Abstract
Amoitone B, a novel compound chemically synthesized as the analogue of cytosporone B, has been proved to own superior affinity with Nur77 than its parent compound and exhibit notable anticancer activity. However, its application is seriously restricted due to the water-insolubility and short biological half-time. The aim of this study was to construct an effective delivery system for Amoitone B to realize sustained release, thus prolong drug circulation time in body and improve the bioavailability. Nanostructured lipid carriers (NLC) act as a new type of colloidal drug delivery system, which offer the advantages of improved drug loading and sustained release. Amoitone B-loaded NLC (AmB-NLC) containing glyceryl monostearate (GMS) and various amounts of medium chain triglycerides (MCT) were successfully prepared by emulsion-evaporation and low temperature-solidification technology with a particle size of about 200 nm and a zeta potential value of about −20 mV. The results of X-ray diffraction and DSC analysis showed amorphous crystalline state of Amoitone B in NLC. Furthermore, the drug entrapment efficacy (EE) was improved compared with solid lipid nanoparticles (SLN). The EE range was from 71.1% to 84.7%, enhanced with the increase of liquid lipid. In vitro drug release studies revealed biphasic drug release patterns with burst release initially and prolonged release afterwards and the release was accelerated with augment of liquid lipid. These results demonstrated that AmB-NLC could be a promising delivery system to control drug release and improve loading capacity, thus prolong drug action time in body and enhance the bioavailability.
Introduction
The orphan receptor Nur77 (also known as TR3, NGFI-B, TIS1 and NAK-1) is a member of the nuclear receptor superfamily, which plays a crucial role in various biological processes such as cell proliferation, metabolism, differentiation and apoptosis (Maruyama et al., Citation1998; Giguère, Citation1999; Li et al., Citation2006). Previous studies have demonstrated that Nur77 is over expressed in numerous cancer cells including stomach (Wu et al., Citation2002), colon (Cho et al., Citation2007), ovarian (Sibayama-Imazu et al., Citation2008) as well as lung (Kolluri et al., Citation2003) cancer cells. Thus, Nur77 acts as a desired target for the treatment of cancer. Cytosporone B (Csn-B), a natural compound extracted from Dothiorella sp. HTF3, has strong affinity to Nur77 and the ability to activate Nur77 directly, which leads to the translocation of Nur77 from caryon to mitochondria, triggering the release of cytochrome C and killing tumor cells (Brady et al., Citation2000; Zhan et al., Citation2008). A series of derivants is synthesized for better activity.
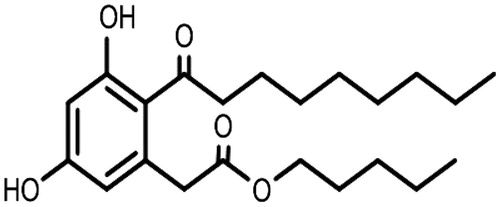
Amoitone B (chemical structure shown in ) is one of the derivants, which has been proved to be the most effective analogue and with the surpassed anticancer activity than its parent compound (Zhan et al., Citation2008; Liu et al., Citation2010). It is regarded as a potential anticancer agent due to its powerful functions for Nur77 and outstanding anticancer activity. Recently, there are more and more attentions paid to the development of Amoitone B. However, the water-insolubility and short biological half-time restrict its successful application. In order to improve the dissolution velocity and saturation solubility, nanocrystal technology was investigated (Hao et al., Citation2012), but there has no research about colloidal drug carriers so far.
Nanostructured lipid carriers (NLC), a novel colloidal delivery system, are developed from solid lipid nanoparticles (SLN) and regarded as the second-generation of lipid nanoparticles (Müller et al., Citation2002a). SLN, consisting of solid liquid, combine the advantages of emulsions, liposomes and polymeric nanoparticles such as fine biocompatibility, good stability, high bioavailability, improved solubility (Dai et al., Citation2010) and controlled drug release (Müller et al., Citation2002b). Moreover, the lipid components of SLN are biodegradable, which can decrease potential toxicity. But the utilization was restricted due to limited loading capacity and expulsion during storage caused by crystallization of lipid matrix (Lin et al., Citation2007).
