Abstract
Now a day’s intranasal (i.n) drug delivery is emerging as a reliable method to bypass the blood–brain barrier (BBB) and deliver a wide range of therapeutic agents including both small and large molecules, growth factors, viral vectors and even stem cells to the brain and has shown therapeutic effects in both animals and humans. This route involves the olfactory or trigeminal nerve systems which initiate in the brain and terminate in the nasal cavity at the olfactory neuroepithelium or respiratory epithelium. They are the only externally exposed portions of the central nervous system (CNS) and therefore represent the most direct method of noninvasive entry into the brain. This approach has been primarily used to explore therapeutic avenues for neurological diseases. The potential for treatment possibilities with olfactory transfer of drugs will increase as more effective formulations and delivery devices are developed. Recently, the apomorphine hydrochloride dry powders have been developed for i.n. delivery (Apomorphine nasal, Lyonase technology, Britannia Pharmaceuticals, Surrey, UK). The results of clinical trial Phase III suggested that the prepared formulation had clinical effect equivalent to subcutaneously administered apomorphine. In coming years, intranasal delivery of drugs will demand more complex and automated delivery devices to ensure accurate and repeatable dosing. Thus, new efforts are needed to make this noninvasive route of delivery more efficient and popular, and it is also predicted that in future a range of intranasal products will be used in diagnosis as well as treatment of CNS diseases. This review will embark the existing evidence of nose-to-brain transport. It also provides insights into the most relevant pre-clinical studies of direct nose–brain delivery and delivery devices which will provide relative success of intranasal delivery system. We have, herein, outlined the relevant aspects of CNS drugs given intranasally to direct the brain in treating CNS disorders like Alzheimer’s disease, depression, migraine, schizophrenia, etc.
Introduction
Oral drug delivery is the most desirable route for drug administration whenever systemic effects are intended (Pires et al., Citation2009). Oral drug delivery methods fail to deliver a number of therapeutic agents to the brain efficiently due to the several drawbacks such as slow onset of action, low bioavailability (40–45%), nausea and incomplete pain relief with recurrence of headaches. Additionally, oral formulations exhibits a short half-life of 1–2 h, with the drug undergoing hepatic first-pass metabolism and being rapidly cleared by the hepatic and the renal system (Jain et al., 2010b). There are barriers in the brain, that is, blood–brain barrier (BBB) which is localized at the level of the endothelial cells and separates brain interstitial fluid (ISF) from blood and blood-cerebrospinal fluid (CSF) barrier (BCSFB), which separates CSF from the choroid plexus interstitial fluid (CP ISF). The two barriers that represent the largest interface between blood and brain extracellular fluids, the BBB and the BCSFB, prevent the free paracellular diffusion of polar molecules by complex morphological features, including tight junctions (TJs) that interconnect the endothelial and epithelial cells, respectively (Redzic, Citation2011). BBB which serves to protect the brain and spinal cord from various noxious substances and pathogens also presents a barrier in the treatment of CNS disorders. Accordingly, a number of invasive strategies like, intraventricular, intraparenchymal, intrathecal delivery (BBB disruption) and noninvasive techniques like chemical modifications, prodrug approach and conjugation of a drug with antibodies or ligands have been used to increase the targeting of CNS drugs (Haque et al., Citation2012). Over the recent decades, intranasal (i.n.) drug delivery is now recognized as a noninvasive route for CNS drugs and reliable alternative to oral and parenteral route (Pires et al., Citation2009). The nasal mucosa emerged as a target tissue for drug delivery and offers many benefits over the oral route due to its large surface area, high blood flow, porous endothelial membrane, avoidance of hepatic first pass metabolism and ready accessibility (Jadhav et al., Citation2007). A wide variety of therapeutic compounds can be administered intranasally for topical, systemic and CNS action (Pires et al., Citation2009).
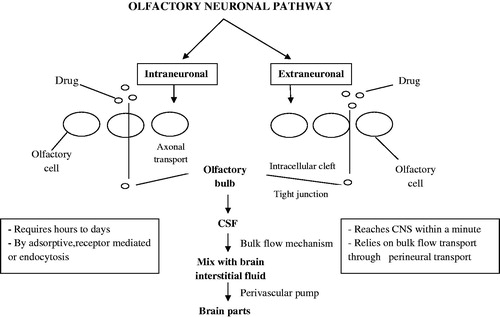
Various drug transport pathways have been described, such as the systemic pathway, in which the drug is absorbed directly into the systemic circulation across the nasal cavity and then across the BBB into the brain; the olfactory pathway, in which the drug passes through the olfactory epithelium into the olfactory bulb and further into the brain tissue or into the cerebrospinal fluid (CSF); and the trigeminal pathway, in which the drug is transported via this nerve system (Khan et al., Citation2010). Olfactory neuronal pathway is further divided into two intraneuronal and extraneuronal pathways. It has been described in . In intraneuronal pathway olfactory neurons in the olfactory epithelium uptake the molecules by processes such as endocytosis, which then reach the olfactory bulb by axonal transport. In extraneuronal pathway, intranasally administered substances first cross the gaps between the olfactory neurons in the olfactory epithelium, which are subsequently transported in to the olfactory bulb. After reaching the olfactory bulb, the substances may enter into other brain regions by diffusion, which may also be facilitated by a “perivascular pump” that is driven by arterial pulsation (Ying, Citation2008). There is difference between the drug delivery by olfactory or trigeminal nerve systems and by nasal mucosa permeation. Once the drug is administered by nasal cavity, the drug permeates through highly vascularized nasal mucosa to systemic circulation and may or may not cross BBB and enter brain. However, olfactory epithelium, olfactory neuron or trigeminal nerves play an important role for targeting drugs to brain.
Nose-to-brain delivery of drug moieties have been attempted by several researchers who exploited the advantages of this route like ease in drug delivery and needle-free drug application without the need of trained personnel, facilitates self-medication, noninvasiveness, essentially painless, avoidance of hepatic first-pass metabolism and thus potential for dose reduction compared to oral dose. The rapid absorption, fast onset of action due to relatively large absorption surface, high vascularization, avoidance of chemical and enzymatic degradation of drugs in the gastrointestinal (g.i.t) fluid, better permeability of lipophilic, low molecular weight drugs through nasal mucosa make this route for administration of drugs such as peptides or protein (Jadhav et al., Citation2007).
Nose-to-brain delivery is possible by the olfactory region located at the roof of nasal cavity, and its neuroepithelium is the only exposed portion of the CNS to the external environment (Haque et al., Citation2012). Drugs delivered intranasally are transported along olfactory sensory neurons to yield significant concentrations in the CSF and olfactory bulb. The olfactory region of nasal mucosa that provides a direct connection between nose and brain is used for targeting of CNS acting drug molecules used in conditions like Alzheimer’s disease, depression, migraine, schizophrenia, etc. (Kumar et al., Citation2008). Although the olfactory route has not been investigated widely in humans because of difficulties in absolute measurements of drug in the CSF or brain tissues, several studies in animals have been published for drugs such as olanzapine, risperidone, buspirone, ropinorole, didanosin, zolmitriptan, sumatriptane, rivastigmine, venlafaxine, clonazepine.
The mathematical parameters like drug targeting index (DTI), direct transport percentage (DTP %), drug targeting efficiency (DTE %) as well as their visualization techniques like gamma scintigraphy are used for evaluating these systems. The degree of drug targeting to brain after i.n. administration can be evaluated by DTI which can be described as the ratio of the value of AUC brain/AUC blood following i.n. administration to that following i.v. administration. The higher the DTI is, the better the drug targeting to brain can be expected after i.n. administration (Wang et al., Citation2003). Brain targeting efficiency, that is, DTE% and DTP% that represents time average partitioning ratio are calculated as follows:
1. Drug targeting efficiency (DTE%) that represents time average partitioning ratio is calculated as follows:
2. Nose-to-brain direct transport percentage (DTP%) are calculated as follows:
Bi.v is the AUC0–240 (brain) following intravenous administration.
