Abstract
In this article, we prepared a dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery by using a degradable pH-responsive ketal derivative, mPEG2000-Isopropylideneglycerol (mPEG-IS, PI) polymer. The purpose of this study is to develop an injectable dual-responsive micellar nanogel system which has a sol-gel phase transition by the stimulation of body temperature with improved stability and biocompatibility as a controlled drug delivery carrier for cancer therapy. The pH-responsive PI was designed with pH-responsive ketal group as hydrophobic moieties and PEG group as hydrophilic moieties. The PI micelles encapsulated paclitaxel (PTX) was fabricated. Then, the PI micelles were formed in a thermo-nanogel. The micellar nanogel could improve the solubility and stability of PTX. The physiochemical properties of PI micelles and micellar nanogel were characterized. The results showed that dual-responsive micellar nanogel could carry out sol-gel transition at 37 °C. The PI polymer can spontaneously self-assemble into micellar structure with size of 100–200 nm. The dual-responsive micellar nanogel could be degraded under lower pH condition. The test in vitro PTX release showed that dual-responsive micellar nanogel could release about 70% for 70 h under pH 5.0 while about 10% release at pH 7.4 and pH 9.0. The dual-responsive micellar nanogel was of lower cytotoxicity and suppressed tumor growth most efficiently. The micellar nanogel will be a new potential dual-responsive drug delivery system for cancer therapy.
Introduction
It is interesting for pH-sensitive delivery system which could respond to the acidic environmental pH condition, such as tumors, inflammatory tissues and phagosomes (Bae et al., Citation2005a). It is useful and important for chemotherapy of cancers in pH-sensitive delivery system. The pH sensitivity could enhance the release of anticancer drugs at the triggering lower pH of endosomes or lysosomes in cytoplasm that could reduce the side cytotoxicity and enhance the efficacy of anticancer drugs (Bae et al., Citation2005b; Ganta et al., Citation2008; Oh et al., Citation2009). As known, the polymer micelles are good vehicles for the poorly soluble anticancer drugs within the core-shell structure. Compared with other delivery systems, such as larger nanoparticles and liposomes, polymeric micelles accumulate more readily in tumors with unique and good properties including small size, pH-and temperature-responsive drug release, systemic stability and unique disposition (Davis et al., Citation2008; Qian et al., Citation2012; Hallaj-Nezhadi et al., Citation2013).
In our previous studies, we reported pH-sensitive hydrazone linkage mPEG2000-Hz-CHEMS (Chen et al., Citation2010a,Citationb, Citation2011, Citation2012). But ketals were more pH-sensitive at the lower pH of tumors than hydrazones and were more stable in the pH 7.4 environment of the blood than hydrazones (Siepmann & Göpferich, Citation2001; von et al., Citation2002).
Many drugs are poorly soluble, such as paclitaxel (PTX), a broad spectrum of carcinomas of the breast, ovary and lung (Singla et al., Citation2002; Heller & Barr, Citation2004), and it is one of the main hurdles for developing clinically useful delivery system. However, due to its low poor solubility, it is formulated with polyethoxylated castor oil and ethanol (Cremophor EL) leading to hypersensitivity of many patients. Thus, a new delivery formulation is needed for PTX (Szebeni et al., Citation1998; Gelderblom et al., Citation2001; Zhang et al., Citation2005; Mo et al., Citation2011a,Citationb).
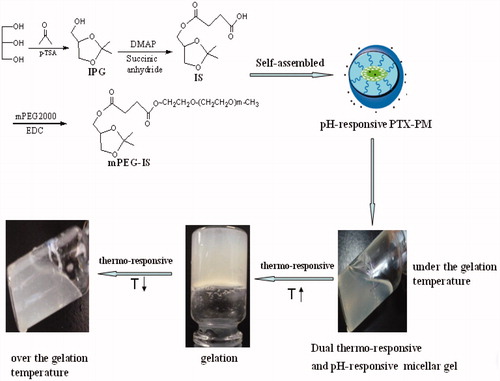
In this article, we prepared a dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery by using a degradable pH-responsive ketal derivative, mPEG2000-Isopropylideneglycerol (mPEG-IS, PI) polymer. The purpose of this study is to develop an injectable dual-responsive micellar nanogel system which has a sol-gel phase transition by the stimulation of body temperature with improved stability and biocompatibility as a controlled drug delivery carrier for cancer therapy. The PI polymer could target drugs to the acidic environments of tumors, inflammatory tissues and phagosomes. It was easy to synthesize the PI polymer. Then, the PI micelle with PTX was added to form a thermo-nanogel. The physicochemical properties, the in vitro release, cytotoxicity and the antitumor efficacy of the dual-responsive self-assembled micellar nanogel were under investigation.
