Abstract
The aim of this study was to explore the nanostructured lipid carriers as a delivery system of biochanin A so as to supply a method to improve its bioavailability. Biochanin A–loaded nanostructured lipid carriers (BCA-NLCs) were prepared by the method of emulsion-evaporation and low temperature solidification. Pharmacokinetics was carried out in rats upon oral administration at a dose of 10 mg/kg. BCA-NLC showed spherical formulation and had mean diameter174.68 ± 0.96 nm, zeta potential −20.9 ± 0.8 mv and entrapment efficiency 97.36 ± 0.14%. DSC and XRD studies indicated that BCA was not in crystal state in NLC. In in vitro release study, the BCA from BCA-NLC exhibited a biphasic release pattern with burst release initially and sustained release afterwards. BCA-NLC showed higher AUC value and circulated in blood for a longer time than BCA suspension. The studies demonstrated that NLC could be a potential delivery system for BCA to improve bioavailability.
Introduction
Biochanin A () is a kind of isoflavone isolated from the red clover ([Trifolium pratense] Leguminosae) (Cassady et al., Citation1988). Pharmacological studies have demonstrated that BCA possesses remarkable biological activities such as antioxidant, anti-inflammatory and antihyperglycemic effects (Kole et al., Citation2011; Yoon et al., Citation2011; Harini et al., Citation2012). In addition, BCA has been widely used as dietary supplement for treating premenstrual syndrome, lowering the cholesterol, improving urine production and preventing osteoporosis (Howes et al., Citation2000; Atkinson et al., Citation2004; Chedeaui et al., Citation2008). However, similar to other flavonoids, a major concern of BCA in application is the poor oral bioavailability (Moon & Morris, Citation2007; Singh et al., Citation2010). Pharmacokinetics studies indicate that BCA exhibited a high clearance and a high volume of distribution in rats and had a poor bioavailability (1–2%) (Moon et al., Citation2006).
Solid lipid nanoparticles (SLNs) were developed at the beginning of the 1990s as an alternative carrier system to nanoemulsions, liposomes and polymeric nanoparticles, and attracted great attention by a deal of research groups all over the world (Haede et al., Citation2011). Compared to traditional carriers, SLN had several advantages, such as good biocompatibility, controlled drug release and improved solubility of drug (Mehnert & Mader, Citation2001). In recent years, much works have been focused on the preparation of SLN of poor water-soluble drugs for improving bioavailability (Chirio et al., Citation2011; Bhandari & Kaur, Citation2013; Huang et al., Citation2013). Nonetheless, there were also some limitations of the SLN system, such as limited drug loading capacity due to the solubility of the drug in the solid lipid and drug expulsion during storage (when lipid crystallizes to the stable β-form) (Chinsriwongkul et al., Citation2011). To overcome these limitations of SLN described above, nanostructured lipid carriers (NLC) were introduced as the SLN of a new generation by blending solid lipid with certain content of liquid lipid. The mixed lipids induced to a matrix with massive crystallization of disturbance which left much imperfection to accommodate drug molecules (Müller et al., Citation2011). Compared to SLN, NLC showed a higher loading capacity and minimize potential expulsion of drug during storage (Mehnert & Mader, Citation2001)
The objective of this study was to prepare a NLC formulation for the oral delivery of BCA. The morphology, particles size, zeta potential, entrapment efficiency and drug loading capacity of BCA-NLC were investigated. Besides, in vitro drug release and pharmacokinetics study on Sprague–Dawley rats were evaluated in detail.
Materials and methods
Biochanin A (>99.0%) was purchased from Shanxi Ci-Yuan Biotechnology Co., Ltd. (Xi-an, China). Pluronic F68 (F68) was obtained from Beijing Feng-li Jing-qiu Commerce and Trade Co., Ltd. (Beijing, China). Glycerin monostearate (GMS) was purchased from Anhui Sheng-ying Pharmaceutical Co., Ltd. (Hefei, China). Tween 80 was purchased from National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China). Lecithin was generally supplied by Anhui Sheng-yuan Pharmaceutical Co., Ltd. (Hefei, China). Medium chain triglycerides (MCT) were purchased from Guangzhou Hong-sheng Chemical Industry Co., Ltd. (Guangzhou, China). Valsartan was purchased from National institute for Food and Drug Control (Beijing, China). Mannitol was purchased from Shanghai Yuan-Ju Biotechnology Co., Ltd. (Shanghai, China). The acetonitrile (HPLC grade) was purchased from the Oceanpak Alexative chemical Co., Ltd. (Gothenburg, Sweden). The water used for all experiments was double-distilled water. All other chemicals were of analytical reagent grade.