Pi.v. is the AUC0–240 (blood) following intravenous administration.
Bi.n is the AUC0–240 (brain) following intranasal administration.
Pi.n. is the AUC0–240 (blood) following intranasal administration (Zhang et al., Citation2004).
Gamma scintigraphy imaging is performed on animal’s brain following i.v. and i.n. administrations to determine the localization of drug in brain and other tissues such as heart, kidney, esophagus, stomach and intestine. Imaging is performed using Single Photon Emission Computerized Tomography (SPECT, LC 75-005, Diacam, Siemens AG, Erlanger, Germany) gamma camera (Md et al., Citation2012).
Conventionally, several dosage forms have been used for intranasal delivery such as liquid drop, liquid spray/nebulizer, aerosol, gel and suspension spray. The nasal cavity presents several barriers like physical removal of formulation by mucocilliary clearance, enzymatic degradation and low permeability of the nasal epithelium. Researchers investigated several methods for the enhancement of drug permeation through the nasal mucosa such as use of mucoadhesive polymers and absorption enhancers to prolong the contact time of drugs with the nasal mucosa and thereby to enhance their permeation. Various investigated nanoformulations are nanoparticles, nanoemulsion, nanosuspension, nanostructured lipid carrier, microemulsion and solid lipid nanoparticles (SLNs). Based on the reports on nanoformulations these formulations could be employed as an effective carrier for the delivery of therapeutic agents through the nose-to-brain route for the treatment of CNS disorders.
In this article, we will discuss the drugs delivered intranasally to direct the brain and compare the efficacy of intranasal drug delivery of a drug with that of other formulations of the same drug (i.e. intravenous (i.v), subcutaneous, oral, transdermal) in treating of CNS disorders.
Drugs investigated/used for nose-to-brain delivery
Bromocriptine
Bromocriptine (BRC) an ergoline derivative and dopamine D2 receptors agonist has been used to delay and minimize the motor fluctuations associated with long-term levodopa treatment in Parkinson’s diseases (PD). BRC has been found to have antioxidant effects, inhibit free radical formation and scavenge free radicals, and retard apoptosis, which is accelerated in PD (Lim et al., Citation2009). Reports on currently available dosage forms, that is, tablet (Sicriptin™), indicated that it could not cross BBB and, after oral administration of BRC, it exhibits low or incomplete absorption and low bioavailability due to high metabolism in the liver (Vautier et al., Citation2006). A number of strategies such as drug delivery and drug targeting systems have been investigated for BRC to prevent drug degradation and loss so as to increase its bioavailability with high concentrations of BRC accumulated in the targeted area (Degim et al., Citation2003). Esposito and coworkers investigated nanolipidic carriers (NLC) of BRC for brain delivery. However, route of administration was invasive requiring surgical expertise which could be disadvantageous for formulations (Esposito et al., Citation2012). Researchers further studied the nanocarrier approach and formulated polymeric nanoparticles (NPs) as a drug carrier system for noninvasive administration of the drug. The administration of formulations by intranasal route was found to be comparatively suitable as compared to other approaches and has some advantages over the conventional routes for administration of formulations such as the ability to circumvent P-glycoprotein (P-gp)-mediated resistance, the possibility of bypassing the BBB without structural modifications and the possibility of employing biocompatible and biodegradable materials (Cho et al., Citation2008). Md and coworkers formulated BRC-loaded chitosan nanoparticles (CS NPs) by ionic gelation of CS with tri-polyphosphate anions and evaluated the ability of distribution of BRC solution and BRC-loaded CS NPs to brain regions and blood, together with the estimation of pharmacokinetic parameters of BRC and BRC-loaded CS NPs in different areas of the body. The brain/blood ratio of 0.47 ± 0.04, 0.69 ± 0.031 and 0.05 ± 0.01 for BRC solution (i.n.), BRC-loaded CS NPs (i.n.) and (i.v.), respectively, at 0.5 h were indicative of direct nose-to-brain transport bypassing the BBB. The gamma scintigraphy imaging of mice brain were performed to determine the localization of drug in brain. The drug targeting index (DTI) and direct transport percentage (DTP %) for BRC-loaded CS NPs following intranasal route were 6.3 ± 0.8 and 84.2 ± 1.9%. These encouraging results suggest that the formulation is a novel effective noninvasive brain drug delivery system of BRC for the treatment of PD (Md et al., Citation2012). In our opinion, this direct nose-to-brain delivery of formulation into the brain will allow for targeted drug delivery and thereby allow numerous other CNS drugs to be delivered to the brain tissue, mostly unchanged and eliminating systemic side effects.
Buspirone HCL
Buspirone hydrochloride is an anxiolytic agent used to treat anxiety caused by alcohol craving or smoking cessation, as well as attention deficit hyperactivity disorder in children (Galichet, Citation2004). BuSpar® (buspirone hydrochloride tablets) is the currently available dosage form which undergoes extensive first-pass metabolism, which leads to low oral bioavailability (4%), short half-life of 2–3 h and a low concentration in the brain (Balo, Citation1994). Hence, researchers worked on the nasal CS drug formulations to provide a longer time for drug transport across the nasal membrane, thereby increasing the brain concentration by avoiding the mucociliary clearance mechanism (Dodane et al., Citation1999). The nasal formulation (Bus-chitosan) prepared using buspirone hydrochloride, CS hydrochloride, hydroxypropyl β-cyclodextrin (HP-β-CD) in sodium chloride solution improved the therapeutic efficacy from nose to brain, avoided first-pass metabolism in the liver and markedly improved the bioavailability to 61%. Besides this, the plasma concentration peak was at 30 min compared with the nasal Bus-Solution formulation in which peak was found to be 60 min. The nasal Bus-chitosan formulation was compared with that for Bus hydrochloride solution after intravenous and intranasal (Bus-Solution) administration in plasma and brain of rats by gamma scintigraphic study. It was found that AUC0–480 in brain after nasal administration of Bus-chitosan formulation was 2.5 times higher than that obtained by intravenous administration and intranasal Bus-plain formulation. The higher DTP and DTI were found with Bus-chitosan formulation as compared to other formulation indicative of direct nose-to-brain transport. These results conclusively demonstrate increased access of buspirone to the blood and brain from intranasal solution formulated with CS and HP-β-CD. In our opinion, there could be a better therapeutic efficacy of nanoformulation of buspirone instead of buspirone solution determined from nose to brain (Khan et al., Citation2009). The small size nanoformulation could help in controlled release of drug and ease of its penetrability across olfactory epithelium for brain targeting.