NLC are composed of the mixture of solid lipid and liquid lipid, possessing the remarkable advantages of SLN. Also, they avoid drug leakage (Dai et al., Citation2010) and obtain higher entrapment efficacy (EE) and loading capacity result from the incorporation of liquid lipid which breaks the ordered crystalline state of solid lipid and enlarges drug storage space (Jenning et al., Citation2000). The characteristics of NLC are changed a lot with augment of liquid lipid. Additionally, NLC can realize passive targeting by altering particle size and achieve active targeting features by modification using proper materials (Jia et al., Citation2010b; Zhao et al., Citation2011). They also can be PEGylated to enhance long circulation (Doktorovová et al., Citation2010; Jia et al., Citation2012). Hence, NLC appear to be an ideal formulation for the efficient delivery of Amoitone B.
There are several methods to prepare NLC, such as microemulsion method, solvent evaporation method, solvent diffusion method, emulsion-evaporation and low-temperature solidification technique, film-dispersion ultrasonic method and high-pressure homogenization (HPH) technique (Hu et al., Citation2006; Dai et al., Citation2010). The HPH technique is generally utilized due to its large scale production ability, whereas the high pressure may result in the coalescence of particles as well as increase degradation rate of drug and carriers (Mehnert & Mäder, Citation2001; Hu et al., Citation2006, Citation2008). Therefore, emulsion-evaporation and low temperature-solidification method with mild condition, simple operation and low energy consumption is developed, which includes emulsification at a high temperature and solidification at 0 °C.
In the present work, AmB-NLC with different amounts of liquid lipid was prepared by the emulsion-evaporation and low temperature-solidification technique. GMS and MCT were selected as the solid and liquid lipid materials, respectively. The emulsifiers were soybean lecithin and poloxamer 188. The physicochemical properties including morphology, particle size, zeta potential, EE, loading capacity, crystalline state and in vitro drug release of NLC were studied in detail, compared with those of SLN. The effects of MCT content on the characteristics of NLC were analyzed specifically.
Materials and methods
Materials
Amoitone B was kindly donated by Xiamen University, China. Glycerol monostearate (GMS) was purchased from Tianjin Damao Chemical agent Co. Ltd., China. Medium chain triglyceride (MCT) was obtained from Croda (Singapore). Poloxamer 188 (F68) was provided by Sigma (St. Louis, MO). Soybean lecithin (Injection grade) was purchased from Shanghai Taiwei Medicine Co. Ltd., China. The methanol (Tianjin Siyou Co., Ltd., China) was of high-performance liquid chromatography grade. All the other reagents and solvents were of analytical grade.
Preparation of AmB-NLC
The NLC were prepared by the method of emulsion-evaporation and low temperature-solidification (Jia et al., Citation2010a). The whole process was described as follows. On one hand, GMS, MCT, soybean lecithin (SL) and Amoitone B powder were weighed accurately and mixed, then dissolved into ethanol (5 mL) absolutely in a water bath at 75 °C to obtain the organic phase. On the other hand, 10 mL aqueous solution containing 250 mg F68 was heated at the same temperature. Then, the organic phase was injected dropwise into the aqueous solution under a mechanical stirrer (ETS-D4, IKA, Germany) with 1200 rpm in a water bath for 2.5 h. The resultant thermal nanoemulsion was dispersed rapidly into 20 mL of distilled water (0–2 °C) in ice bath with stirring at 1000 rpm for 2 h. Consequently, the AmB-NLC dispersions were achieved from the supernatant after centrifugation at 3500 rpm for 10 min. The formulations of AmB-NLC were shown in .
Table 1. Preparation of AmB-NLC.
