Abstract
Long-circulating nanoliposomes (LCNs) containing Baicalein (BAI) were prepared by diethyl ether injection method in this paper. Using soybean phosphatidylcholine (SPC) and cholesterol (CHOL) as the carrier ingredients, BAI-loaded LCNs were formulated in optimized condition with encapsulation efficiency (EE) of 41.5 ± 4.77%. The average diameter of BAI-loaded LCNs was ∼709 nm determined by dynamic light scattering (PDI 0.43). Drug storage stability study showed that BAI-loaded LCNs were highly stable in phosphate-buffered saline (PBS, pH 7.4) at 4 °C. Additionally, the pharmacokinetic behaviors of BAI-loaded LCNs were studied using Kunming mice as experimental animals. Parallelly, free BAI solution was studied as control. As expected, it is found that LCNs-encapsulated BAI yields greater BAI oral bioavailability with 4.52 times of free BAI. These results suggest the potential applications of LCNs for the delivery of BAI.
Introduction
Baicalein (BAI, 5,6,7-trihydroxyflavone) is obtained from Traditional Chinese Medicine – Scutellaria baicalensis with diverse interesting bioactivities such as anti-inflammation (Lee et al., Citation2011), attenuating oxidative stress (Choi et al., Citation2010; Stavniichuk et al., Citation2011) and anticancer (Wang et al., Citation2010; Takahashi et al., Citation2011). In China, combined with other herbs, BAI has been widely used in the treatment of inflammation, fever, cough, dysentery, jaundice, hepatitis and hypertension for many years (Zhang et al., Citation2001). Recently considerable attention has been focused on BAI because of its important role in improving cerebral blood flow volume. However, clinical use of free BAI has been limited by its low oral bioavailability (Lai et al., Citation2003; He et al., Citation2011). Recently, some work has been done to improve the oral bioavailability of BAI. Liu and co-workers (Citation2006) reported that the oral bioavailability of BAI formulated with hydroxypropyl-β-cyclodextrin was 1.65-fold enhanced. A self-microemulsifying drug delivery system (SMEDDS) of BAI has been established and the in vivo results showed that the absorption of BAI from SMEDDS resulted in about 200.7% increase in relative bioavailability compared with that of BAI suspension (Liu et al., Citation2012).
Liposomes, with a well biocompatibility and good biodegradability due to the similar structure as cell-surface phospholipid bilayer, have been recently used as a popular nanovesicle for administration of oral drugs including both small molecules and protein owing to its excellent drug-loading rate, targeting and slow releasing action as well as enhanced bioavailability (Tan et al., Citation2010; Huang et al., Citation2011; Park et al., Citation2011; Song et al., Citation2011). In particular, it has been proven that liposomal drugs show better oral bioavailability and long-circulating property (Maud et al., Citation2003; Guan et al., Citation2011; Isacchi et al., Citation2011; Vural et al., Citation2011). Tan et al. (Citation2012) demonstrated that the relative oral bioavailability of evodiamine-phospholipid complex can be significantly increased to 218.82% in comparison with that of free evodiamine. Although liposomes show the advantages described above, there is no report to employ liposomes to deliver BAI and systematically investigate the pharmacokinetic behavior of BAI liposomes in vivo.
In this work, BAI-loaded long-circulating nanoliposomes (LCNs) were prepared by using diethyl ether injection method, and a simple, sensitive as well as rapid high-performance liquid chromatograph (HPLC) method was established to examine its pharmacokinetic behavior in vivo. The data obtained indicated that the oral bioavailability of BAI can be 4.52-fold enhanced by liposomes encapsulation, showing a great potential application of LCNs for BAI-involved disease treatment.
Materials and methods
Chemicals
Both baicalein (BAI, purity >98%) and soybean phosphatidylcholine (SPC, purity >94%) were purchased from Linuo Biological Technology Co., Ltd. (Zhengzhou, Henan Province, China). Cholesterol (CHOL) was obtained from Xi’an Chemical Reagent Company (Xi’an, Shanxi Province, China). The solvents used for the chromatographic analysis were of HPLC grade. All other reagents and solvents were of analytical grade and used directly.