Materials and methods
Materials
Chitosan was provided by the Shanghai Lanji Co. Ltd., China, with deacetylation degrees of 97% and viscosity average molecular weight of 65 000 D. mPEG2000, 1Ethyl3(3dimethyllaminopropyl) (EDC), 4-(dimethyl amino) pyridine (DMAP) and Succinic anhydride were obtained from Sigma Chemical Co. (St. Louis, MO). MTT was obtained from Sigma Chemical Co. (St Louis, MO). All other reagents were of analytical grade and supplied by Sinopharm Group Chemical Reagent Corp. HEK293 cells and hepatoma solidity (Heps) cell were obtained from the Yantai Science & Biotechnology Co. Ltd, Yantai, China. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotics (100 U/mL penicillin G and 0.1 mg/mL streptomycin). Cells were maintained at 37 °C in a humidified incubator containing 5% CO2.
Synthesis and characterization of PI
The synthesis of PI was shown in . PI was synthesized with three steps. Firstly, glycerin (9.2 g, 100 mmol), acetone (30 mL, 400 mmol), n-hexane (30 mL), p-toluenesulfonic acid (p-TSA) (0.314 g, 1.824 mmol) were added in the 100 mL flask. After reaction of 48 h, the solvent was removed and the residue was concentrated and purified with column chromatography. The purified 1,2-Isopropylideneglycerol (IPG) was obtained. Secondly, IPG (3.29 g, 24 mmol) and succinic anhydride (2.0 g, 20 mmol) were dissolved in dichloromethane (40 mL). EDC (1.2 g, 6 mmol) and DMAP (0.5 g, 4 mmol) were added. After reaction of 12 h, the solvent was removed and the residue was concentrated and purified with diethyl ether and acetone three times. A light amber fluid (IS) was obtained. Thirdly, IS (0.7 g, 3 mmol) was suspended in 20 mL dichloromethane with DMAP (0.0732 g, 0.6 mmol) and EDC (0.2 g, 1 mmol) stirring at room temperature for 30 min, and then mPEG2000 (0.5 g) was added to the solution. After reaction of 12 h, the solvent was removed and the residue was concentrated. The crystals mPEG-IS (PI) that formed were collected with diethyl ether (20 mL). A white PI powder was obtained. The chemical structures of the PI derivative were determined by FT-IR and 1H-NMR.
Preparation of PTX-loaded PI micelles (PTX-PM)
The pH-responsive PI and PTX (1:1) were dissolved in chloroform in a 25 mL flask. Then, after the chloroform removed, the PI micelle (PTX-PM) was prepared with adding pH 7.4 PBS under the ultrasound (Park et al., Citation2002).
Characterization of PTX-PM
The size of micelles was measured by Zetasizer 3000 HS instrument. Transmission electron microscopy (TEM) observation was carried out in the micellar solution, which was negatively stained with 0.01% phosphotungstic acid and placed on a copper grid coated with film.
Preparation of dual-responsive gels
The dual-responsive micellar nanogel was obtained with ratios of CS and GP from 1:1 to 8:1. Chitosan (0.1 g) was dissolved in acetic acid and the clear 2% (W/V) chitosan solution was obtained. GP (0.56 g/mL) solution was added into the chitosan solution drop by drop under stirring and stored at 4 °C. The gelation temperature and pH-responsive gel test were also tested.