AmB-NLC dispersions were freeze-dried for long-term stability. Mannitol (5%, m/V) was used as a cryoprotectant in the freeze-drying process. First, the NLC dispersions were pre-frozen with an ultra cold freezer (DW-86 L, Haier, China) at −80 °C for 24 h. Then, the samples were freeze-dried at −50 °C for 48 h utilizing a freeze dryer (FD-1000, EYELA, Japan). The final powders were gathered for the further usage.
Transmission electron microscopy observation
Transmission electron microscopy (TEM) (H-7000, Hitachi, Japan) was applied to examine the morphology of AmB-NLC. A drop of the sample was spread on a 200-mesh copper grid, and then negatively stained with 2% phosphotungstic acid for 30 s. After dried at room temperature, it was observed by TEM.
Particle size and zeta potential analysis
The particle size and zeta potential of AmB-NLC were evaluated by the DelsaTM Nano C Particle Analyzer (Beckman Coulter Inc.). All the samples were diluted with distilled water to obtain a suitable concentration for measurement and every sample was examined in triplicate.
Entrapment efficiency and drug loading determination
It is vital to determine entrapment efficiency (EE) and drug loading (DL) precisely because they are two crucial properties to evaluate preparation technology. Given the water insolubility of AmB, the free drug in formulation was ignorable (Zhai et al., Citation2013). So the drug in preparation was approximately equal to that entrapped into NLC. Briefly, 1 mL of initial AmB-NLC dispersions was disrupted with 4 mL of methanol, sonicated for 10 min to make the drug release thoroughly from the nanoparticles and then centrifuged at 3500 rpm for 15 min. The supernatant was collected as the sample for further examination.
The Agilent 1200 HPLC system (Agilent, Santa Clara, CA) was used to determine Amoitone B content. A Hypersil-ODS2 column (4.60 mm × 250 mm, 5 µm) (Elite, China) was used. The mobile phase was methanol/water (90:10, V/V) with a flow rate of 1.0 mL/min and the test wavelength was 300 nm. The standard curve of peak area against concentration of Amoitone B (μg/mL) was shown as follow: y = 19.01 x −1.6744 (y = peak area, x = Amoitone B concentration). The range was 0.1–60 μg/mL with a correlation coefficient of r = 0.9999. The EE and DL were calculated by the following equations:
Wtotal, Wlipid and Wloaded were the weight of total drug added when preparing AmB-NLC, weight of lipids utilized when preparing AmB-NLC and weight of drug entrapped into AmB-NLC, respectively.
The reason why we ignored the effect of ethanol was that we had heated at 75 °C for 2.5 h when preparing AmB-NLC, so we could consider that the ethanol had volatilized absolutely. Furthermore, we tried to examine the free drug in the supernatant by ultra-filtered centrifugation. In brief, AmB-NLC dispersions were added into Amicon Ultra-4 ultrafiltration device and centrifuged at 3500 rpm for 15 min. So, the free drug in aqueous solution was separated from NLC. A total of 1 ml filtrates were withdrawn and tested by HPLC. As a result, we did not detect drug peak. So we considered the drug content in the supernatant was negligible.
X-ray diffraction analysis
Wide-angle X-ray scattering (WAXS) investigations were performed by an X-ray diffractometer (D/max r-B, Rigaku, Tokyo, Japan). A CuKα radiation source was utilized and the wavelength was set at 1.5405 Å. Standard runs using a 40 kV and 100 mA in this process. Samples were performed with a scanning rate of 0.026°/min and the scanning range of the 2θ from the initial angle 3° to the final angle 45°.
Differential scanning calorimeter analysis
For further determining the crystalline state of NLC, differential scanning calorimeter (DSC) was performed using DSC-41 apparatus. A total of 10 mg of Amoitone B, the lipid, the surfactants, mannitol, the physical mixture and AmB-NLC freeze-dried powder were measured, respectively. For DSC measurement, the sample was put in an aluminum pan. A scanning rate of 10 °C/min was employed in the 50–500 °C temperature range and α-Al2O3 was used as a reference.