Preparation of BCA-NLC
In this study, the BCA-NLC was prepared by the method of emulsion-evaporation and low temperature solidification (Dai et al., Citation2010). The pressure of our work place was atmospheric pressure. BCA (5 mg), solid lipid (GMS, 80 mg), liquid lipid (MCT, 20 μL) and lecithin (150 mg) were dissolved into 5 mL mixed organic solvents (acetone: ethanol, 1:1, v/v) with heating at 75–80 °C to get lipid phase. The F68 (150 mg) and Tween 80 (150 mg) were completely dissolved into water (10 mL), heated to the same temperature as the lipid phase to obtain a clear aqueous phase. After the two phases became same isothermal, the lipid phase was poured into the aqueous phase under magnetic stirring (1000 ± 25 rpm) to obtain a clear homogenous emulsion. After 3 h, the hot emulsion was then poured into 30 mL of cold water (0∼4 °C) and stirred at 1000 ± 25 rpm in an ice-bath for 2 h to obtain a fine dispersion of the BCA-NLC. The obtained dispersion was then freeze-dried using a lyophilizer (LGJ0.5, Beijing Si-huan Scientific Instrument, Beijing, China) to obtain a fine powder. Mannitol (5%, w/v) was selected as the cryoprotectant in the process of free-drying.
Transmission electron microscope (TEM) investigation
The morphology of the BCA-NLC was examined using a negative-staining method. A drop sample was spread on a copper grid coating and stained by 2% phosphotungstic acid (PTA) solution. Then the grid was dried in the air for 5 min, and observed by the TEM (H-1000, Hitachi, Japan).
Particles size and zeta potential
The particles size, polydispersity index (PI) and zeta potential of BCA-NLC were measured by the Zetasizer (Nano-ZS90, Malvern Instruments, UK) at 25 °C. Before the measurements, all samples were diluted for 10-fold by water. Approximately 1 mL diluted BCA-NLC dispersion was poured into disposable polystyrene cuvette under a fixed angle of 90° using a He-Ne laser at a wavelength of 633 nm.
HPLC analysis of BCA
The content of BCA was determined by high performance liquid chromatography (HPLC). The HPLC composed binary LC-15C pumps (Shimadzu, Japan) and a SPD-15C UV detector (Shimadzu, Japan). Data processing was performed with LC-Solution software. The analytical column was Cosmosil C18 (4.6 × 250 mm, 5 μm). The injection volume was 20 μL; the detecting wavelength of UV detector was set at 260 nm; the mobile phase was a mixture of acetonitrile: 0.1% phosphoric acid (52:48, v/v) at a flow rate was 1 mL/min; The column temperature was 30 °C. The recovery of BCA in three concentrations (1.6 μg/mL, 2.0 μg/mL and 2.4 μg/mL) was 99.02%, 98.92% and 98.60%. BCA possessed good linearity in the range of 0.0050–1 μg/mL (r = 0.9999). The HPLC methodology was used for detecting EE and drug release profiles.
Determination entrapment efficiency (EE) and drug loading capacity (DL)
The EE and DL were determined by the method of centrifugation and ultrafiltration. One milliliter BCA-NLC dispersion was placed into ultrafiltration tube (100 k, Amicon Ultra-4, Millipore, USA) and centrifuged (LC-4016, Anhui USTC Instruments Co., Ltd., China) at 3500 rpm for 30 min. The aqueous phase collected at the bottom ultrafiltration tube was injected into HPLC to determine the BCA content (denoted by Wfree). The total drug contained in BCA-NLC was quantified as following, 1 mL sample from the same batch was dissolved into methanol and stirred for 5 min to make drug release thoroughly from the nanoparticles, the obtained suspension was then filtered through 0.45 μm membrane; the filtrate was analyzed by HPLC to determine the total content of BCA (denoted by Wtotal). The EE and DL were calculated by following equations
Where Wtotal, Wfree and Wlipid were the weight of the total drug in the system, the weight of free drug analyzed in the filtrate and the weight lipid added in the system, respectively.