Clonazepam
Clonazepam is a benzodiazepine derivative having anxiolytic, muscle relaxant, antionvulsant, sedative and hypnotic properties (Jenner et al., Citation1986). It is the drug of choice in suppression of myoclonic seizures and acts by facilitating the inhibitory neurotransmittor transmission, gamma amino butyric acid, in the brain by a direct effect on benzodiazepine receptors (Rey et al., Citation1999). Presently, clonazepam is available in tablet (marketed as Linotril or Klonopin) and injectable dosage forms. These formulations result in limited drug uptake across the BBB due to release of clonazepam into the peripheral circulation, thereby enhancing its distribution to the non-targeted sites. Previous studies have demonstrated that direct transport of drugs to the brain circumventing the BBB following intranasal administration provides a unique feature and better option to target drugs to brain. Researchers investigated various intranasal formulations using mucoadhesive agents and their benefits in enhancing nose-to-brain drug transport. Brain-targeted clonazepine microemulsion (CME)/clonazepine mucoadhesive microemulsion (CMME) were prepared by using medium chain triglyceride as an oil, polyoxyethylene-35-ricinoleate as a surfactant, polysorbate 80 as a cosurfactant and propylene glycol as an anhydrous continuous phase. CMME was prepared by addition of polycarbophil to CME. The brain/blood uptake ratios of intranasal CMME, CME, clonazepam solution (CS) with intravenous CME at 0.50 h were found to be 0.67, 0.50, 0.48 and 0.13, respectively, indicating more effective targeting with intranasal administration and best targeting of the brain with intranasal CMME. Brain/blood ratio at all sampling points up to 8 h following intranasal administration of CMME compared to intravenous was found to be twofold higher, indicating larger extent of distribution of the drug in brain. This investigation demonstrated more rapid and larger extent of transport of clonazepam into the rat brain with intranasal CMME, which may prove useful in treating acute status epileptics. In our perspective, the intranasal delivery of clonazepam is a promising route for brain targeting. However, more preclinical and clinical studies should also be performed in near future to establish these formulations in the market on the basis of low risk/high benefit ratio as compared to high risk/low benefit ratio in their present forms (Vyas et al., 2006b).
Deferoxamine
Deferoxamine (DFO) is an FDA-approved high-affinity iron chelator used to treat acute iron poisoning and acts by binding free iron in the bloodstream and enhancing its elimination in the urine and treats ischemic stroke damage, as well as intracerebral hemorrhage (Freret et al., Citation2006). There are certain evidences which indicate that dysregulation of brain iron metabolism and transport causes neuronal damage and cell death in ischemic stroke. Desferrioxamine Mesilate 500 mg and 2 g Powder for Injection are available in market. DFO is generally not injected intravenously for two reasons. Firstly, it is a small molecule and is eliminated rapidly through the kidney. Secondly, the injection of an intravenous bolus can cause acute hypotension that is rapid and can be lethal (Dragsten et al., Citation2000). Subcutaneous and intramuscular injections of DFO are often associated with local injection site reactions. These negative attributes of DFO have limited its utility as a neuroprotective agent. However, a noninvasive intranasal method for the administration of DFO has recently emerged as a rapid way to bypass the BBB and target DFO to the brain, reduce its systemic exposure and treat stroke damage after middle cerebral artery occlusion (MCAO) in rats (Dhanda et al., Citation2005). Researchers administered DFO intranasally at a dose of 6 mg and the result was compared with intravenous administration, and it has been found that intranasal DFO resulted in significantly higher brain concentrations (0.9 to 18.5 μM) at 30 min as compared to intravenous administration (0.1 to 0.5 μM, p < 0.05). Intranasal administration of DFO increased targeting to the frontal cortex by 271-fold when compared to intravenous delivery. These results suggest that intranasal administration of DFO is a promising noninvasive alternative to systemic administration for the treatment of ischemic stroke (Hanson et al., Citation2009).
Didanosine
Didanosine (2′,3′-dideoxyinosine, ddI), an reverse transcriptase inhibitor effective against HIV, as part of highly active antiretroviral therapy (HAART) has primarily been administered orally (Hartman et al., Citation1990) and available as tablet and capsule form (didanosine, Videx/VidexEC). It exhibits several disadvantages related to oral delivery including, low and highly variable bioavailability and limited CNS penetration, metabolism in the g.i.t., first-pass liver metabolism, and its highly polar nature resulting consequently in low intestinal permeability (Anderson et al., Citation1995). The above oral drawbacks can be overcome by delivering the antiviral drugs ddI intranasally efficiently to the CNS to avoid first-pass effect and hence improve bioavailability. Researchers prepared ddI-loaded CS-NPs through ionotropic gelation of CS with tripolyphosphonate anions, and the NPs were administered intranasally to rats compared to ddI solution which was administered intranasally and intravenously. The concentrations of ddI in blood, CSF and brain tissues were analyzed by ultra performance liquid chromatography mass spectroscopy (UPLC/MS). The results of ddI were compared after intranasal administration of ddI NPs or solution with intravenous administration of ddI aqueous solution for higher brain/plasma, olfactory blood/plasma and CSF/plasma concentration ratios (p < 0.05) of intranasally administered ddI NPs or solution was observed. Thus, both the intranasal route of administration and formulation of ddI in CS-NPs increased delivery of ddI to CSF and brain. These results suggested that ddI can reach CNS compartments via a direct pathway initiating in the nasal cavity and does not include systemic circulation (Al-Ghananeem et al., Citation2010). In our view, CS NPs possibly enhance the drug absorption and improve the efficacy of ddI uptake from the nasal cavity to brain.
Duloxetine
Duloxetine (DLX) hydrochloride is a potent dual reuptake inhibitor of both serotonin and norepinephrine (SNRI) (Bymaster et al., Citation2001) and it is efficacious in the treatment of both emotional and painful physical symptoms in patients with major depressive disorder (MDD). Currently available dosage forms, that is, tablet and capsule of DLX in market (Dlx Tab, Dulane Cap), exhibit low systemic bioavailability due to extensive first-pass metabolism, which indicates availability of even lower amount in the brain due to the presence of BBB. Moreover the non-targeted delivery results in numerous side effects (Jain et al., Citation2011). Nose to brain delivery of drugs not only provides a direct delivery to brain but also bypasses BBB and first-pass metabolism. Researchers formulated nanostructured lipid carrier (NLC) containing duloxetine (DLX-NLC) using homogenization-ultrasonication method. NLC consists of a mixture of spatially different lipids in which solid lipid is blended with liquid lipid (oil) (Muchow et al., Citation2008), and it is a colloidal drug carrier which shows better drug delivery characteristics than the carriers including polymeric nanoparticles, fat emulsions, liposomes and SLNs and exhibit very low cytotoxicity relative to polymeric NPs (Yang et al., Citation1999). The authors evaluated the permeability of DLX-NLC through porcine nasal mucosal membrane so that it can be targeted to the brain through nose-to-brain route of drug delivery. Both the formulations (DLX-NLC dispersion and lyophilized DLX-NLC) exhibited sustained release of DLX. The in vitro release results revealed that DLX was released from DLX-loaded NLC formulations for a prolonged period of time, thus maintaining the DLX level and, hence, antidepressant effect for a longer period of time. Storage temperature affected the NLC stability and it was found that DLX-NLCs were more stable at 4 °C than room temperature. Moreover, the lyophilized NLCs were more stable than the NLC dispersions. The result of permeation studies through porcine nasal mucosal membrane confirmed the possibility of delivery of drug-loaded NLCs through nasal route. Based on these studies, it was concluded that the NLC can be employed as an effective carrier for the delivery of therapeutic substances even through the nose-to-brain route for the treatment of CNS disorders (Alam et al., Citation2011).
Alam and coworkers also compared the efficacy of DLX-loaded NLC with DLX solution pharmacodynamically following intranasal administration and estimated DLX concentration in brain and blood after administration to albino Wistar rats either intranasally or orally in solution form (DLX solution) or after encapsulation in NLC (DLX-NLC). NLC was prepared using the liquid lipid propylene glycol monocaprylate mixed with melted solid lipid (glyceryl monostearate) and pluronic F-68 (poloxamer) as surfactant solution. It has been found that intranasal DLX-NLC treatment exhibited improved behavioral analysis results such as swimming, climbing and immobility as compared to the DLX solution treatment after 24 h of study. Studies conclusively demonstrated higher concentration of intranasal DLX-NLC in brain compared with DLX solution. Thus, intranasal DLX-NLC was found to be a promising formulation for the treatment of depression (Alam et al., Citation2012).