In vitro release study
The release of AmB-NLC was examined by the dialysis method. In short, proper volume of NLC dispersion (containing 1 mg of Amoitone B) was added to the dialysis bag and then the dialysis bag was placed into 200 mL release medium (15% ethanol in PBS, pH 7.4), stirred at 100 rpm on a magnetic stirrer at 37 °C. A total of 1 mL of solution was withdrawn and fresh release medium at equal volume was added quickly to maintain the constant volume (200 mL) at the predetermined intervals. The samples were filtered with 0.22 μm filters and then assayed by HPLC method under the same analytic conditions described above. The results of triplicate measurements were used to calculate cumulative drug release.
Stability test
Lyophilized powder of AmB-NLC with 15% MCT content was stored at 4 °C for a period. The powder was re-dispersed with distilled water by vortexing before the determination of particle size and zeta potential. The drug content and EE were measured by the method described above.
Results and discussion
TEM examination
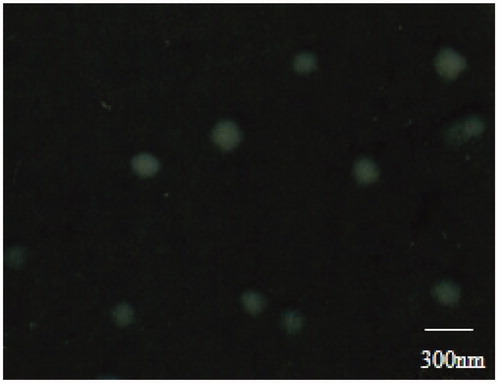
The morphology of AmB-NLC was showed in and it was obvious that the nanoparticles were in spherical shape and non-adherent among each other. The particle size was about 200 nm with a uniform distribution. From the picture, we could draw the conclusion that the NLC was prepared successfully in terms of morphology and the formulation was stable due to the uniform distribution.
Particle size and zeta potential analysis
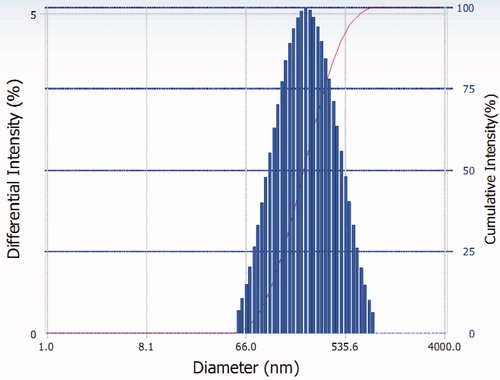
Particle size and zeta potential are two key indexes to evaluate the nanoparticles. The results were listed in . The particle size distribution of AmB-NLC was displayed in , from which we could see a narrow distribution for AmB-NLC. Zeta potential analysis was an effective way to estimate the storage stability of nanoparticles. It was reported that the formulation had good stability when its zeta potential was above −30 mV (critical value) due to the powerful static repulsion produced by ionic surfactants which prevented aggregation among particles. In other words, the higher zeta potential implied the better stability within limits. In our study, the zeta potential was about −20 mV. Although it was below the critical value, we also could say the formulation has the long-term stability as a result of the use of nonionic surfactants which offered strong steritical hindrance (Müller & Jacobs, Citation2002; Jia et al., Citation2010b). The non-ionic surfactant used in this work was F68 and aggregation of nanoparticle was avoided due to sufficient steritical hindrance. In a word, a stable formulation had been obtained.
Table 2. Results of AmB-NLC (n = 3).
Drug EE and DL capacity
EE and DL are two important characteristics for preparation evaluation. According to a great deal of literatures, the incorporation of liquid lipid could break the regular crystal structure of solid lipid and the disordered structure was able to enlarge storage space of drug, which led to the improvement of drug EE and DL capacity (Jenning et al., Citation2000; Dai et al., Citation2010). In present study, formulations with different liquid lipid content were investigated and the results were listed in . Taking samples 8, 4, and 5 as examples, it was obvious that the EE and DL were 52.2%, 71.1%, 78.8% and 2.61%, 3.55%, 3.94%, respectively. Thus, with the augment of MCT proportion, the EE and DL were enhanced gradually, which was owing to the higher solubility of drug in liquid lipid as well as the enlarged space for drug.