Preparation of BAI-loaded LCNs
Selection of preparation method
BAI-loaded LCNs were prepared respectively following film dispersion method (Bangham et al., Citation1965), reverse phase evaporation method (Szoka & Papahadjopoulos, Citation1978) and diethyl ether injection method (Batzri & Korn, Citation1973). The best one with higher encapsulation efficiency (EE) will be selected to use in the following study.
Optimization of preparation conditions
An orthogonal experiment was performed to optimize the preparation conditions. Considered factors were involved in: (A) mass ratio of SPC and CHOL; (B) dosage of BAI; (C) volume of aqueous solution (0.1 M phosphate buffer, pH 7.4); (D) volume of diethyl ether. The orthogonal designs L9(43) were presented in with EE of BAI-loaded LCNs as evaluation standard.
Table1. The orthogonal designs L9(43) for optimize preparation conditions of BAI-loaded LCNs.
Preparation of BAI-loaded LCNs
According to the results of orthogonal experiment, BAI-loaded LCNs were prepared as follows: 0.020 g of BAI dissolved in 20 mL of phosphate buffer (pH 7.4) was named as solution 1, and 0.480 g of SPC as well as 0.060 g of CHOL mixed in 15 mL of diethyl ether was prepared (defined as solution 2). Then, solution 1 was added dropwise into solution 2. After heated in water-bath to remove diethyl ether, the liposome suspensions were ultrasonic distributed uniformly and stored at 4 °C for the following studies.
Characterization of BAI-loaded LCNs
A total of 2.5 mL of BAI-loaded LCNs solution was respectively transferred into two centrifugal tubes numbered for 1 and 2. Subsequently, 5.0 mL of anhydrous alcohol was added into tube 2. The absorption of free BAI at 278 nm in tube 1 and tube 2 was measured by UV-Vis spectrometer after centrifugation at 12 000 rpm for 30 min at 4 °C. Their concentrations (defined as C1 and C2) are calculated according to the linear regression equation (A = 0.000174354 + 0.03087C with the correlation coefficient of 0.999). Therefore, EE was calculated from the following equation:
BAI-loaded LCNs were evaluated for storage stability (0.1 M phosphate buffer, pH 7.4, at 4 °C). Drug leakage from liposomes was detected at predetermined times (24 h, 1 week, 1 month). A change in the amount of entrapped drug was interpreted as an indicator of liposomal instability.
The average particle size and size distribution of BAI-loaded LCNs were determined using dynamic light scattering (DLS) techniques with a Nano-ZS ZEN3600 instrument (MALVERN Instruments Ltd, Malvern, Worcestershire, UK).
Pharmacokinetics
Apparatus and chromatographic conditions
An Agilent 1100 liquid chromatography system (Agilent Technologies Inc, Santa Clara, CA), equipped with quaternary solvent deliver system and DAD detector, was used. A Agilent TC-C18 (4.6 × 150 mm, 5.0 μm) connected with a Agilent TC-C18 guard column (4.6 × 12.5 mm, 5 μm) at a temperature of 30 °C was applied for all analyses. Detection wavelength was set at 278 nm. The mobile phase consisted of (A) acetonitrile, (B) water and (C) 0.1% trifluoroacetic acid (33:47:20, V:V:V). The flow rate was 0.8 mL/min and aliquots of 20 μL were injected.
Method validation
The method was validated for linearity, precision, accuracy and stability following the International Conference on Harmonization (ICH) guideline.
Sample preparation
Aliquots of 30 µL blood plasma and 15 µL internal standard solutions (30 µg/mL) were added into a centrifuge tube. The sample mixture was mixed with 40 µL of 35% perchloric acid, vortex-mixed for 20 s, and then, the precipitate was removed by centrifugation at 12 000 rpm for 10 min. 20 µL supernatant was injected into the HPLC system. The ratio of the peak area of the sample relative to that of the internal standard was calculated for the quantitative analysis.
Calibration curves
Different amount of BAI was added to mice blank plasma and the final concentration was controlled to 0.05, 0.10, 0.25, 0.50, 1.00, 2.00, 3.00, 4.00 µg/mL for BAI. These standard samples were treated according to the above-mentioned method. Linearity of BAI in mice plasma was determined with three injections for each concentration and plotted using linear regression of the mean peak area versus concentration.