Differential scanning calorimetry (DSC) analysis
The interaction of BCA with lipids and the state of BCA in the NLC formulation were determined using DSC (Q2000, TA Instrument, USA). Approximately, 100 mg of the samples were added into aluminum pan and sealed tightly. Empty aluminum pan was used as reference to determine the baseline. The scanning rate of all samples was 5 °C/min ranging from 25 °C to 250 °C. The DSC thermograms for BCA, F68, GMS, lecithin, mannitol, their physical mixture and BCA-NLC lyophilized powders were recorded.
X-ray diffraction (XRD) analysis
The XRD was measured by an X-ray diffractometer (XD-3X, Persee, China) with a Cu Kα radiation source. The diffraction patterns were measured with a voltage of 36 kV and a current of 24 mA between 2θ = 2° and 2θ = 50° using a scan speed of 0.02°/s.
Stability analysis
BCA-NLC dispersion was kept at 4 °C and 25 °C, respectively. The BCA-NLC were sampling after 15, 20 and 30 days. The changes of particle size and EE were determined to evaluate physical stability of BCA-NLC.
In vitro release study
In vitro release of BCA from BCA suspension and BCA-NLC were investigated by the method of dialysis bag diffusion. Into the pretreated dialysis bag (21 mm 10K, Biosharp, USA), 2 mL of BCA-NLC dispersion was placed and both the ends of bags were tied to prevent any leakage. Later, dialysis bag were carefully immersed into receptor compartment containing 400 mL of phosphate buffer (PH 7.4), stirred at 100 ± 5 rpm and maintain at a temperature of 37 ± 0.5 °C. At regular time intervals, 2 mL of the sample from the dissolution medium was collected for analysis and replaced with a same volume of the fresh medium. The content of the BCA released to the medium was detected by HPLC.
Pharmacokinetics study
All animal experiments were carried out according to the principles of Laboratory Animal Care (People’s Republic of China). All rats were supplied by Experiment Animal Center of Anhui Medical University (Hefei China) and housed at a temperature of 20 ± 2 °C and 45–50% relative humidity. Pharmacokinetics study of BCA-NLC was compared with BCA suspension. Prior to the experiment, 12 Sprague–Dawley rats (180–220 g) were randomly divided into two groups (group 1 and group 2) and fasted overnight, but allowed to free access to water. BCA suspension was prepared by dissolving the BCA into 0.5% CMC-Na solution, the lyophilized BCA-NLC powder was reconstituted by water. The BCA suspension and BCA-NLC (equivalent to the dose of 10 mg/kg of BCA) were administered orally to group 1 and group 2 rats, respectively. At defined time intervals (0.083, 0.25, 0.5, 1, 1.5, 2, 4, 8, 12, 24 h), 0.6 mL of blood samples from the angulus oculi of rats was collected and centrifuged for 20 min (3000 rpm), the blood samples was then stored at −20 °C.
The plasma samples were processed as following procedures. Two hundred microliter of the plasma sample was mixed with 20 μL internal-standard (valsartan) solution (25 μg/mL). One milliliter of acetidin was added into plasma sample followed by vortexing for 5 min. After centrifugation for 10 min (3000 rpm), the organic layer was transferred to a new tube and evaporated under a stream of high purity nitrogen gas. The residue was reconstituted with 100 μL of mobile phase by vortexing for 5 min, 20 μL of the sample solution was injected into the HPLC system.
Statistics
All values obtained from this study were expressed as mean ± S.D. Statistical significance was determined by Student’s t-test, with p < 0.05 indicating significant difference. The main pharmacokinetics parameters were acquired by the software 3p97.