X-ray diffraction
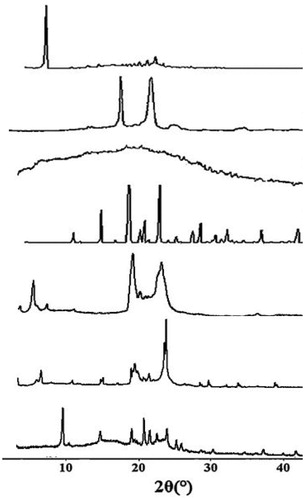
XRD is employed extensively to analyze the changes of nanoparticles crystalline state. In order to detect whether the crystalline structure of Amoitone B in NLC was changed or not, the XRD test was carried out. showed that the characteristic peaks of Amoitone B (a) (6.27°), F68 (b) (19.04° and 23.15°) and GMS (c) (5.53°, 19.40° and 23.41°) could be found in the curve of the physical mixture while the characteristic peak of Amoitone B was disappeared in the curve of NLC, indicating a disordered crystalline state of drug in NLC. In addition, the peak intensities of GMS and F68 were remarkably weakened in the AmB-NLC freeze-dried powder. These results further proved that Amoitone B had been incorporated into the lipid matrix in amorphous or disordered structure.
Differential scanning calorimeter
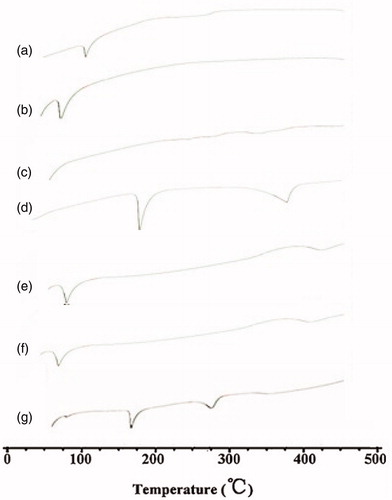
The DSC patterns of Amoitone B (a), F68 (b), soybean lecithin (c), mannitol (d), GMS (e), the physical mixture (f) and the AmB-NLC freeze-dried powder (g) were revealed in , from what we could see that characteristic peaks of Amoitone B (106.4 °C), GMS (62.7 °C) and F68 (57.3 °C) could be found in the curve of the physical mixture, implying the original crystalline states of them which remained unchanged when mixed physically. The characteristic peak of Amoitone B, however, disappeared in the curve of NLC, meaning a non-crystal form of the drug in NLC. This phenomenon further illustrated that Amoitone B had been incorporated or absorbed thoroughly in NLC, demonstrating the formation of AmB-NLC.
In vitro release studies
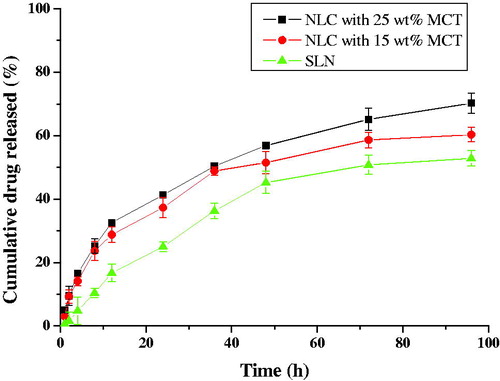
The in vitro release of drug was studied to confirm whether the controlled-release process was achieved successfully. Theoretically, conditions in vitro release studies should be consistent with that in vivo as much as possible. Simultaneously, the feasibility of the experiment should be taken into account. Amoitone B is insoluble in water. Considering the limit of detection of the high-performance liquid chromatograph (Agilent 1200) in our laboratory, improving the solubility of Amoitone B in PBS (pH 7.4) is very important to detect the concentration of Amoitone B in the dissolution medium more accurately. Therefore, PBS (pH 7.4) with 15% ethanol was selected as the dissolution medium to improve the solubility of Amoitone B and gain the sink conditions. Taking samples 6 and 7 as examples, the release curves of AmB-NLC were displayed in , which revealed a two-stage process. A biphasic drug release pattern was observed. First, a fast drug release was found at the initial 12 h, with about 30% of total Amoitone B released during this period. Then followed a sustained release and approximate 70% of the Amoitone B released in 96 h. The release curve of AmB-SLN was also shown in which implied a more sustained release.