Measurement of gelation temperature
In brief, 1 mL of each polymer solution was transferred to each 4 mL vial, and the vials were kept at 4 °C overnight. The vials were then placed in a water bath and equilibrated at 15 °C for 15 min. The sol-gel and gel-sol transitions of the polymer solutions were tested by the tube inversion method in the temperature range of 15–80 °C while the polymer solutions were heated at a rate of 2 °C per every 5 min. The transition temperature at which a polymer solution stopped flowing after a tube inversion was recorded as the gelation temperature (Chen et al., Citation2010a, Citation2011).
pH-responsive gel test
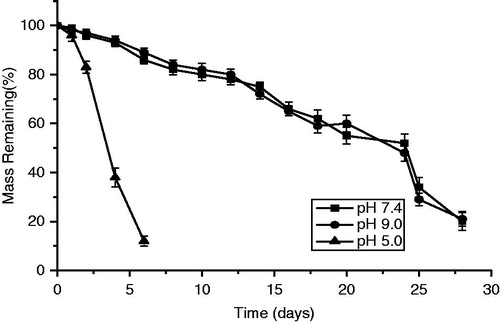
The pH-responsive PTX-PM (25%) was prepared at a concentration of in phosphate-buffered saline (pH 7.4). One milliliter of polymer solution was set in 5 mL vials, and the vials were put at 4 °C overnight. Later, the polymer solutions were incubated at 37 °C for 10 minutes for gelation. After gelation, 3 mL of phosphate buffer (pH 5.0, 7.4, and 9.0) was added to the vials. After gel erosion for a predetermined time, the weights of the gels were measured (Chen et al., Citation2010a, Citation2011).
In vitro drug release
The in vitro release of PTX from gels was determined using a dialysis bag placed in a sealed glass vial under constant shaking. 500 μg of PTX was packed into the dialysis bags. The dialysis bags containing dual-responsive gels were placed in a water bath at 37 °C until the gel formed. The release medium was 45 mL of 0.1 M phosphate buffer (pH 5, 7.4, and 9), providing sink conditions for PTX. The medium was maintained at 37 °C and shaken at 100 rpm. At various time intervals, 1 mL of dissolution fluid was collected. The sample filtrate was analyzed by HPLC. The mobile phase was a mixture of methanol and water (75:25 v/v). The column was a Kromasil C18 (4.6 × 250 mm). The flow rate was 1.0 mL/min, the detection wavelength was 227 nm (ultraviolet detector, HP1100, Agilent), the column temperature was 30 °C and the injected volume of the sample was 20 μL (Chen et al., Citation2010a, Citation2011; Yuk et al., Citation2011).
In vitro cytotoxicity assay
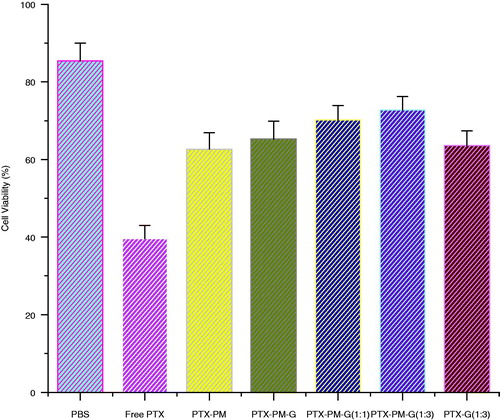
To evaluate the cytotoxicity of the dual-responsive gels by MTT assay, HEK293 cells were seeded at a density of 1 × 104 cells/well in a 96-well plate overnight. A 100 μL aliquot of PTX solution (0.5 mg/mL), PTX-loaded pH-responsive micelles(PTX-PM), PTX-loaded dual-responsive gels, diluted PTX-loaded dual-responsive gels (1:2, 1:4 in phosphate-buffered saline) and free PTX in diluted gel (1:4 in phosphate-buffered saline) were added to each well and incubated overnight; positive controls (50 μg as PTX) and negative controls (phosphate-buffered saline alone) were placed in additional wells and incubated overnight. After incubation, 100 μL of 5 mg/mL MTT in Dulbecco’s Modified Eagle’s Medium was added to each well and incubated for four hours at 37 °C. To dissolve the formazan crystal, 100 μL of MTT solubilization solution was added. After shaking the 96-well plate for 30 minutes, absorbance was measured at 570 nm using a microplate reader (Chen et al., Citation2010a, Citation2011; Yuk et al., Citation2011).