Results and discussion
Preparation and characterization of BCA-NLC
In this study, BCA-NLC was successfully prepared by the method of emulsion evaporation and low temperature solidification. The advantages of this method were easy handling and fast production process without technically sophisticated equipment (Das & Chaudhury, Citation2011). From literature, it was evident that the type and amount of emulsifier influence the size of the particles and their stability. The amount of the emulsifier should be optimum to cover the surface of the nanoparticles (Manjunath et al., Citation2005). In this study, Tween 80 and lecithin were used in combination with Poluronic F68 as emulsifiers. The combined use of two or more emulsifying agents appeared to produce mixed surfactant films at the particle size. The mixed surfactants covered the surface efficiently as well as produced sufficient viscosity to promote stability (Müller et al., Citation2000). MCT was used as the liquid lipid of the matrix which made the NLC different from the SLN. SLNs were produced from solid lipid only and after preparation at least a part of the particles crystallized in a higher energy modification (α and β′). During storage, these modifications could transform to the low energy (β modification), Due to its high degree of order, the number of imperfections in the crystal lattice was reduced leading to drug expulsion. By creating a less ordered solid lipid matrix, that is, by blending a solid lipid with a liquid lipid, a higher active load of the particles could be achieved. In general, the drug molecules could be located in between the fatty acid chains or between the lipid layers and also in imperfections of the lipid matrix. In case of very similar lipid molecules, especially when highly purified monoacid glycerides were used, the drug loading was very limited and drug expulsion occurred within a short time due to the formation of the well-ordered β modification. Therefore, the production of NLC yielded an increase of drug loading capacity in the particles and also minimized the drug expulsion during storage (Pardeike et al., Citation2009).
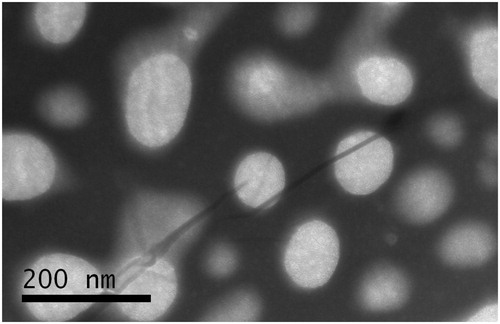
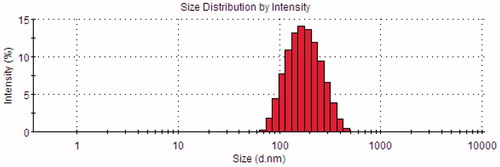
The morphology of BCA-NLC determined by TEM was shown in . It showed that the particles were nearly spherical in shape and uniformly distributed. The diameters of the particles were ∼200 nm, which was in good agreement with the results obtained from the measurement by the nanosizer ().
The mean diameter of BCA-NLC was 174.68 ± 0.96 nm. The PI was 0.177, indicating that the distribution of the particles was quite narrow. Particle size played a crucial role in the gastrointestinal uptake and their clearance by the reticuloendothelial system. Hence, the precise determination of the particle size was very important. Usually, particle size less than 300 nm was advisable for the intestinal transport (Haede et al., Citation2011).
The determination of the zeta potential was a much helpful way to predict the physical storage stability of colloidal systems (Das & Chaudhury., Citation2011). In this study, the zeta potential value of BCA-NLC was −20.9 ± 0.8 mv (n = 3). In general, the zeta potential value was above the critical value of −30 mv, implying a long-term stability of colloidal systems. Despite the zeta potential value of the BCA-NLC was blow 30 mv, nanoparticles still could have the same long-term stability, for the sterically stability layer was sufficiently thick (Silvander, Citation2002; Manjunath et al., Citation2005). In this study, the thick stability layer was composed by the use of F68, which is located on the surface of the nanoparticles to form sterically stabilizing layer.
EE and DL were two appraisal factors for preparation technology (Lee et al., Citation2007). In this study, the EE and DL of BCA-NLC were 97.36 ± 0.14% and 4.18 ± 0.24%, respectively. These high EE and DL could be attributed to the use a combination of highly ordered with less ordered lipids, which caused numerous crystal defects in solid matrix and provided much imperfections to accommodate more drug molecules (Mukherjee et al., Citation2009).