Erythropoietin
Erythropoietin (EPO) is a glycoprotein hormone that regulates erythropoiesis, or red blood cell production, and possesses neuroprotective effects on hypoxic/ischemic cerebral damages and experimental subarachnoid hemorrhage (Ehrenreich et al., Citation2004). Currently, systemic (intraperitoneal or subcutaneous) and intracerebroventricular (i.c.v.) injections of EPO are available (Epoetin Alfa Injection) for treatment of cerebral ischemia (Maiese et al., Citation2004). Systemic injections have some drawbacks which include inability to penetrate through BBB and undesirable systemic effects on other tissues, while i.c.v. injection can cause brain damages and infections and is not suitable for clinical use (Brines et al., Citation2000). Researchers evaluated neuroprotective effect by administering recombinant human EPO (rhEPO) intranasally to rats with focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO). It has been found that rhEPO administered intranasally at doses of 4.8, 12 and 24 U (administered 10 min after MCAO and 1 h after reperfusion) reduced infarct volume, brain swelling and cell damage in the ischemic hemispheres, and improved behavioral dysfunction 24 h after cerebral ischemia. Intraperitoneal rhEPO (5000 U/kg) also showed the protective effect, but the heat-inactivated rhEPO did not show any effect. It has been reported that the pharmacological availability of rhEPO after intranasal administration compared with intravenous administration was about 4% to 7% in rats as evaluated by measuring percentage circulating reticulocytes of red blood cells or by residual circulating reticulocytes using a microcell counter. Thus, intranasal administration of relatively small doses of rhEPO protects rats from acute injury after focal cerebral ischemia, suggesting that intranasal rhEPO may be a more effective and safer administration route for treatment of ischemic or other brain diseases (Yu et al., Citation2005).
Estradiol
17 b-Estradiol (E2), the most potent female sex hormone, belongs to the family of steroid hormones. Apart from its influence on primary and secondary sexual characteristics, it has profound effects on learning, memory and mood as well as neurodevelopmental and neurodegenerative processes or regulation of brain development. Long-term estrogen replacement has proved to be beneficial in the prevention and treatment of AD (Schupf et al., Citation2006). Nasal mucociliary clearance is one of the most important limiting factors for nasal drug delivery. It limits the time for drug absorption after nasal drug administration, hence limiting the bioavailability (Critchley et al., Citation1994). Researchers worked on bioadhesive polymers. The chitosan (CS) was used for overcoming this limitation and thereby improving bioavailability of intranasally administered drugs. Wang and associates prepared E2-loaded CS-NPs to reduce nasal mucociliary clearance and enhancing permeation of E2 into the nasal mucosa, and then improving the E2 bioavailability, especially for brain targeting. They investigated the levels of E2 in blood and CSF in Wistar rats at the dose of 0.48 mg/kg following intranasal administration of E2 CS-NPs and compared with intravenous administration. The plasma levels achieved following intranasal administration were significantly lower than those after intravenous administration, while CSF concentrations achieved after intranasal administration were significantly higher than those after intravenous administration. The higher DTI (3.2) and DTP% (68.4%) were found with E2 CS-NPs following intranasal as compared to intravenous administration indicative of better brain uptake of E2 CS-NPs. In our opinion, due to higher permeation of E2 into the nasal mucosa, bioavailability of E2 was improved especially for brain targeting. The results indicated that the E2 CS-NPs could be a novel drug delivery system for the treatment of AD via nose to brain (Wang et al., Citation2008).
Growth hormone-releasing neuropeptide (hexarelin)
Growth hormone (GH), which is secreted by the pituitary gland, stimulates the growth and cell reproduction in humans and other animals. The growth hormone-releasing peptides (GHRP) are a family of synthetic, five to seven amino acid peptides that selectively stimulate GH secretion via specific G protein-coupled receptors both directly from the pituitary gland and through stimulation of the hypothalamus (Korbonits et al., Citation1999). GHRP cannot be administered orally due to obstacles like low permeability through the g.i. mucosa because of their polar nature, chemical and enzymatic inactivation in the g.i.t. and hepatic first-pass effect leading to lower bioavailability. Intranasal delivery is an alternative method of administering GHRP to overcome the above obstacles because of the relatively high absorption efficiency of the drugs through the nasal mucosa with a high permeability and relatively lower enzymatic activity, hence increasing in the bioavailability of drugs (4.8 ± 0.9%) than that obtained after oral administration (0.3 ± 0.1%) (Ghigo et al., Citation1994). Researchers prepared hexarelin nasal solution by using an aqueous cosolvent vehicle consisting of ethanol, propylene glycol and n-tridecyl-β-d-maltoside as a permeation enhancer and investigated the plasma pharmacokinetics and brain distribution profiles of the hydrophilic GHRP, HEX after intravenous and intranasal administration to rabbits at a dose of 1 mg/kg. The levels of HEX achieved in brain and CSF following intranasal administration were approximately 1.6 times greater than those attained after intravenous administration despite the intranasal plasma levels being significantly lower than the intravenous plasma levels. These results revealed that the hydrophilic neuropeptide, HEX attained significant concentration into the CSF and brain tissues from the nasal cavity and may provide a promising and durable therapeutic option for the treatment of GH deficiency (Yu & Kim, Citation2009).
It was also concluded that, intranasal administration produces a relatively high absorption efficiency for HEX as compared to other route. However, problems associated with intranasal administration of solution form of hydrophilic peptides such as poor and slow diffusion through the nasal mucosal membrane, thereby preventing desired levels of the therapeutic agent being achieved by means of simple transnasal administration restrict their application. This therefore necessitates the development of any other formulation or nano-formulation for convenient, efficient and effective intranasal delivery to enhance the direct transport of therapeutics from nose to brain.
Interferon beta
Interferons (IFNs) are anti-inflammatory cytokines that have antiviral, antitumor and immune regulatory actions. There are two classes of interferons, type I and type II. Type I IFN proteins include IFNα and IFNβ and type-II interferon includes IFNγ (Platanias & Fish, Citation1999). Type I interferons have immune modulatory effect due to which they are suggested for treatment of various diseases, such as multiple sclerosis (MS), AD, arthritis, hepatitis and cancer. Interferon beta-1b (IFNh-1b) is used for the treatment of MS. MS is a chronic, inflammatory, demyelinating disease of the CNS, most commonly found in the periventricular white matter, brain stem, cerebellum, optic nerves and spinal cord (Anderson et al., Citation1992). For treating the neurological diseases, the IFNβ protein need to get pass through the BBB. Currently, IFNβ is administered either by intramuscular (Avonex®, Biogen Idec, Zug, Switzerland) or subcutaneous injections (Betaferon®/Betaseron® (Bayer HealthCare Pharmaceuticals, CA)/Extavia® (Novartis, Basel, Switzerland)), both routes being associated with negative side effects such as injection site necrosis and poor delivery to the CNS. Therefore, researchers studied intranasal administration which offers a noninvasive method to directly deliver IFNβ-1b to the CNS. They examined intranasally administered IFNβ-1b concentrations delivered to the CNS, and compared CNS, lymph node and peripheral tissue concentrations following intranasal versus intravenous administration of 125I-labeled IFNβ-1b. Studies conclusively demonstrated higher CNS levels and lower peripheral organ levels following intranasal administration of IFNh-1b at similar blood levels compared to intravenous which yielded 88–98% lower CNS levels and 100–165% greater peripheral organ levels. Autoradiography confirmed much greater delivery to the CNS with intranasal administration. It shows that intranasal administration offers a noninvasive method of drug delivery for MS that bypasses the BBB and directly targets the CNS and lymph nodes (Rossa et al., Citation2004).