In vivo antitumor efficacy
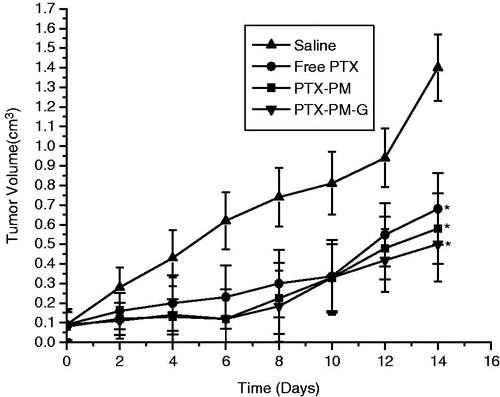
The BALB/c mice model with inoculation of approximately 1 × 106 hepatoma solidity cell was prepared. The BALB/c mice at the right flank using a 1.0 mL syringe kept in an SPF facility and had free access to food and water. As the tumor volume grew 100–200 mm3, PTX solution, PTX-loaded pH-responsive micelles (PTX-PM), PTX-loaded dual-responsive gels were intravenously administered (30 mg/kg) by tail vein every other day. Then all the mice were weighed and sacrificed by separation and measurement of the tumor with normal saline as the control group (Chen et al., Citation2010a, Citation2011; Yuk et al., Citation2011).
Statistical analysis
The results were expressed as the mean ± standard deviation (n = 3) and the statistical analysis was performed using SPSS software. p < 0.05 was considered to be statistically significant.
Results and discussion
Synthesis and characterization of PI
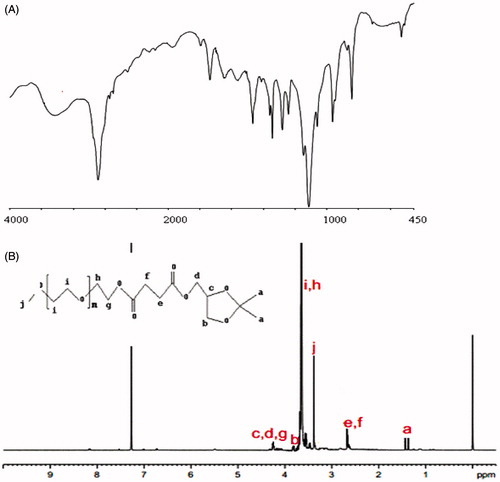
The structure changes of PI were confirmed by FT-IR spectra (). The result showed new peaks at 3434, 2888, 1467 cm−1 attributed to PEG chain, 1343, 1114 cm−1 attributed to IPG chain, 1738 cm−1 assigned to the ester linkage between PEG and IPG group. These results suggest that PEG and IPG group were part of the PI structure. showed the 1H-NMR spectra IPG (A) and PI (B), which can be found in the spectrum of .
Preparation and characterization of PTX -PM
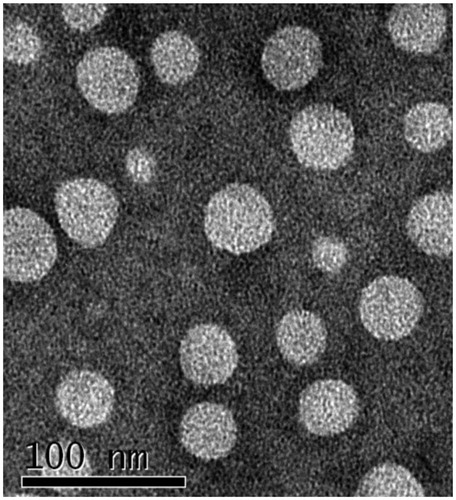
The pH-responsive PTX-PM was tested with average diameter 100–200 nm (). As shown the TEM in , PTX-PM was discrete round structures with entrapment efficiency (EE) about 52%. showed the particle sizes of PTX-PM before and after freeze-drying was almost constant.
Table 1. The physicochemical parameters of PTX-PM before freeze-drying and after freeze-drying.
Characterization of dual-responsive micellar nanogel
In the previous study, a thermosensitive poloxamer gel for vaginal delivery and temperature and ultrasound dual-sensitive liposomal microbubble gel were characterized with FT-IR and DSC which was similar with dual-responsive micellar gel (data were not shown). With temperature-sensitive gelling, the concentration of CS/GP (4:1, v/v) was optimal formulation to form micellar nanogel ().