Crystallinity and polymorphism analysis
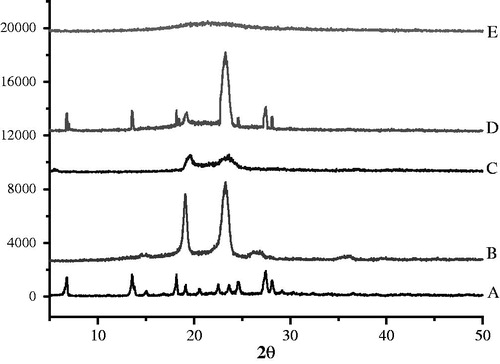
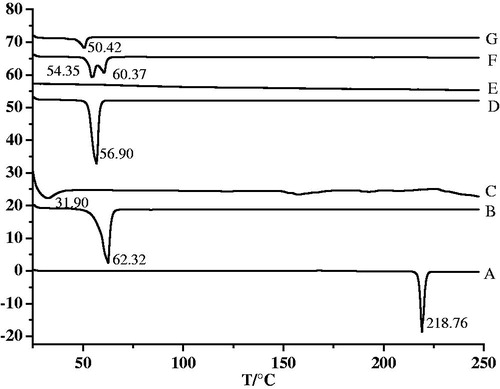
DSC and XRD were two widely used techniques to determine the crystallinity and polymorphic behavior of the components of the SLN/NLC (Das & Chaudhury, Citation2011). DSC used the fact that different lipid modifications possessed different melting points and melting enthalpies. By the means of XRD, it was possible to assess the length of the long and short spacings of the lipid lattice (Müller et al., Citation2000). exhibited the DSC thermograms of the BCA, F68, GMS, lecithin, mannitol, their physical mixture and BCA-NLC lyophilized powders. The typical peak of F68 (62.32 °C, B), GMS (56.90 °C, C) could be found in physical mixture (E), indicating these components remained in their initial crystalline state, while these peaks were disappeared in pattern of lyophilized BCA-NLC (E); besides, a new peak (50.42 °C) appeared in BCA-NLC. These results suggested BCA and individual components lost their crystalline nature when incorporated into NLC.
Figure 4. The Differential scanning calorimetry curves: BCA (A), F68 (B), lecithin (C), GMS (D), mannitol (E), physical mixture (F) and lyophilized BCA-NLC (G).
To identify the physical state of BCA incorporated in NLC, the X-ray diffraction was performed and patterns of BCA (A), F68 (B), GMS (C), physical mixture (D) and lyophilized BCA-NLC powders (E) were illustrated in . From the diagram of BCA, it was clear that BCA showed highly crystalline nature for that BCA exhibited typical sharp peaks at 2θ equaled 6.8°, 13.4°, 18.2° and 27.5°. These peaks were also appeared in the pattern of physical mixture, which indicated that the BCA was in good crystal. On the contrary, these shark peaks were disappeared in the pattern of BCA-NLC. The absent crystallinity of BCA in the nanoparticles confirmed that BCA was encapsulated into or absorbed in the surface of nanoparticles, which was mainly dispersed into the lipid matrix with molecularity state not crystallinity form (Dai et al., Citation2010). The diffraction pattern of GMS showed sharp peaks at 2θ equals 19.5° and 23.4°, F68 presented shark peaks at 2θ equals 19.1° and 23.2°. Compared with the physical mixture, the peaks intensities of both GMS and F68 were significantly decreased in NLC, suggesting that the degree of the crystallinity was lower in NLC than the raw materials. The results were confirmed with the DSC analysis.
Stability study
The data of the particle size and EE analyzed after 15, 20 and 30 days are shown in . From the table, BCA-NLC dispersion possessed high stability both in low and room temperature with respect of no significant changes in particle size (p > 0.05) and EE (p > 0.05) in 30 days. The physical stability of NLC-caring drugs may be expected to reduce in vivo the local adverse effect of some drugs on the gastrointestinal tract and to protect biodegradable molecules (Bargoni et al., Citation1998).
Table 1. The physical stability of BCA-NLC at 4 °C and 25 °C.