Risperidone
Risperidone (RSP) is a dopamine antagonist of the second generation and an approved antipsychotic drug belonging to the chemical class of benzisoxazole derivative possessing antiserotonergic, antiadrenergic and antihistaminergic properties used mainly to treat schizophrenia. The efficacy of neuroleptics is thought to be due to antagonism of dopamine receptors in the mesolimbic and mesofrontal systems. Currently, RSP is commercially available as an oral liquid (Risperidal®) and orally disintegrating tablet (Risperidal® M-TAB). These dosage forms exhibit low bioavailability due to extensive first-pass metabolism and non-targeted delivery results in numerous side effects. Kumar and coworkers have formulated risperidone nanoemulsion (RNE) and mucoadhesive nanoemulsion (RMNE) and delivered through intranasal route. RNE was prepared using glyceryl monocaprylate as the oily phase, tween 80 as surfactant, a mixture of transcutol and propylene glycol as cosurfactant and distilled water as the aqueous phase. RMNE was prepared by addition of chitosan to RNE. It improved the bioavailability by preventing first-pass metabolism and also provided targeting to the receptor site and bypassed the BBB to achieve desired drug concentration at the site of action, hence preventing availability of drug at non-targeting sites and reducing the side effects. The authors examined biodistribution of RNE, RMNE and risperidone solution (RS) in the brain and blood of Swiss albino rats following i.n. and i.v. administration using optimized technetium-labeled (99mTc-labeled) RSP formulations. The brain/blood uptake ratio of 0.617, 0.754, 0.948 and 0.054 obtained for RS (i.n.), RNE (i.n.), RMNE (i.n.) and RNE (i.v.), respectively, at 0.5 h are indicative of the superiority of mucoadhesive nanoemulsion administered intranasally over the intranasal and intravenous administration of RNE. The rapid and larger extent of transport of RSP by RMNE was indicated by higher drug transport efficiency (DTE%) and DTP% for mucoadhesive nanoemulsions as compared to the RS (i.n.), RNE (i.n.) and RNE (i.v.) into the rat brain (Kumar et al., Citation2008). The study conducted in rats clearly demonstrated effectiveness of intranasal delivery for brain targeting. In our view, the prepared nanoparticles could reduce the side effects, dose and frequency of administration, and possibly even the cost of the therapy.
Researchers also prepared RSP-loaded solid lipid nanoparticles (RSLNs) by solvent emulsification-solvent evaporation method and explored the possibility of brain targeting by nose-to-brain delivery. They performed the pharmacodynamic study of RSLNs, by paw test using Perspex platform which showed higher hindlimb retraction time (HRT) values as compared with RSP solution (RS), indicating the superiority of RSLNs over the RS for brain targeting. The pharmacokinetics and biodistribution studies in mice showed that brain/blood ratio at 1 h post-administration of RSLNs (i.n.) was found to be 1.36 ± 0.06 (nearly 10 - and 5-fold higher) as compared with 0.17 ± 0.05 for RS (i.v.) and 0.78 ± 0.07 for RSLNs (i.v.), respectively. Gamma scintigraphy imaging of mice brain following i.n. administration confirmed the localization of drug in brain. These results confirmed the existence of direct nose-to-brain delivery route for NPs administered to the nasal cavity (Patel et al., Citation2011). In our opinion, SLN has lipidic excipients which made the formulation more lipophilic and in turn helped in its transcellular absorption through olfactory bulb.
Rivastigmine
Rivastigmine (RHT) is a drug candidate for Alzheimer’s disease (AD). AD is a progressive neurodegenerative disorder of the CNS and is the most common cause of dementia (Ray & Lahiri, Citation2009) in which there is a progressive deterioration of intellectual and social functions, memory loss, personality changes and inability for self-care. RHT is inhibitor of both acetylcholinestrase (AChE) and butrylcholinestrase (BuChE) enzyme and is 4–17 times more specific for inhibiting AChE in brain as compared to heart and blood (Kosasa et al., Citation1999). However, limitation with its oral drug (PrSandoz®) delivery restricts the entry of drug into brain due to its hydrophilicity, thereby necessitating to improve it. Wilson and associates have worked on RHT-loaded poly(n-butyl cyanoacrylate) NPs for brain targeting through intravenous route but the results of surfactant-coated poly(n-butylcyanoacrylate) NPs showed possibility of distribution to non-targeted site (Wilson et al., Citation2008). Therefore, further investigation has been carried out to deliver a wide range of drugs to targeting areas of the body for controlled drug release and site-specific drug targeting by developing polymeric NPs. Fazil and coworkers prepared RHT-loaded CS-NPs by ionic gelation method to enhance the uptake of RHT to the brain via intranasal, hence improve the bioavailability, avoid first-pass metabolism and avoid the distribution to non-targeted sites thus leading to decreased peripheral side effects. They investigated CS-NPs by confocal laser scanning microscopy technique using rhodamine-123 as a marker. They compared the result after administration of RHT (i.v.), RHT (i.n.) and CS-RHT NPs (i.n) to confirm their brain-targeting efficiency and also determined the levels of RHT in plasma and brain tissues. The brain/blood ratio of RHT for different formulations which were 0.235, 0.790 and 1.712 of RHT (i.v.), RHT (i.n.) and CS-RHT NPs (i.n), respectively, at 30 min are indicative of higher brain concentration after intranasal administration of CS-NPs and direct nose-to-brain transport of loaded drug, thereby bypassing the BBB as compared to intravenous administration of RHT solution and intranasal administration of RHT solution. The higher DTE 355 ± 13.52% and DTP 1.80 ± 6.71% were found with CS-RHT NPs as compared to other formulation indicative of better brain uptake of CS-RHT NPs. In our perspective, these encouraging results suggest that CS-RHT NPs could be a novel colloidal drug delivery system for the treatment of AD via nose-to-brain delivery (Fazil et al., Citation2012).
Ropinirole
Ropinirole a dopamine D2 agonist of the non-ergoline class is prescribed mainly for PD, restless leg syndrome and extrapyramidal symptoms either alone or as an adjunct to reduce “on–off” fluctuations in levodopa response. Oral ropinirole (Ropin) therapy is associated with nausea, vomiting and gastrointestinal disturbances and exhibits low bioavailability (50%), due to extensive metabolism in the liver (Galichet, Citation2004). Nasal delivery of ropinirole minimizes gastrointestinal disturbances and adverse hepatic effects associated with oral administration and consequently may increase its brain concentration, thereby resulting into better management of PD. Researchers developed mucoadhesive temperature-mediated in situ gel formulations using CS and hydroxyl propyl methyl cellulose as gelling agent to enhance intranasal delivery of the ropinirole to the brain and hence increase its bioavailability up to 82%. They evaluated the brain uptake of ropinirole in albino rats following intranasal administration of 99mTc ropinirole in situ gel, intranasal ropinirole solution and intravenous ropinirole solution. Studies conclusively demonstrated high brain DTP and DTI >1 of radiolabeled (99mTC) ropinirole in situ gel nasal formulation over the intranasal ropinirole solution and intravenous ropinirole solution. It confirms direct nose-to-brain transport of the intranasal in situ gel formulation of ropinirole (Khan et al., Citation2010).
Mustafa and coworkers formulated a thermodynamically stable and infinite dilutable nanoemulsion (o/w) encapsulating ropinirole and Sefsol 218 (10% v/v), tween 80 (18% v/v), Transcutol (18% v/v) and water (54% v/v) as matrix, surfactant, cosurfactant and aqueous phase, respectively. They constructed ternary phase diagrams to locate the area of nanoemulsion for different selected systems. They studied ex vivo nasal permeation of drug from nanoemulsion formulations and found highly significant (p < 0.005) permeation result as compared to ropinirole suspension. From in vitro and ex vivo results, it was concluded that the developed nanoemulsion formulation might be a promising carrier for intranasal delivery of ropinirole for the better management of PD (Mustafa et al., Citation2012).
