Abstract
Availability of proper concentration of medicament on to the corneal surface is a challenging task. Many novel formulations, i.e. hydrogels, nanoparticles, ocuserts, etc. had been tested to improve ocular bioavailability, out of which our group found, in situ gel and polymeric nanoparticle are the most interesting approach to achieve ocular retention. We found that in situ gel stay only for 12 h and poly(lactic-co-glycolic acid (PLGA) nanoparticles are non mucoadhesive in nature so we try to combine both these formulations and termed it as “Nanoparticle laden in situ gel”. Here we prepare nanoparticle laden in situ gel containing levofloxacin encapsulated PLGA nanoparticle, incorporated in chitosan in situ gel and evaluated its ocular retention by gamma scintigraphy in rabbits. The observations of acquired gamma camera images showed good retention over the entire precorneal area. From static and dynamic gamma scintigraphy evaluation, we can be interpret that developed nanoparticle laden in situ gel formulation cleared at a very slow rate and remained at corneal surface for longer duration than marketed formulation, in situ gel and nanosuspension alone.
Introduction
In spite of incessant research in ocular drug delivery systems, proper bioavailability in ocular region is a demanding task. A number of ocular diseases like glaucoma, conjunctivitis and dry eye syndrome necessitate frequent drug administration. The physiological constraints imposed by the protective mechanisms of the eye lead to poor absorption of drugs, resulting in a short duration of the therapeutic effect. When a drug solution is dropped into the eye, the natural tear drainage and blinking action of the eye result in a 10-fold reduction in the drug concentration within 4–20 min (Maurice, Citation1987; Schoenwald, Citation1990). This compels the clinician to recommend a frequent dosing at an extremely high concentration which results in pulse type dosing and in several cases it may lead to side effects of ophthalmic products. The availability of medicament at corneal surface would be considerably improved if the precorneal residence time of drugs could be increased. Our group found in situ gel and nanoparticles to be promising formulations. Every formulation has its limitation, i.e. chitosan in situ gel is mucoadhesive but remains for 12–15 h (Gupta et al., Citation2009) whereas poly(lactic-co-glycolic acid (PLGA) nanoparticles are non-mucoadhesive in nature, so retaining them on eye is challenging (Nanjawade et al., Citation2007). In curiosity to try combination of both these formulation strategies we developed the “nanoparticle laden in situ gel”. This system can be formulated as a nanosuspension in liquid dosage form suitable to be administered by instillation into the eye which, upon exposure to physiological conditions, changes to the gel phase, thus increasing the pre-corneal residence time of the nanoparticles and enhancing ocular retention. In our present work, we are preparing levofloxacin nanoparticle laden in situ gel and evaluating its ocular retention on rabbits using gamma scintigraphy.
Materials and methods
Materials
Levofloxacin was received as gift sample from Micro Labs Ltd. (Chandigarh, India); PLGA (50:50), IV = 0.2 dl/g, was obtained from Purac Biomaterial, Singapore. Poly vinyl alcohol (PVA, MW 95 000) was obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Chitosan (practical grade, 75–85% deacetylated, molecular weight 150 kDa) was obtained as kind gift from M/s, India Sea Foods, India. All other chemicals were of analytical grade.
Preparation of nanoparticle laden in situ gel
Levofloxacin nanoparticles were prepared as per the previous published work (Gupta et al., Citation2010). Concisely, nanoprecipitation technique was used to prepare PLGA nanoparticles. Drug and polymer ratio was maintained at 1:10 (drug 10 mg, PLGA 100 mg) and dissolved in acetone (5 ml) at room temperature. The resulted solution was slowly dropped with constant speed (0.5 ml/min) into 20 ml of 1.5% w/v PVA solution with continuous stirring at 1800 rpm. Acetone and some water were evaporated, and the remaining aqueous suspension was collected. The nanosuspension was then centrifuged at 18 000 rpm, 20 °C for 1 h (Remi, Mumbai, India). Nanoparticles were collected after three wash with distilled water and then lyophilized by means of Christ Alpha 1–4 lyophilizer (Christ, Osterode am Harz, Germany) using 1%w/v mannitol as lyoprotectant.
Whereas, in situ gel base is prepared by dissolving 0.5%w/v chitosan in 1% v/v acetic acid and pH was adjusted to 5.5–6.0 by phosphate buffer system (Gupta et al., Citation2009).
For preparation of the nanoparticle laden in situ gel, weighed quantity of drug loaded freeze dried nanoparticles (equivalent to the prescribed dose of levofloxacin 0.5% w/v) were taken and dispersed in the respective 1 ml of in situ gel base (0.5%w/v chitosan solution). Freeze dried nanoparticles were weighed on the basis of its drug loading (Gupta et al., Citation2010). Formulation was tested for initial physicochemical characterization i.e. clarity, osmolarity, gelation pH, particle size of nanoparticles and viscosity.
In vivo ocular retention study – gamma scintigraphy
Gamma scintigraphy is used to assess in vivo precorneal drainage of the developed formulation. Male New Zealand albino rabbits weighing 1.8–2.5 kg of either sex and free from any signs of ocular inflammation or gross abnormality were used in the study. Animals were procured from the animal house of Institute of Nuclear Medicine & Allied Sciences (INMAS) Delhi, India and all study protocols were approved by local institutional Animal Ethics committee. Levofloxacin was radiolabeled with Tc-99 m by direct labelling method using stannous tartarate in concentration of 200 μg at pH 6.5. Gamma camera (Millenium VG, Milwaukee, WI), autotuned to detect the 140 keV radiation of Tc99m was used for scintigraphy study. Rabbits were anesthetized using ketamine HCl injection given intramuscularly in a dose of 15 mg/kg body weight. The rabbits were positioned 5 cm in front of the probe and 50 µl of the radiolabeled formulation was instilled onto the left corneal surface of the rabbits. Recording was continued for 30 min with a frame of 5 s using 128 × 128 pixel matrix. Individual 60 frames (60 × 30 s) were also captured by dynamic imaging process. Region of interest (ROI) was selected on the image and time–activity curve was plotted to calculate the rate of drainage from eye. A single whole body static image was also taken after 5 h of instillation of drug/formulation. Each formulation was tested on three rabbits.