Precision
The measurement of intra- and inter-day variability was carried out to determine the repeatability of the developed assay method. The intra-day repeatability was examined on three individual sample solutions (0.10, 1.00, 3.00 µg/mL) and were determined for six replicates respectively within one day, and inter-day repeatability was determined for three independent days. The relative standard deviation (R.S.D.) was taken as a measure of repeatability.
Accuracy
Known quantities of standard solution were added into the mice blank plasma, and then the resultant samples were treated and analyzed by the established HPLC method. The added standard solution was prepared in the concentration range of calibration curve with three different concentration levels (0.10, 1.00, 3.00 µg/mL) and sextuplicate experiments were performed at each level. The percentage recoveries were evaluated by calculating the ratio of detected amount versus added amount.
Animals and experimental design
Male Kunming mice, weighing approximately 20 ± 2 g, were obtained from laboratory animal center in Huazhong University of Science and Technology. The research protocol of the animal experimentation was approved by the Institutional Animal Ethics Committee, Henan University of Science and Technology, Luo Yang, China. They were placed in animal house, Medical School, Henan University of Science and Technology, Luo Yang, China. Housing conditions were thermostatically maintained at 22 ± 2 °C with constant humidity (55 ± 2%) and a 12-h light–dark cycle, which has in keeping with national standard (Laboratory Animal-Requirements of Environment and Housing Facilities) (GB 14925—2010).
110 mice were randomly divided into 11 groups, 10 of each group. Each group was intragastric given by BAI-loaded LCNs with a dosage of 60 mg/kg body weight (loaded BAI). 1–200 mg/kg of oral administration dose for BAI was generally used (Chen et al., Citation2011; Huang et al., Citation2012; Gandhi, Citation2013) and BAI showed linear pharmacokinetics in rats within the dosage range from 20 to 100 mg/kg (Che et al., Citation2007). After a single dose of liposomes, the mice were narcotized and blood was taken from the veins at various times (0.5 h, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 16 h, 20 h, 24 h). Heparin was used as an anticoagulant to prepare plasma. The concentration of BAI in plasma was measured by HPLC. In addition, the pharmacokinetic features of free BAI solution (60 mg/kg body weight, in 50% ethanol–water solution) were examined with another 110 mice at the same condition.
Data analysis
Data are presented as mean ± SD (standard deviation). Pharmacokinetic parameters were obtained using the Drug & Statistics 1.0 (Mathematical Pharmacology Professional Committee of China, Shanghai, China). Distribution and elimination data were represented by the following parameters: plasma half-life (T1/2); area under the curve (AUC0--∞); total body clearance (CL/F); apparent volume (V1/L); peak concentration (Cmax).
Results and discussions
Characterization of BAI-loaded LCNs
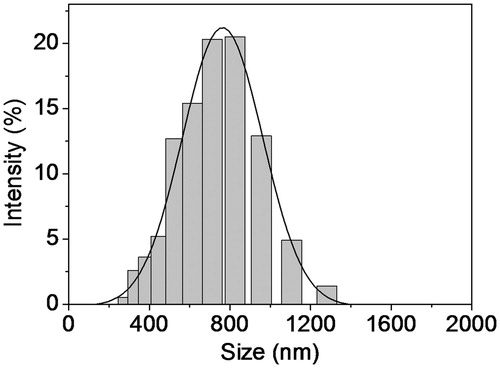
By comparing EE of three methods for preparing BAI-loaded LCNs, the diethyl ether injection method has a higher EE under the same conditions. Therefore, diethyl ether injection method was selected to prepare BAI-loaded LCNs in the following experiments. The results of orthogonal experiments are shown in . The optimized conditions for preparing BAI-loaded LCNs are as follows: mass ratio of SPC and CHOL is 8:1, 0.020 g of BAI should be dissolved in 20 mL of 0.1 M phosphate buffer solution (pH 7.4), and 0.060 g of CHOL in 15 mL of diethyl ether. Under these optimized conditions, three parallel experiments were performed to prepare BAI-loaded LCNs, and the calculated average EE was up to 41.5 ± 4.77%. Using EE as the evaluation principle, it is found that BAI-loaded LCNs prepared in our experiment was very stable for at least 1 month at 4 °C in 0.1 M phosphate buffer solution (pH 7.4) and ∼95% of drug is still entrapped. From the result of DLS analysis presented in , the average diameter of BAI-loaded LCNs was ∼709 nm (polydisperse index, PDI 0.43) with no obvious aggregation.
