Abstract
The aim of this was to investigate and compare the chemosensitizing effect of some pharmaceutical excipients (TPGS, Pluronic P85 and chitosan) by evaluating the cytotoxicity of the chemotherapeutic drug Hydroxy Camptothecin (HCPT) loaded into PLGA nanoparticles. Different nanoparticles formulations were developed and evaluated for size, zeta potential, morphology, loading and encapsulation efficiency as well as in vitro drug release. The cytotoxicity of the nanoparticles was evaluated by MTT assay in A549 (human lung carcinoma cell line) and HT29 (human colon carcinoma cell line) whereas their cellular uptake was determined by confocal laser scanning microscopy and microfluorimetry assay. The results revealed that nanoparticles possessed a desirable nanometric size (revealed by dynamic light scattering measurements and TEM) with appreciable HCPT encapsulation (>48%) and negative surface charge that was switched to positive upon coating with chitosan. The nanoparticles adopted a sustained release phase preceded by initial burst of HCPT that was reduced by chitosan coating. The cytotoxicity of the nanoparticles in A549 and HT29 cells was significantly augmented compared to simple drug solution and basic nanoparticles without excipients. The excipients could be ranked according to their IC50 lowering effect in the following order [TPGS (sixfold lower IC50) > Pluronic P85 > Chitosan]. The augmented cytotoxicity and chemosensitizing effect might be attributed to overcoming drug efflux (in case of TPGS 1000 or Pluronic P85) and/or maximizing internalization by cancer cells (chitosan coating). Acting as chemopotentiators, the studied excipients could have potential in reducing therapeutic HCPT doses and minimizing adverse effects in lung and colon chemotherapy.
Introduction
Lung and colorectal carcinoma are common worldwide (GLOBOCAN, Citation2008). Chemotherapy treatment suffers from lack of selective tumor tissue uptake of the anticancer drugs resulting in many side effects. Pharmaceutical nanotechnology for the delivery of conventional anticancer agents has been explored to solve this problem. The leaky vasculature and reduced lymphatic drainage of the tumor tissue was found to increase capacity for the uptake and retain of nanoparticles of up to 400 nm (Davaran et al., Citation2006; Gindy & Prud'homme, Citation2009; Gu et al., Citation2009; Yu et al., Citation2009); an effect renowned as the enhanced permeability and retention (EPR) effect (Mahor et al., Citation2010; Shaji & Jain, Citation2010; Zaki & Tirelli, Citation2010). Not only can the nanoparticles accumulate at the tumor site as a result of the EPR effect (passive targeting) (Kumari et al., Citation2009) but also bypass multidrug resistance (MDR) (Hu & Zhang, Citation2009). Nevertheless, various attempts have been made to maximize selectivity with tumor cells; of these one could mention active targeting (by attaching to nanoparticles surface a ligand or aptamer which is recognized by cancer cell-surface receptors (Zhang et al., Citation2007b; Acharya et al., Citation2009; Betancourt et al., Citation2009), nanoparticles surface charge modification to enhance interaction with cancer cell membrane (Taetz et al., Citation2009; Yang et al., Citation2009a) and/or coadministration/incorporation of efflux inhibitors (herbs, drugs or pharmaceutical excipients (Batrakova et al., Citation2004; Collnot et al., Citation2006; Shah et al., Citation2009; Zhou et al., Citation2009).