Sumatriptan
Sumatriptan (ST) is a highly selective 5-HT1D receptor agonist belonging to the triptan class prescribed for the treatment of acute migraine that can contract intracranial artery and redistribute blood and improve cerebral blood flow (Tfelt-Hansen et al., Citation2000). Currently, it is available in the market as oral tablets (PrIMITREX DF®, GSK, Brentford, UK), subcutaneous injection (PrIMITREX®) and an intranasal spray (PrIMITREX®). However, the current dosage forms exhibit a number of limitations, such as low oral bioavailability (14%) partly due to incomplete absorption and short plasma elimination half-life (∼2 h), protein binding (14–21%) and hepatic first-pass effect (Perry & Markham, Citation1998). Researchers have attempted to design novel dosage forms (micellar nanocarrier of sumatriptan) with an aim to overcome the limitations of the currently available therapies that would provide a higher retention of the formulation in the nasal cavity, thus offering a more efficient nose-to-brain delivery of the drug by exploiting their structural advantage of displaying core–shell architecture. The inner core is composed of hydrophobic regions of the surfactants (amphiphiles), which facilitates the solubilization of poorly soluble moieties, such as sumatriptan and the hydrophilic blocks of the amphiphiles suppresses opsonization by blood components, thus resisting phagocytosis by macrophages and decreasing clearance by the reticuloendothelial system, resulting in prolonged circulation times (Letchford & Burt, Citation2007). In vivo biodistribution studies in rats demonstrated that the intranasal administration of 99 mTc-ST micellar nanocarrier resulted in a targeted delivery to the brain, particularly to the olfactory regions, when compared with the results of the intravenous and nasal ST solutions (Jain et al., 2010b).
Vyas and coworkers developed microemulsion of ST that could provide rapid transport of drug across nasal mucosa and longer residence time in the nasal cavity. They examined the biodistribution of sumatriptan microemulsion (SME), sumatriptan succinate microemusion (SSME), sumatriptan solution (SSS) and marketed product (SMP) in the brain and blood of Swiss albino rats following intranasal and intravenous administrations using optimized technetium-labeled (99mTc labeled) ST formulations. SME and SSME were prepared using medium chain triglyceride (MCT) as an oil, caprylocaproyl macrogol glyceride as surfactant, mixture (1:1 wt/wt) of purified diethylene glycol monoethyl ether and fatty acid ester of polyglycerol as cosurfactant and distilled water as aqueous phase. Brain/blood uptake ratios at 0.5 h following intravenous administration of SME and intranasal administrations of SME, SMME and SSS were found to be 0.20, 0.50, 0.60 and 0.26, respectively, suggesting effective transport of drug following intranasal administration of microemulsions. The result demonstrated higher DTE and DTP for mucoadhesive microemulsions thus indicating more effective brain targeting following intranasal administration (Vyas et al., 2006a). In our view, the enhanced bioavailability and transport of ST following intranasal administration of SMME could help in decreasing the dose and frequency of dosing thereby and possibly maximizing the therapeutic index.
Venlafaxine
Venlafaxine (VLF) is an antidepressant of serotonin and norepinephrine reuptake inhibitors (SNRI) and is used for the treatment of major depressive disorder (MDD) and anxiety disorder. It inhibits central serotonin and norepinephrine neuronal reuptake increasing the diminished levels of neurotransmitters like serotonin and norepinephrine in the synaptic cleft between neurons in the brain (Wilson et al., Citation2007). VLF is commercially available as immediate and controlled release tablets and capsules (Effexor, Effexor XR) but oral therapy exhibits number of drawbacks, such as slow onset of action, low bioavailability (40–45%), elimination half-life of 4–5 h along with side effects like tachycardia, increased blood pressure, fatigue, headache, dizziness, sexual dysfunction, dry mouth. Therefore there is a need to maintain effective therapeutic concentration in the brain by using other therapies or routes to treat depression (Stahl et al., Citation2005). Researchers prepared VLF-loaded CS-NPs to enhance the uptake of VLF to brain via intranasal delivery and investigated the localization of CS-NPs in brain and other organs qualitatively by confocal laser scanning microscopy technique using rhodamine-123 (ROD-123) as marker. Moreover, CS-NPs possess advantages of small particle size, enhanced permeability across nasal mucosa and ability to encapsulate various ingredients. The authors compared the result after administration of VLF-loaded CS-NPs i.n. and VLF solution i.n. and i.v. to confirm their brain-targeting efficiency and also determined the levels of VLF in plasma and brain tissues. The brain/blood ratios of VLF were found to be 0.0293, 0.0700 and 0.1612 for VLF (i.v.), VLF (i.n.) and VLF CS-NPs (i.n.), respectively, at 0.5 h, and the higher DTE 508.59 and DTP 80.34 of VLF CS-NPs as compared to other formulations indicated better brain uptake of VLF CS-NPs. On the basis of these research findings, it was concluded that VLF-loaded CH-NPs could be an efficacious drug delivery system for nose-to-brain targeting in the treatment of depression (Haque et al., Citation2012).
Zolmitriptan
Zolmitriptan is a selective serotonin receptor agonist exhibiting a potent symptomatic antimigraine effect. Zolmitriptan mimics the action of serotonin by directly stimulating the serotonin receptors in the brain and thus acts peripherally inhibiting dilatation and inflammation of cranial vessels. Currently, Zolmitriptan is available commercially as a swallowable tablet (ZOMIG®), an oral disintegrating tablet (ZOMIG-ZMT®) and a nasal spray (ZOMIG nasal spray), in doses of 2.5 and 5 mg (Vyas et al., Citation2005). But, the oral zolmitriptan exhibits drawbacks, such as slow onset of action, low bioavailability (40–45%), nausea and incomplete pain relief with recurrence of headaches, short half-life (1–2 h), first-pass metabolism (Goadsby & Yates, Citation2006). Administration of the nasal solution of the drug in the spray form resulted in a quicker onset of action, providing relief in headache within 15 min of administration. However, clinical evidences show no significant improvement in other pharmacokinetic parameters, such as half-life, bioavailability and therapeutic gain, over the oral dosage forms (Bigal et al., Citation2003). Researchers have attempted the formulation of mucoadhesive microemulsion dosage form of zolmitriptan. They prepared micellar nanocarriers [formulated using PF127 (Pluronic® F127) in combination with PEG-400 (Polyethylene glycol-400)] and TPGS (d-α-tocopheryl polyethylene glycol). Micellar nanocarriers of the drug were developed to exploit their advantages, such as low particle size, enhanced permeability across nasal mucosa, suitable flow properties and ability to incorporate varying ingredients, which would allow targeting of the solubilized moiety, longevity and higher retention effects in the desired areas, in turn providing an enhanced drug action (Torchilin, Citation2007). Biodistribution studies showed the superiority of the developed nanocarrier for brain targeting, in turn providing an enhanced drug action when compared with the oral tablets, intravenous, nasal spray, intranasal mucoadhesive microemulsion (Jain et al., 2010a).
This review has discussed the relevant aspects of CNS drugs given intranasally to direct the brain targeting CNS disorders like Alzheimer’s disease, depression, migraine, schizophrenia, etc., and also has discussed the comparison of intranasal drug delivery of a drug with that of other formulation of the same drug (i.e. intravenous, subcutaneous, oral, transdermal) in the treatment of CNS disorders. The summary of various formulations used in nose to brain targeting is listed in .
Table 1. Various brain-targeted intranasal formulations of CNS drugs investigated in different research labs.