Table 2. Results of orthogonal experiments.
Pharmacokinetics at single dose in mice
Under our designed experimental conditions for HPLC analysis, BAI and the internal standard can be completely separated with a retention time of 4.5 and 3.8 min, respectively. The calibration curve was drawn by plotting the peak area ratio of BAI to internal standard versus standard concentration of BAI. The peak area response was linear over the range 0.05–4.00 μg/mL with a regression equation, Y = 0.547X − 0.00353. The correlation coefficient was 0.999. The detection limit for ATX (LOD) was 0.0080 μg/mL with a signal-to-noise = 3. The limit of quantification was 0.05 μg/mL with a variation coefficient of 2.7%.
From the results shown in and , the developed analytical method was reproducible with good accuracy. The intra- and inter-day variations were all less than 4% and the percentage recoveries were in the range of 94%–105% with R.S.D less than 2%.
Table 3. Results of intra- and inter-day variability for validation of the proposed method for BAI detection.
Table 4. Results of relative recovery and absolute recovery of BAI in mice plasma (n = 6).
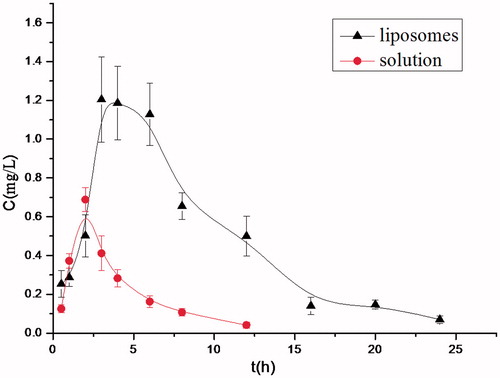
After oral administration of BAI-loaded LCNs in mice, BAI could be detected in plasma. BAI plasma concentration–time curve is shown in . The concentration of BAI had a dynamic change in plasma as time goes by. It can be seen that plasma concentration of BAI reached the peak value 3 h later, and sustained at higher level for 6 h. After 24 h, a little amount of BAI was still present. Whereas, for the free BAI solution, the peak time is 2 h, and the concentration of BAI in mice plasma drastically declined to a relatively low level. All these results strongly demonstrated that the BAI-loaded LCNs prepared in this work show remarkable sustained-release property.
Pharmacokinetic parameters of BAI-loaded LCNs and free BAI solution are shown in . It can be seen that AUC0--∞ of liposomes is 4.52 times of the solution. Using liposome as the drug carrier, the value of CL/F and V1/F decreased significantly, suggesting that the clearing time of BAI in plasma was prolonged, and the retention time was extended. Namely, the oral bioavailability of BAI was 4.52-fold enhanced after it was entrapped into liposomes. It is supposed that hepatoenteral circulation of BAI is possibly present and the first-pass effect may be avoided (Li et al., Citation2005).
Table 5. Pharmacokinetic parameters of BAI-loaded LCNs and free BAI solution in mice plasma (n = 10).
Conclusions
BAI-loaded LCNs with suitable encapsulation efficiency (EE) and good stability were prepared by diethyl ether injection method in this work. Moreover, a simple, sensitive and rapid HPLC method has been developed to quantify BAI in mice plasma. Pharmacokinetics of BAI liposomes have indicated that plasma concentration of BAI in mice detected by HPLC is appropriate for bioequivalence research, and hence, it is suitable for pharmacokinetic studies of BAI.
