control Rel 55:3–12