Experimental factors considered while delivering drug for intranasal delivery
It is very important to consider various factors such as head position, delivery volume and method of administration which can influence the deposition of formulation within the nasal cavity and pathways involved to the CNS after intranasal administration. It has been found that different head position of the animal/patient can alter the administration of formulation in to the CNS. During intranasal delivery, the drainage of formulation in to the esophagus and trachea should be considered to attain maximum concentration in to the CSF and olfactory bulb. The most promising position for brain targeting is with head down and forward position; however, this position can be uncomfortable and inconvenient to patients which could result in compliance issues. Different administration devices have been used during intranasal delivery, such as sprays, nose droppers or needle-less syringes, to target drug to different regions of the nasal cavity. Several researchers administered intranasal drug solution in anesthetized mice and rats using pipette for delivering drops to each nostrils to allow the solution to be absorbed in to the nasal epithelium. Other administration method involve OptiMist, that is, breath-actuated device that targets liquid formulation to olfactory epithelium for direct transport in to the CNS and ViaNase device that targets a nasal spray to the olfactory and respiratory epithelium of the nasal cavity. Delivery volume is also important factor to be considered for delivering therapeutics. Rats receive a total volume of 40–100 µl, mice receive a total volume of 5–10 µl and in humans, the nasal cavity has a volume of 25 cm3 and receives 400 µl resulting in CNS effects. Efficient delivery to the CNS can be achieved by selecting the right combination of head position, formulation and delivery device to target the therapeutics to specific region of the nasal cavity (Dhuria et al., Citation2010). Overview of Nasal delivery devices with some key marketed products are given in .
Table 2. Overview of nasal delivery devices with key marketed products.
Future prospective of intranasal delivery to the CNS
Effective noninvasive treatment of neurological diseases is often limited due to the presence of biochemical dynamic barriers: the BBB and blood–cerebrospinal fluid barrier (BCSFB) (Wong et al., Citation2012). BBB represents an insurmountable obstacle for a large number of drugs including antibiotics, antineoplastic agents and various CNS-active drugs like neuropeptides. This creates a considerable threat for the therapy of cerebral diseases (Chakraborty et al., Citation2009). It is increasingly clear that crossing of BBB and drug delivery to CNS is a complex and challenging task requiring close collaboration and common efforts among researchers of several scientific areas including pharmaceutical sciences, biological chemistry, physiology and pharmacology (Denora et al., Citation2009). Therefore, treatment of neurological diseases has become one of the most significant challenges. Recent advances in nanotechnology have provided promising solutions to this challenge (Wong et al., Citation2012). From the works done in the last few years, we can conclude that nanotechnology is receiving increasing attention in an efficient manner. Several nanocarriers, for example, polymeric nanoparticles, solid lipid nanoparticles, liposomes, micelles, dendrimers, nanogels, nanoemulsions and nanosuspensions have been studied for the delivery of CNS therapeutics (Wong et al., Citation2012). It is very likely that in the near future more drugs intended for CNS disorders in the form of nasal formulation will come in the market (Putheti et al., Citation2009). But this functional drug delivery mechanism to the brain is a potential area of research. There are some challenging tasks which have to be faced during intranasal delivery, especially of large molecular weight polar drugs such as peptides and proteins, low membrane permeability, possibility of an enzymatic degradation of the molecule in the lumen of the nasal cavity and mucocilliary clearance. These challenges should be improved by focusing on bioadhesive excipients and absorption enhancers in the formulation to overcome the rapid mucocilliary clearance, enzymatic degradation and low membrane permeability, thereby improving the bioavailability of drugs incorporated. Nanoparticle-based drug delivery technology which presently exists should be improved further, so it can be safe, effective, target oriented and also cost-effective. Future development of CNS nanomedicines needs to focus on increasing their drug-trafficking performance and specificity for brain tissue using novel targeting moieties, improving their BBB permeability and reducing their neurotoxicity (Wong et al., Citation2012). Clinical studies of direct nose-to-brain delivery and delivery devices have not been carried out which if successful would prove the relative success of intranasal delivery system as compared to other dosage forms. Therefore, there is need to focus on clinical trials and preclinical trials to improve the intranasal delivery system. Mechanism of delivery of drug to direct brain is also not clear so attention to basic research should be paid to clear the confusion as to how exactly the drug passes through nose to specific brain areas for treatment of neurological and psychiatric diseases. Additionally, there is need to be focus on formulation strategies, drug delivery devices, invention of new excipients to improve the nasal bioavailability, study of mucoadhesive property of polymers to avoid expulsion of drugs so that they can be retained for longer period and show maximum effects. Besides this, toxicodynamic studies of drug and excipients and nanotoxicity of nanocarriers also need to be evaluated extensively (Putheti et al., Citation2009).
Declaration of interest
The authors state no conflict of interest and have received no payment in the preparation of this manuscript.
The authors are grateful to All India Council of Technical Education (AICTE), Government of India, for providing fellowship to Deepti Mittal as financial assistance and are also grateful to Department of Science and Technology (DST), New Delhi, for providing DST INSPIRE Fellowship to Shadab Md as financial assistance.
References
- Alam MI, Baboota S, Ahuja A, et al. (2012). Intranasal administration of nanostructured lipid carriers containing CNS acting drug: pharmacodynamic studies and estimation in blood and brain. J Psychiatr Res 46:1133–8
- Alam MI, Baboota S, Ahuja A, et al. (2011). Nanostructured lipid carrier containing CNS acting drug: formulation, optimization and evaluation. Curr Nanosci 7:1014–27
- Al-Ghananeem AM, Saeed H, Florence R, et al. (2010). Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by aids viruses. J Drug Target 18:381–8
- Anderson BD, Morgan ME, Singhal D. (1995). Enhanced oral bioavailability of DDI after administration of 6-Cl-ddP, an adenosine deaminase-activated prodrug, to chronically catheterized rats. Pharm Res 12:1126–33
- Anderson DW, Ellenberg JH, Leventhal CM, et al. (1992). Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol 31:333–6
- Balo R. (1994). Buspirone in the treatment of separation anxiety in an adolescent boy. J Clin Psychopharmacol 14:360–1
- Bigal ME, Bordini CA, Antoniazzi AL, Speciali JG. (2003). The triptan formulations: a critical evaluation. Arq Neuropsiquiatr 61:313–20
- Brines ML, Ghezzi P, Keenan S, et al. (2000). Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97:10526–31
- Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, et al. (2001). Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacol 25:871–80
- Chakraborty C, Sarkar B, Hsu CH, et al. (2009). Future prospects of nanoparticles on brain targeted drug delivery. J Neurooncol 93:285–6
- Cho K, Wang X, Nie S, et al. (2008). Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14:1310–16
- Critchley H, Davis SS, Farraj NF. (1994). Nasal absorption of desmopressin in rats and sheep. Effect of a bioadhesive microsphere delivery system. J Pharm Pharmacol 46:651–6