In vitro releasing test and cytotoxicity assay
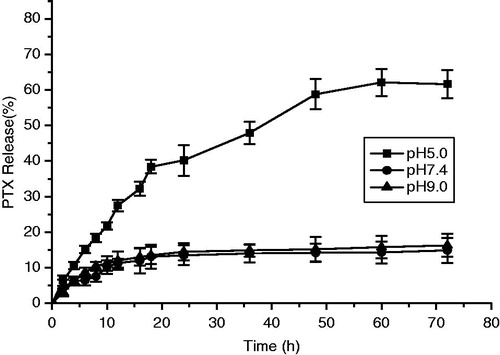
As shown in , the cumulative release of PTX suggested that the dual-responsive micellar nanogel presented a sustained release in pH 7.4 and 9.0 buffers but a rapid release behavior at pH 5.0 buffers. Thus, the dual-responsive micellar nanogel is interesting for pH-sensitive PTX delivery system. The test in vitro PTX release showed that dual-responsive micellar nanogel could release about 70% for 70 h under pH 5.0 while about 10% release at pH 7.4 and pH 9.0. The pH-responsive test for dual-responsive gel is tested in . PTX-loaded dual-responsive gel in the pH 5.0 study was eroded nearly in five days, whereas the gels in the pH 7.4 and 9.0 studies eroded in around 5 weeks.
As shown in , the results indicated that encapsulated PTX in dual-responsive micellar nanogel and PTX-PM were less toxic than the free PTX. There was not any significant difference between the PTX formulations. The PTX-loaded dual-responsive gel is interesting for pH-responsive drug delivery in different pH.
In vivo antitumor efficacy
The antitumor efficacy of PTX-loaded dual-responsive micellar gel in vivo was evaluated in tumor-bearing BALB/c mice. As shown in , there was no difference between the groups in 1 week; however, after the next week, there was different. This could be the reason of slow-release of PTX from the dual-responsive micellar nanogel in vivo under the body temperature and pH condition. The tumor volume of the saline control group was excessively enlarged, while the other groups were much smaller. The dual-responsive micellar gel group suppressed tumor growth most efficiently, followed by the PTX-PM group.
Discussion
In the previous study, we developed thermosensitive poloxamer gel for vaginal delivery. We also developed a biocompatible chitosan/glycerol phosphate (CS/GP) gel as a carrier for liposomal microbubble. However, in this study, we developed a novel pH-responsive micellar nanogel based on CS/GP. The mechanism could be explained that thermoresponsive chitosan/glycerol phosphate (CS/GP) was liquid at room temperature but gels at 37 °C. (Chen et al., Citation2012, Citation2013). Then, the pH-responsive PTX-PM was added into the thermoresponsive CS/GP gel and the dual thermoresponsive and pH-responsive self-assembled micellar nanogel was fabricated.
There was not much report about ketal polymer. Several new acid-sensitive polyketals were reported with good pH-degradable properties, such as PCADK (von Burkersroda et al., Citation2002), PPADK (Heffernan & Murthy, 2005) and PK3 (Fiore et al., Citation2010), that can be used to intracellular drug delivery. However, it is difficult to obtain the polyketal polymers. At the same time, most of the polyketals were used to the microparticles with the long degradation rate. Thus, in our research, a novel pH-responsive ketal polymer based micellar nanogel was obtained and could be used to pH-responsive drug delivery system. Based on the antitumor efficacy in vitro and in vivo, there was no significant difference between the PTX formulations. Simultaneously, this phenomenon can be explained the novel micellar nanogel could enhance the solubility of PTX and could be further used to temperature and pH dual-responsive drug delivery system.
Conclusion
In this article, we prepared a dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery by using a degradable pH-responsive ketal derivative, mPEG2000-Isopropylideneglycerol (mPEG-IS, PI) polymer. The purpose of this study is to develop an injectable dual-responsive micellar nanogel system which has a sol-gel phase transition by the stimulation of body temperature with improved stability and biocompatibility as a controlled drug delivery carrier for cancer therapy. The micellar nanogel could improve the solubility and stability of PTX. The physiochemical properties of PI micelles and micellar nanogel were characterized. The results showed that dual-responsive micellar nanogel could carry out sol-gel transition at 37 °C. The PI polymer can spontaneously self-assemble into micellar structure with size of 100–200 nm. The dual-responsive micellar nanogel could be degraded under lower pH condition. The test in vitro PTX release showed that dual-responsive micellar nanogel could release about 70% for 70 h under pH 5.0 while about 10% release at pH 7.4 and pH 9.0. The dual-responsive micellar nanogel was with lower cytotoxicity assay and suppressed tumor growth most efficiently, indicating the potential to have a therapeutic effect for cancer therapy. The micellar nanogel will be a new potential dual-responsive drug delivery system.