In vitro release study
The release profiles of BCA from BCA suspension and BCA-NLC were shown in . It was evident that the BCA suspension showed quick release of BCA with a cumulative release of more than 95% within 4 h. For the BCA-NLC, BCA exhibited relative biphasic drug release pattern with a burst release at initial 4 h, which was evident that there was some drug molecules attracting on the surface of nanoparticle, and then a slow release was observed in a prolong way. The percentage of accumulating release of BCA-NLC over 48 h was more than 98%. The mechanism of enhanced solubility and dissolution could be explained by the change of the drug crystallinity to amorphous state, reduction of the particle size and the solubilizing effect of hydrophilic effect (Zur Muhlen et al., Citation1998; Han et al., Citation2011). The drug release patterns () of BCA from BCA suspension and BCA-NLC were analyzed by zero-order kinetics, first-order kinetics, Higuchi, Weibull, Nibbergull and Biexponential equations. The BCA-NLC was fitted into the two-stage exponential kinetic model, equation was 1 − Q% = 0.978e−0.185t +0.412e−0.06t (rα = 0.9978, rβ = 0.9887). In equation, Q% was the percentage of accumulate drug release and t was the time of drug release. It was possible to modify the release profiles as a function of lipid matrix, surfactant concentration and production parameters (e.g. temperature) (Müller et al., Citation2000). At the beginning of preparation, both the lipids and BCA were dissolved into organic solvents (acetone and ethanol). With the increasing temperature of water phase and the use of emulsifier, the solubility of BCA in water phase would increase, which indicated that BCA might migrate from lipids to water. During the process of low temperature solidifying, the solubility of BCA in water phase decreased continuously that meant a re-partition of BCA into the lipid phase. When the temperature reached the recrystallization temperature of the solid lipid (GMS), it would rapidly solidify to form a solid lipid core in which the liquid lipid (MCT) was randomly distributed. At the time, the BCA which were present in lipid at this temperature would be encapsulated in lipid core. Continuing to reduce the temperature, the already crystallized core was not accessible anymore for BCA; thus, a fraction of BCA might accumulate in outer shell of the particle or absorbed on the surface of nanoparticle. So, the drug in the outer shell or on the surface of nanoparticles was released in a way of burst release at the initial stage. In contrast, the drug incorporated in the core was released in a sustained way (Müller et al., Citation2002; Jia et al., Citation2010; Yang et al., 2010).
Figure 6. In vitro release profiles of BCA from different vehicles. (A) BCA suspension; (B) BCA-NLC.
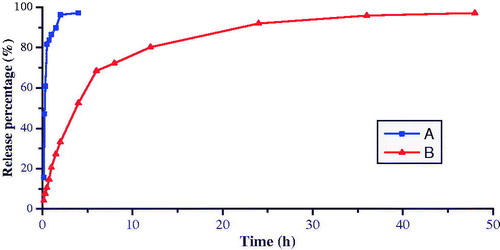
Table 2. The regression equation of BCA released from BCA suspension and BCA-NLC in vitro.
Pharmacokinetics study
In this study, BCA has good linearity ranged from 0.02 to 4.0 μg/mL (r = 0.9998), and the lower limit of quantification (LQQ) of BCA was 0.02 μg/mL. The recovery of BCA from plasma at three concentrations (0.05, 0.5 and 1.5 μg/mL) ranged from 86.01% and 105.35%. The recovery of valsartan at 1.0 μg/mL was 94.36 ± 4.62% (n = 5). The BCA blood concentration-time profiles of the two formulations were shown in . Compared with BCA suspension, a remarkable increase was observed in plasma concentration of BCA in the formulation NLC. After oral administration, plasma level of BCA eliminated rapidly both in BCA suspension and BCA-NLC. In addition, a second peak was found around 5 h in BCA-NLC. The first peak of NLC was attained within 1–2 h, indicating that NLC was transported quickly from the gastrointestinal tract into systematic circulation. Subsequently, concentration of NLC started to decrease due to macrophage uptake and distribution of NLC among different organs. The second peak appeared at around 5 h after the appearance of first peak; nevertheless, its concentration was appreciably lower than that of the first peak. It was due to release and distribution of NLC from particular organs into systemic circulation (Haede et al., Citation2011).
Figure 7. Plasma concentration-time profiles of BCA suspension and BCA-NLC after oral administration in rats. Results are expressed as the mean ± SD of six rats.