Figure 6. In vitro drug release profiles of formulations with different liquid lipid content in PBS (pH7.4) (n = 3).
The mechanism of nanoparticles release was considered as the following three forms. First, the dissolution and diffusion of the drug absorbed in the surface of nanoparticles led to the burst release. Second, the corrosion of lipid materials, which depended on numerous factors such as the pH, the temperature, the properties of materials, and so on. Third, the degradation of nanoparticle materials, in which process the hydrolysis and enzymolysis happened and the drug was released from nanoparticles. For the NLC, the release process was complicated, including several kinds of concomitant forms.
The phenomenon of could be explained by the developmental process of NLC. Originally, the drug was dispersed in the emulsion droplet of the initial nanoemulsion due to its water-insolubility. During the cooling of nanoemulsion, the drug was precipitated first because of its high melting point, and then the solid lipid was rapidly solidified into a solid lipid core containing most of the drug. At the same time, the liquid lipid was encapsulated in or scattered randomly to be the outer shell. A few drugs was dispersed or absorbed in the surface. When contacting the release solution, the superficial drug was freed quickly, leading to the burst release in the initial stage. Then with the degradation and erosion of lipid matrix, the drug incorporated into nanoparticles core was released in a prolonged way (Müller et al., Citation2000; Dai et al., Citation2010).
It was obvious that the release rate was accelerated with increasing of MCT content. The reason might be that the liquid lipid had higher dissolving capacity for lipophilic drug and most MCT was distributed at the surface of NLC, so more drugs were at the shell of NLC and the release was facilitated thanks to the augment of MCT (Zur Mühlen et al., Citation1996; Hu et al., Citation2002).
Storage stability of NLC
There was no agglomeration found in the freeze-dried powder of AmB-NLC after 3 months storage at 4 °C. The size was 241.2 ± 4.4 nm (n = 3) and zeta potential was −18.4 ± 0.2 mV (n = 3), which showed no significant changes and the EE and drug content were 71.5 ± 1.1% (n = 3) and 99.4 ± 0.27% (n = 3), respectively. The results indicated a good stability of AmB-NLC.
Conclusion
Amoitone B, a newly synthesized compound with remarkable antitumor activity, is regarded as a promising agent for tumor therapy. In this study, we provided a formulation for the pharmaceutical-prepared development of the drug. Amoitone B-loaded NLC were prepared successfully by the emulsion-evaporation and low temperature-solidification technology to realize sustained release and improved loading capacity. Numerous characteristics of NLC with different contents of MCT were investigated in detail. The incorporation of liquid lipid into solid lipid matrix led to increased EE and DL as well as enhanced storage stability. In vitro release evaluation was carried out, proving a burst release first and the prolonged release subsequently and it was accelerated with increasing of liquid lipid. XRD and DSC measurements revealed the amorphous or less ordered form of Amoitone B in NLC. Therefore, NLC can be a promising dosage form for parenteral delivery of Amoitone B for cancer therapy. Furthermore, further investigation on cytotoxicity in vitro and the pharmacokinetic study in vivo are in progress.
Declaration of interest
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this article. This work was supported by the National Basic Research Program of China (973 Program), No. 2009CB930300.