Results and discussion
In our present work, we have prepared levofloxacin loaded PLGA nanoparticle laden chitosan in situ gel. As an approximation, 60 mg of levofloxacin nanoparticles (∼5 mg of levofloxacin) was mixed with 1 ml of in situ gel. Final composition of LNG is given in . The developed nanoparticles laden in situ gel was then evaluated for various physicochemical characteristics (). Particle size and polydispersity index (PI) of levofloxacin nanoparticles were found to be 199 nm and 0.344 nm, respectively. The preparation was well dispersed with slight turbidity. After reconstitution of freeze dried nanoparticle in in situ gel, no difference is found in gelation pH and viscosity of the formulation.
Table 1. Final composition of levofloxacin nanoparticles laden in situ gel (LNG).
Table 2. Physicochemical characterization of levofloxacin nanoparticles laden in situ gel.
Gamma scintigraphy
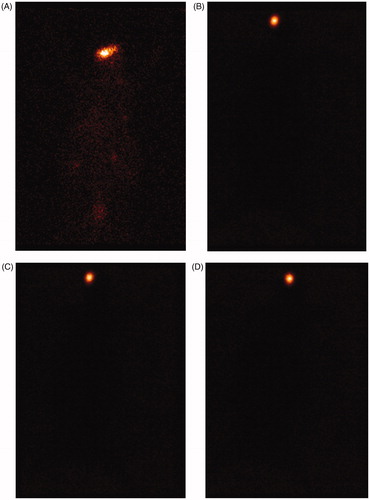
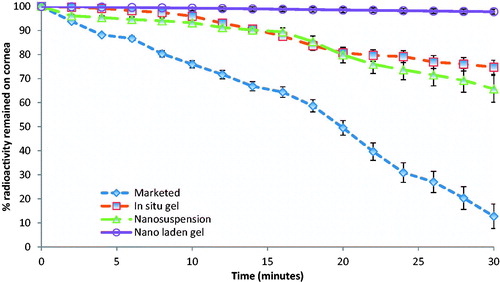
For scintigraphic studies, levofloxacin was radiolabeled with radionuclide Tc-99 m. The Tc-99 m–levofloxacin complex was found to give the labeling efficiency of 95.2%. After administration of the radiolabeled formulation, a good spreading was observed over the entire precorneal area. Marketed formulation cleared very rapidly from the corneal region and reached in to systemic circulation via nasolachrymal drainage system as significant activity was recorded in kidney and bladder after 5 h of ocular administration (), whereas formulations, i.e. in situ gel (LG), nanosuspension (LN) and nanoparticle laden in situ gel (LNG) were retained at corneal surface for longer duration. No significant radioactivity was observed in systemic circulation (kidney and bladder) even after 5 h (). To differentiate between retention times of formulations (LG, LN, LNG) and marketed eye drop solution, we have carried out dynamic studies for 30 min. The time–activity curve of the remaining activity on the corneal surface as a function of time was plotted (). The marketed formulation shows a quick fall in radioactivity counts with respect to time on corneal surface as compared to LG, LN and LNG showing that the nanoparticle laden in situ gel retained for longer duration giving extended release as compared to all other formulations.
Conclusion
PLGA nanoparticles of levofloxacin, due to nano-size, are able to provide prolong retention on the eye than the marketed formulation. PLGA is an anionic polymer and hence it is non mucoadhesive in nature. To enhance the efficacy of the levofloxacin nanoparticulate formulation, we incorporate them in mucoadhesive chitosan in situ gel to make nanoparticle laden in situ gel which helps in retention of nanoparticles on the eye. A good spreading and retention of formulation was observed in gamma scintigraphy studies. In time–activity curve, there is a minimal fall in counts/s of formulation as compared to rapid fall of marketed formulation further shows that the nanoparticles laden in situ gel have better retention and bioavailability. However, more work need to be carried out to prove efficacy of the new system “nanoparticle laden in situ gel”.
Declaration of interest
The authors report no conflict of interest.
Acknowledgements
Authors are thankful to Council of Scientific and Industrial Research (CSIR) for providing SRF fellowship to Mr Himanshu Gupta for carrying out this research work.
References
- Gupta H, Aqil M, Khar RK, et al. (2009). Development and characterization of Tc-99m timolol maleate for evaluating efficacy of in situ ocular drug delivery system. AAPS Pharm Sci 10:540–6
- Gupta H, Aqil M, Khar RK, et al. (2010). Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J Drug Target 19:409–17
- Maurice DM. (1987). Kinetics of topical applied drugs. In: Saettone MS, Bucci P, Speiser P, eds. Ophthalmic drug delivery, biopharmaceutical, technological and clinical aspects. Fidia Research Series. Padova, Italy: Liviana Press, Vol. 11, 19–26
- Nanjawade BK, Manvi FV, Manjappa AS. (2007). In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Rel 122:119–34
- Schoenwald RD. (1990). Ocular drug delivery: pharmacokinetic considerations. Clin Pharmacokinet 18:255–69