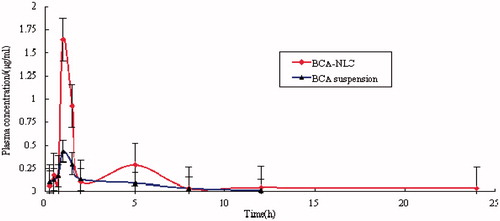
The obtained pharmacokinetics parameters obtained by a non-compartmental analysis are exhibited in . The Cmax value of BCA-NLC was significantly higher than that obtained with the BCA suspension (p < 0.05). The relative bioavailability of BCA could be exhibited by the area under the curve (AUC) for the given dose in the formulation. In this study, the AUC0–∞ after oral administration of BCA-NLC was 3.58 ± 2.10 μg/mL/h, which was approximately sevenfold higher than that of BCA suspension (0.51 ± 0.25 μg/mL/h). Compared with the BCA-suspension, the t1/2 and mean residence time (MRT) of BCA-NLC were significantly increased for 3.2-fold and 2.3-fold in plasma respectively, which may be due to sustained release of BCA from BCA-NLC.
Table 3. The pharmacokinetics parameters of the BCA suspension and BCA-NLC upon oral administration at a dose of 10 mg/kg (n = 6), (mean ± S.D.).
The CLz/F was significantly decreased in the formulation of BCA-NLC (p < 0.01). This meant a quick reduction of BCA, which may be transferred to various organs of lymphatic systems in the body (Haede et al., Citation2011). The possible mechanism of NLC uptake was intracellular uptake via the M-cells of Peyer’s patches in the gut. Through M cell uptake, drugs could be effectively transported to systemic circulation through intestinal lymphatics via thoracic lymph duct. Thus, NLC could effectively improve AUC0–∞ and MRT. Compared with BCA suspension, the results indicated that systemic absorption of BCA was significantly enhanced by incorporating into NLC. The NLC showed a promising potential for enhancing oral bioavailability of poorly water-soluble drugs.
The possible reasons for low bioavailability of BCA were its poor water solubility and extensive metabolism to glucuronide and sulfate conjugates both in the intestine and liver (Moon et al., 2007). In this study, the formulation of NLC successfully improved the AUC0–∞ of BCA in rats; the possible mechanism could be summarized as following. Firstly, after oral administration of BCA-NLC, the lipid matrix was degraded by the enzymes in the gut forming surface active mono- and diacylglycerols which could solubilize the BCA. Subsequently, BCA-NLC interacted with bile salt to form mixed micelles which promoted BCA transport to the systemic circulation through intestinal lymphatics, thus avoiding the hepatic first-pass effect and increasing plasma concentration of BCA (Florence, Citation1997). Lastly, particle size and surface properties of NLC formulation are also important parameters that further affect the absorption process. The particle size of BCA-NLC was almost less than 200 nm, which exhibits very high uptake and are easily released from Peyer’s patches, thereby facilitating BCA transport to lymphatic system (Bargoni et al., Citation1998).
Conclusion
In this work, the BCA-NLC with a high drug incorporation was successfully prepared by the method of emulsion evaporation and low temperature solidification. The characterization of the BCA-NLC was evaluated in detail. Due to the use of liquid lipid, the matrix of BCA-NLC was in less-ordered state, which greatly improved the drug loading capacity and storage stability. In vitro release study, BCA from BCA-NLC exhibited biphasic drug release patterns with burst release at the initial 4 h and followed by sustained release. In addition, the dissolution rate of BCA was also significantly improved. Pharmacokinetics study suggested that BCA-NLC showed higher AUC value and had a prolonged residence time of drug in the systemic blood circulation. These results indicated that the formulation of NLC could be used as a potential vehicle of BCA for improving bioavailability to achieve its biological activities.
Declaration of interest
These authors have reported no conflicts of interest.
These authors acknowledge the financial support of Undergraduate Scientific and Technological Innovation Foundation of Anhui University of Traditional Chinese Medicine (201207).