References
- Brady SF, Wagenaar MM, Singh MP, et al. (2000). The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org Lett 2:4043–6
- Cho SD, Yoon K, Chintharlapalli S, et al. (2007). Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor–dependent and nuclear receptor–independent pathways. Cancer Res 67:674–83
- Dai WT, Zhang DR, Duan CX, et al. (2010). Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul 27:234–41
- Doktorovová S, Araújo J, Garcia ML, et al. (2010). Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC). Colloids Surf B Biointerfaces 75:538–42
- Giguère V. (1999). Orphan nuclear receptors: from gene to function. Endocr Rev 20:689–725
- Hao LL, Wang XY, Zhang DR, et al. (2012). Studies on the preparation, characterization and pharmacokinetics of Amoitone B nanocrystals. Int J Pharm 433:157–64
- Hu FQ, Jiang SP, Du YZ, et al. (2006). Preparation and characteristics of monostearin nanostructured lipid carriers. Int J Pharm 314:83–9
- Hu FQ, Yuan H, Zhang HH, Fang M. (2002). Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization. Int J Pharm 239:121–8
- Hu FQ, Zhang Y, Du YZ, Yuan H. (2008). Nimodipine loaded lipid nanospheres prepared by solvent diffusion method in a drug saturated aqueous system. Int J Pharm. 348:146–52
- Jenning V, Thünemann AF, Gohla SH. (2000). Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm 199:167–77
- Jia LJ, Shen JY, Zhang DR, et al. (2012). In vitro and in vivo evaluation of oridonin-loaded long circulating nanostructured lipid carriers. Int J Biol Macromol 50:523–9
- Jia LJ, Zhang DR, Li ZY, et al. (2010a). Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv 17:11–18
- Jia LJ, Zhang DD, Li ZY, et al. (2010b). Nanostructured lipid carriers for parenteral delivery of silybin: biodistribution and pharmacokinetic studies. Colloids Surf B Biointerfaces 80:213–18
- Kolluri SK, Bruey-Sedano N, Cao X, et al. (2003). Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol 23:8651–67
- Li Q, Ke N, Sundaram R, Wong-Staal F. (2006). NR4A1, 2, 3 an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol 21:533–40
- Lin XH, Li XW, Zheng LQ, et al. (2007). Preparation and characterization of monocaprate nanostructured lipid carriers. Colloid Surface A 311:106–11
- Liu JJ, Zeng HN, Zhang LR, et al. (2010). A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Res 70:3628–37
- Maruyama K, Tsukada T, Ohkura N, et al. (1998). The NGFI-B subfamily of the nuclear receptor superfamily (review). Int J Oncol 12:1237–43
- Mehnert W, Mäder K. (2001). Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–96
- Müller R, Jacobs C. (2002). Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm 237:151–61
- Müller RH, Mäder K, Gohla S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm 50:161–77
- Müller R, Radtke M, Wissing S. (2002a). Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 242:121–8
- Müller R, Radtke M, Wissing S. (2002b). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54:S131–55
- Sibayama-Imazu T, Fujisawa Y, Masuda Y, et al. (2008). Induction of apoptosis in PA-1 ovarian cancer cells by vitamin K2 is associated with an increase in the level of TR3/Nur77 and its accumulation in mitochondria and nuclei. J Cancer Res Clin 134:803–12
- Wu Q, Liu S, Ye XF, et al. (2002). Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis 23:1583–92
- Zhai YJ, Guo SS, Liu CH, et al. (2013). Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloid Surface A 429:24–30
- Zhan YY, Du XP, Chen HZ, et al. (2008). Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol 4:548–56
- Zhao X, Zhao YL, Geng LL, et al. (2011). Pharmacokinetics and tissue distribution of docetaxel by liquid chromatography–mass spectrometry: Evaluation of folate receptor-targeting amphiphilic copolymer modified nanostructured lipid carrier. J Chromatogr B 879:3721–7
- Zur Mühlen A, Zur Mühlen E, Niehus H, Mehnert W. (1996). Atomic force microscopy studies of solid lipid nanoparticles. Pharmaceut Res 13:1411–16