Declaration of interest
The authors report no conflicts of interest in this work.
This study is financially supported by the National Basic Research Program of China (973 Plan, 2012CB724003), the National Natural Science Foundation of China (81302718), Taishan Scholar Project (to Dr. Fenghua Fu), Open Project Program of State Key Laboratory of Long-acting and Targeting Drug Delivery System (YX12H071), A Technology Development Program Projects of Shandong Province (2011YD18075).
References
- Bae Y, Jang WD, Nishiyama N, et al. (2005a). Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol Biosys 1:242–50
- Bae Y, Nishiyama N, Fukushima S, et al. (2005b). Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug Chem 16:122–30
- Chen D, Jiang X, Liu J, et al. (2010a). In vivo evaluation of novel pH-sensitive mPEG-Hz-Chol conjugate in liposomes: pharmacokinetics, tissue distribution, efficacy assessment. Artif Cells Blood Substit Immobil Biotechnol 38:136–42
- Chen D, Jiang X, Huang Y, et al. (2010b). pH-Sensitive mPEG-Hz-cholesterol conjugates as a liposome delivery system. J Bioact Compat Polym 25:527–42
- Chen D, Liu W, Shen Y, et al. (2011). Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomedicine 6:2053–61
- Chen D, Sun K, Mu H, et al. (2012). pH and temperature dual-sensitive liposome gel based on novel cleavable mPEG-Hz-CHEMS polymeric vaginal delivery system. Int J Nanomedicine 7:2621–30
- Chen D, Hong Y, Hong M, et al. (2013). Novel chitosan derivative for temperature and ultrasound dual-sensitive liposomal microbubble gel. Carbohydrate Polymers 94:17–23
- Davis ME, Chen ZG, Shin DM. (2008). Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–82
- Fiore VF, Lofton MC, Roser-Page S, et al. (2010). Polyketal microparticles for therapeutic delivery to the lung. Biomaterials 31:810–17
- Ganta S, Devalapally H, Shahiwala A, Amiji M. (2008). A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 126:187–204
- Gelderblom H, Verweij J, Nooter K, Sparreboom A. (2001). Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37:1590–8
- Heller J, Barr J. (2004). Poly(ortho esters)-from concept to reality. Biomacromolecules 5:1625–32
- Heffernan M, Murthy N. (2005). Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem 16:1340–2
- Hallaj-Nezhadi S, Dass CR, Lotfipour F. (2013). Intraperitoneal delivery of nanoparticles for cancer gene therapy. Future Oncol 9:59–68
- Mo R, Xiao Y, Sun M, et al. (2011a). Enhancing effect of N-octyl-O-sulfate chitosan on etoposide absorption. Int J Pharm 409:38–45
- Mo R, Jin X, Li N, et al. (2011b). The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials 32:4609–20
- Oh KT, Kim D, You HH, et al. (2009). pH-sensitive properties of surface charge-switched multifunctional polymeric micelle. Int J Pharm 376:134–40
- Park JS, Oh YK, Yoon H, et al. (2002). In situ gelling and mucoadhesive polymer vehicles for controlled intranasal delivery of plasmid DNA. J Biomed Mater Res 59:144–51
- Qian WY, Sun DM, Zhu RR, et al. (2012). pH-sensitive strontium carbonate nanoparticles as new anticancer vehicles for controlled etoposide release. Int J Nanomedicine 7:5781–92
- Siepmann J, Göpferich A. (2001). Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev 48:229–47
- Singla AK, Garg A, Aggarwal D. (2002). Paclitaxel and its formulations. Int J Pharm 235:179–92
- Szebeni J, Muggia FM, Alving CR. (1998). Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst 90:300–6
- von Burkersroda F, Schedl L, Göpferich A. (2002). Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 23:4221–31
- Yuk SH, Oh KS, Koo H, et al. (2011). Multi-core vesicle nanoparticles based on vesicle fusion for delivery of chemotherapic drugs. Biomaterials 32:7924–31
- Zhang JA, Anyarambhatla G, Ma L, et al. (2005). Development and characterization of a novel Cremophor EL free liposome-base paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm 59:177–87