- Degim IT, Acartürk F, Erdogan D, Lortlar ND. (2003). Transdermal administration of bromocriptine. Biol Pharm Bull 26:501–5
- Denora N, Trapani A, Laquintana V, et al. (2009). Recent advances in medicinal chemistry and pharmaceutical technology strategies for drug delivery to the brain. Curr Top Med Chem 9:182–96
- Dhanda DS, Frey WH II, Leopold D, Kompella UB. (2005). Approaches for drug deposition in the human olfactory epithelium. Drug Del Tech 5:64–72
- Dhuria SV, Hanson LR, Frey WH. (2010). Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99:1654–73
- Dodane V, Amin Khan M, Merwin JR. (1999). Effect of chitosan on epithelial permeability and structure. Int J Pharm 182:21–32
- Dragsten PR, Hallaway PE, Hanson GJ, et al. (2000). First human studies with a high-molecular-weight iron chelator. J Lab Clin Med 135:57–65
- Ehrenreich H, Timner W, Siren AL. (2004). A novel role for an established player: anemia drug erythropoietin for the treatment of cerebral hypoxia/ischemia. Transfus Apher Sci 31:39–44
- Esposito E, Mariani P, Laura Ravani L, et al. (2012). Nanoparticulate lipid dispersions for bromocriptine delivery: characterization and in vivo study. Eur J Pharm Biopharm 80:306–14
- Fazil M, Shadab M, Haque S, et al. (2012). Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci 47:6–15
- Freret T, Valable S, Chazalviel L, et al. (2006). Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur J Neurosci 23:1757–65
- Galichet LY. (2004). Clarke’s Analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material. London, UK: Pharmaceutical Press. 2:726–7
- Ghigo E, Arvat E, Gianotti L, et al. (1994). Growth hormone-releasing activity of hexarelin, a newsynthetic hexapeptide, after intravenous, subcutaneous, intranasal, and oral administration in man. J Clin Endocrinol Metab 78:693–8
- Goadsby PJ, Yates R. (2006). Zolmitriptan intranasal: a review of the pharmacokinetics and clinical efficacy. Headache 46:138–49
- Hanson LR, Roeytenberg A, Martinez PM, et al. (2009). Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J pharmacol Exp Ther 330:679–86
- Haque S, Shadab M, Fazil M, et al. (2012). Venlafaxine loaded chitosan NPs for brain targeting, Pharmacokinetic and pharmacodynamic evaluation. Carbo Polym 89:72–9
- Hartman NR, Yarchoan R, Pluda JM, et al. (1990). Pharmacokinetics of 2′,3′-dideoxyadenosine and 2′,3′-dideoxyinosine in patients with severe human immunodeficiency virus infection. Clin Pharmacol Ther 47:647–54
- Jadhav KR, Gambhire MN, Shaikh IM. (2007). Nasal drug delivery system- Factors affecting and application. Curr Drug Ther 2:27–38
- Jain A, Jain SK. (2011). Drug targeting to the brain – a review. Curr Nanosci 7:21–36
- Jain R, Nabar S, Dandekar P, Patravale V. (2010a). Micellar nanocarriers: potential nose-to-brain delivery of zolmitriptan as novel migraine therapy. Pharm Res 27:655–64
- Jain R, Nabar S, Dandekar P, et al. (2010b). Formulation and evaluation of novel micellar nanocarrier for nasal delivery of sumatriptan. Nanomedicine (Lond) 5:575–87
- Jenner P, Pratt JA, Marsden CD. (1986). Mechanism of action of clonazepam in myoclonus in relation to effects on GABA and 5-HT. Adv Neurol 43:629–43
- Khan S, Patil K, Bobade N, et al. (2010). Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J Drug Target 18:223–34
- Khan S, Patil K, Yeole P, Gaikwad R. (2009). Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats. J Pharm Pharmacol 61:669–75
- Korbonits M, Little JA, Forsling ML, et al. (1999). The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol 11:521–8
- Kosasa T, Kuriya Y, Matsui K, Yarnanishi Y. (1999). Inhibitory effects of donepezil hydrochloride (E2020) on cholinesterase activity in brain and peripheral tissues of young and aged rat. Eur J Pharmacol 386:7–13
- Kumar M, Misra A, Babbar AK, et al. (2008). Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm 358:285–91
- Letchford K, Burt H. (2007). A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 65:259–69
- Lim JH, Kim SS, Boo DH, et al. (2009). Protective effect of bromocriptine against BH4-induced Cath. A cell death involving up-regulation of antioxidant enzymes. Neurosci Lett 451:185–9
- Maiese K, Li F, Chong ZZ. (2004). Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci 25:577–83
- Md S, Khan RA, Mustafa G, et al. (2012). Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci 48:393–405
- Muchow M, Maincent P, Müller RH. (2008). Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm 34:1394–405
- Mustafa G, Baboota S, Ali J, Ahuja A. (2012). Formulation development of chitosan coated intra nasal ropinirole nanoemulsion for better management option of Parkinson: an in vitro-ex vivo evaluation. Curr Nanosci 29:293–300
- Patel S, Chavhan S, Soni H, et al. (2011). Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target 19:468–74
- Perry CM, Markham A. (1998). Sumatriptan: an updated review of its use in migraine. Drugs 55:889–922
- Pires A, Fortuna A, Alves G, Falcao A. (2009). Intranasal drug delivery: how, why and what for. J Pharm Pharm Sci 12:288–311
- Platanias LC, Fish EN. (1999). Signaling pathways activated by interferons. Exp Hematol 27:1583–92
- Putheti R, Patil MC, Obire O. (2009). Nasal drug delivery in pharmaceutical and biotechnology: present and future. J Sci Tec 4:1–21
- Ray B, Lahiri DK. (2009). Neuroinflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol 9:434–44
- Redzic Z. (2011). Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8:1--23
- Rey E, Treluer JM, Pons G. (1999). Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Focus on delivery routes. Clin Pharmacokinet 36:409–24
- Rossa TM, Martineza PM, Rennera JC, et al. (2004). Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol 151:66–77
- Schupf N, Winsten S, Patel B, et al. (2006). Bioavailable estradiol and age at onset of Alzheimer’s disease in postmenopausal women with Down syndrome. Neurosci Lett 406:298–302
- Stahl SM, Grady MM, Moret C, Briley M. (2005). SNRIs: their pharmacology clinical efficacy and tolerability in comparison with other classes of antidepressants. CNS Spectr 10:732–47
- Tfelt-Hansen P, De Vries P, Saxena PR. (2000). Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 60:1259–87
- Torchilin VP. (2007). Micellar nanocarriers: pharmaceutical perspectives. Pharm Res 24:1–16
- Vautier S, Lacomblez L, Chacun H, et al. (2006). Interactions between the dopamine agonist, bromocriptine and the efflux protein, P-glycoprotein at the blood–brain barrier in the mouse. Eur J Pharm Sci 27:167–74
- Vyas TK, Babbar AK, Sharma RK, Misra A. (2005). Intranasal mucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J Drug Target 13:317–24
- Vyas TK, Babbar AK, Sharma RK, et al. (2006a). Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci 95:570–80
- Vyas TK, Babbar AK, Sharma RK, et al. (2006b). Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS Pharm Sci Tech 7:E49–57
- Wang F, Jiang X, Lu W. (2003). Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int J Pharm 263:1–7
- Wang X, Chi N, Tang X. (2008). Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm 70:735–40
- Wilson AD, Howell C, Waring WS. (2007). Venlafaxine ingestion is associated with rhabdomyolysis in adults: a case series. J Toxicol Sci 32:97–101
- Wilson B, Samanta MK, Santhi K, et al. (2008). Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res 1200:159–68
- Wong HL, Wu XY, Bendayan R. (2012). Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev 64:686–700
- Yang S, Zhu J, Lu Y, et al. (1999). Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm Res 16:751–7
- Ying W. (2008). The nose may help the brain: intranasal drug delivery for treating neurological diseases. Future Neurol 3:1–4
- Yu H, Kim K. (2009). Direct nose-to-brain transfer of a growth hormone releasing neuropeptide, Hexarelin after intranasal administration to rabbits. Int J Pharm 378:73–9
- Yu YP, Xu Q, Zhang Q, et al. (2005). Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett 387:5–10
- Zhang Q, Jiang X, Jiang W, et al. (2004). Preparation of nimodipine loaded microemulsion for intranasal delivery and evaluation of the targeting efficiency to brain. Int J Pharm 275:85–96