References
- Atkinson C, Compston JE, Day NE, et al. (2004). The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 79:326–33
- Bargoni A, Cavalli R, Caputo O, et al. (1998). Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res 15:745–50
- Bhandari R, Kaur IP. (2013). Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid solid lipid nanoparticles. Int J Pharm 441:202–12
- Cassady JM, Zennie TM, Chae YH, et al. (1988). Use of a mammalian cell culture benzo(a) pyrene metabolism assay for the detection of potential anticarcinogens from natural products: inhibition of metabolism by biochanin A, an isoflavone from Trifolium pratense L. Cancer Res 48:6257–61
- Chedeaui P, San Miquel G, Hidalgo L, et al. (2008). Effect of Trifolium pratense-derived isoflavones on the lipid profile of postmenopausal women with increased body mass index. Gynecol Endocrinol 24:620–4
- Chinsriwongkul A, Chareanputtakhun P, Ngawhirunpat T, et al. (2011). Nanostructured lipid carriers (NLC) for parenteral delivery of an anticancer drug. AAPS Pharm Sci Tech 13:150–8
- Chirio D, Gallarate M, Peira E, et al. (2011). Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J Mroencapsul 28:637–48
- Dai W, Zhang D, Duan C, et al. (2010). Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul 27:234–41
- Das S, Chaudhury A. (2011). Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharmasci Tech 12:62–76
- Florence AT. (1997). The oral absorption of micro-and nanoparticulates: neither exceptional nor unusual. Pharm Res 14:259–66
- Haede H, Das M, Jain S. (2011). Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv 8:1407–24
- Han HK, Lee BJ, Lee HK. (2011). Enhanced dissolution and bioavailability of biochanin A via the preparation of solid dispersion in vitro and in vivo evaluation. Int J Pharm 415:89–94
- Harini R, Ezhumalai M, Pugalendi KV. (2012). Antihyperglycemic effect of biochanin A, a soy isoflavone, one steptozotocindiabetic rats. Eur J Pharmacol 676:89–94
- Huang X, Chen YJ, Peng DY, et al. (2013). Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids Surf B Biointerfaces 102:391–7
- Howes JB, Sullivan D, Lai N, et al. (2000). The effects of dietary supplement with isoflavones from red clover on the lipoprotein profiles of post menopausal women with mild to moderate hypercholesterolaemia. Atherosclerosis 152:143–7
- Jia LJ Zhang DR, Li ZY, et al. (2010). Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv 17:11–8
- Kole L, Giri B, Manna SK, et al. (2011). Biochanin A, an isoflavon, showed antiproliferative and anti- inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur J Pharmacol 25:8–15
- Lee MK, Lim SJ, Kim CK. (2007). Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 28:2137–46
- Manjunath K, Reddy JS, Venkateswarlu V. (2005). Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol 27:127–44
- Mehnert W, Mader K. (2001). Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–96
- Müller RH, Radtke M, Wissing SA. (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54:131–55
- Müller RH, Mader K, Gohla S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm 50:161–77
- Müller RH, Sheqokar R, Keck CM. (2011). 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial application. Curr Drug Discov Technol 8:207–27
- Moon YJ, Morris ME. (2007). Pharmacokinetics and bioavailability of the bioflavonoid biochanin a: effects and quercetin and EGCG on bioavailability disposition in rats. Mole Pharm 4:865–72
- Moon YJ, Sagawa K, Frederick K, et al. (2006). Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J 8:433–42
- Mukherjee S, Ray S, Thakur RS. (2009). Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci 71:349–58
- Pardeike J, Hommoss A, Müller RH. (2009). Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm 366:170–84
- Silvander M. (2002). Steric stabilization of liposomes – a review. Progr Colloid Polym Sci 120:35–40
- Singh SP, Wahajuddin Ali MM, et al. (2010). High-throughput quantification of isoflavones, biochanin A and genistein, and their conjugates in female rat plasma using LC-ESI-MS/MS: application in pharmacokinetic study. J Sep Sci 33:3326–34
- Yoon KN, Alam N, Lee KR, et al. (2011). Antioxidant and antityroinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules 6:2334–47
- Zur Muhlen A, Schwarz C, Mehnert W. (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery – drug release and release mechanism. Eur J Pharm Biopharm 45:149–55