It has proved that the oral bioavailability of BAI-loaded LCNs was 4.52-fold enhanced compared with BAI solution. Plasma concentration of BAI-loaded LCNs can reach the peak value 3 h later and be sustained within 6 h. It is reported that the oral bioavailability of BAI formulated with hydroxypropyl-β-cyclodextrin and with self-microemulsion was enhanced only 1.65-fold and 2.0-fold, respectively (Liu et al., Citation2006, Citation2012). Apparently, in this paper, formulation BAI with phospholipid liposomes improved its oral bioavailability more significantly and directly, implying a great potential application of LCNs for the BAI-mediated disease treatment.
Declaration of interest
The authors report no declarations of interest.
We acknowledge the financial support from the Natural Science Foundation of the Education Department of Henan Province (2011A350001) and the Scientific and Technological Project of Henan Province (122102340567).
References
- Bangham AD, Standish MM, Watkins JC. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238–52
- Batzri S, Korn ED. (1973). Single bilayer liposomes prepared without sonication. Biochim Biophys Acta 298:1015–19
- Che QM, Yang L, Chen Y, et al. (2007). Comparison of pharmacokinetics between different doses of baicalein in rats. Chin J New Drugs 16:604–6
- Chen LL, Dou J, Su ZZ, et al. (2011). Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res 91:314–20
- Choi JH, Choi AY, Yoon H, et al. (2010). Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and CHOP induction. Exp Mol Med 42:811–22
- Gandhi NM. (2013). Baicalein protects mice against radiation-induced DNA damages and genotoxicity. Mol Cell Biochem 379:277–81
- Guan P, Lu Y, Qi J, et al. (2011). Enhanced oral bioavailability of cyclosporine A by liposomes containing a bile salt. Int J Nanomedicine 6:965–74
- He X, Pei L, Tong HH, Zheng Y. (2011). Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with Pluronic F68 to improve dissolution and oral bioavailability. AAPS PharmSciTech 12:104–13
- Huang HL, Wang YJ, Zhang QY, et al. (2012). Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J Gastroenterol 18:6605–13
- Huang YB, Tsai MJ, Wu PC, et al. (2011). Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J Drug Target 19:709–18
- Isacchi B, Arrigucci S, la Marca G, et al. (2011). Conventional and long-circulating liposomes of artemisinin: preparation, characterization, and pharmacokinetic profile in mice. J Liposome Res 21:237–44
- Lai MY, Hsiu SL, Tsai SY, et al. (2003). Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol 55:205–9
- Lee EK, Kim JM, Choi J, et al. (2011). Modulation of NF-kappaB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Free Radic Res 45:507–17
- Li Z, Ge L, Qi C, et al. (2005). Role of intestinal first-pass metabolism of baicalein in its absorption process. Pharm Res 22:1050–8
- Liu J, Qiu L, Gao J, Jin Y. (2006). Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-beta-cyclodextrin. Int J Pharm 312:137–43
- Liu WL, Tian R, Hu WJ, et al. (2012). Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia 83:1532–9
- Maud C, Fabienne N, Nicole C, et al. (2003). Marine lipid-based liposomes increase in vivo FA bioavailability. Lipids 38:551–9
- Park SJ, Choi SG, Davaa E, Park JS. (2011). Encapsulation enhancement and stabilization of insulin in cationic liposomes. Int J Pharm 415:267–72
- Song YK, Hyun SY, Kim HT, et al. (2011). Transdermal delivery of low molecular weight heparin loaded in flexible liposomes with bioavailability enhancement: comparison with ethosomes. J Microencapsul 28:151–8
- Stavniichuk R, Drel VR, Shevalye H, et al. (2011). Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. Exp Neurol 230:106–13
- Szoka Jr F, Papahadjopoulos D. (1978). Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA 75:4194–8
- Takahashi H, Chen MC, Pham H, et al. (2011). Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta 1813: 1465–74
- Tan ML, Choong PF, Dass CR. (2010). Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 31:184–93
- Tan QY, Liu S, Chen XL, et al. (2012). Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS PharmSciTech 13:535–45
- Vural I, Sarisozen C, Olmez SS. (2011). Chitosan coated furosemide liposomes for improved bioavailability. J Biomed Nanotechnol 7:426–30
- Wang L, Ling Y, Chen Y, et al. (2010). Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett 297:2–8
- Zhang XL, Li ZF, Liu XG. (2001). Review in pharmacological study of Baicalein. Chin Pharmacol Bull 17:711–13