10-Hydroxycamptothecin (HCPT) is a potent analogue of the cytotoxic alkaloid Camptothecin that was first isolated from Camptotheca acuminata or happy tree of China. It acts by inhibition of the nuclear enzyme topoisomerase I (Top I) (Hatefi & Amsden, Citation2002). Hydroxycamptothecin (HCPT) has demonstrated antitumor activity toward a wide range of cancer including lung and colorectal cancer. However, its clinical use is limited by cancer cell resistance due to efflux mechanism and poor stability in physiological milieu (Hofheinz et al., Citation2005). To overcome these limitations, polymeric nanoparticulate delivery systems are sought of due to their ability to protect the drug from premature degradation; prevent drugs from prematurely interacting with the biological environment; enhance absorption of the drugs into a selected tissue (e.g. solid tumor); control the pharmacokinetic and drug tissue distribution profile and improve their intracellular penetration. In this study, the biodegradable and biocompatible FDA-approved polymer: PLGA was selected to stabilize the HCPT in the nanoparticles due to its ability to afford an acidic microenvironment (Shenderova et al., Citation1999). This property was shown to stabilize the lactone species of HCPT (responsible for chemotherapeutic effect) (Shenderova et al., Citation1999). It was shown to reduce the reversible pH-sensitive interconversion of HCPT from the potent lactone form (stable below pH 5) to the poorly active carboxylate form (stable above pH 8) for more than 10 weeks (>95% lactone) under simulated physiological milieu (Shenderova et al., Citation1997). In vivo, the nanoparticles can (i) release their contents in close proximity to the target cells; (ii) attach to the membrane of the cell and act as an extracellular sustained-release drug depot; or (iii) internalize into the cell. This study aims at comparing the chemosensitizing effect of some pharmaceutical excipients. The cytotoxicity and cellular uptake of these nanosystems will be evaluated on lung carcinoma cells A549 and colon carcinoma cell HT29 to elucidate how these excipients act differently
Materials and methods
10-Hydroxy camptothecin (HCPT) was purchased from Qingdao Green-Extract Biology and Technology Co., Ltd., Qingdao, China. Poly lactide-co-glycolide (PLGA) (lactide:glycolide) 50:50, acid terminated; inherent viscosity, 0.18 dL/g; Mw, 4000 Da) was a kind gift from Purac Biochem (Gorinchem, Netherlands). Polyvinyl alcohol (Mw 6000 Da), Fluoresceinamine, D-α-tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS 1000 or simply, TPGS 1000), Chitosan (CS; low molecular weight: 495 kDa), Dimethyl sulfoxide (DMSO), DCC (dicyclohexylcarbodiimide) and NHS (N-hydroxysuccinimide) were purchased from Sigma (St. Louis, MO, USA). Pluronic P85 was a kind gift from BASF Corporation, NJ, USA.
Cell Culture
A549 (Human Caucasian lung carcinoma) and HT29 (Human Caucasian colon adenocarcinoma) cells (ECACC, Salisbury, UK) were maintained as, respectively adherent cell cultures at 37 °C in a humidified atmosphere (5% CO2) in Dulbecco modified Eagle’s minimal essential medium (DMEM, 25 mM glucose) supplemented with 2 mM glutamine (Gibco) and 10% heat-inactivated fetal calf serum (FCS) (Invitrogen, Paisley, UK). Antibiotic solution composed of 100 IU/ml penicillin and 100 IU/ml streptomycin (Gibco) was added to the culture medium during cell maintenance. For monolayer formation, the cells were detached using trypsin–EDTA (Invitrogen, Paisley, UK) consisting of 2.5% (w/v) of trypsin and 0.2% (w/v) EDTA in PBS. The cells were suspended in DMEM containing FCS to a count of 105 living cells/ml as determined by trypan blue exclusion stain and a hemocytometer. For cytotoxicity and cellular uptake experiments, aliquots of the cells were seeded in 96-well plates and incubated till confluency. For confocal microscopy, cells were seeded on coverslips in a six-well plate.
Preparation of HCPT-loaded PLGA nanoparticles
HCPT nanoparticles were prepared by emulsification solvent evaporation method using PLGA polymer and different emulsifiers as previously reported (Zhang & Feng, Citation2006a). Briefly, 1 ml solution of HCPT in dichloromethane (DCM) was used (1 mg/ml) and added to PLGA solution in DCM (50 mg/ml); guided by preliminary work, a drug to polymer ratio 1:25 was optimal. The previous organic phase was vortex mixed; then added to 50 ml aqueous solution of different emulsifiers, namely, PVA (1%) (Zaki et al., Citation2013), TPGS (0.1%) (Zhang & Feng, Citation2006a; Feng et al., Citation2007) or Pluronic P85 (0.1%) (Johnson et al., Citation2002). The composition and codes of nanoparticles formulations are shown in . The dispersions were homogenized using high shear homogenizer at 48 000 rpm. The organic phase was then evaporated on a magnetic stirrer at room temperature. For separation of nanoparticles from unincorporated drug, ultracentrifugation (Beckman, XL-90, Ramsey, Minnesota, USA) was performed at 40 000 rpm and 4 °C for 1 h.
Table 1. Composition and codes of nanoparticles prepared by emulsification solvent evaporation method.
Uncoated and chitosan-coated nanoparticles were prepared by the nanoprecipitation after slight modification (Derakhshandeh et al., Citation2007). Briefly, PLGA polymer and 1 mg of HCPT were dissolved in 20 ml acetone at a polymer:drug ratio 1:30. The organic phase was added dropwise (0.5 ml/min) into PVA aqueous solution (1%; acidic pH 3 adjusted by 0.1 N HCl to stabilize the lactone form of HCPT). In another set of experiments, chitosan-coated PLGA nanoparticles were prepared as previously described (Nafee et al., Citation2007) by dissolving the CS (0.2%, w/v) in the external aqueous phase during the nanoparticles manufacturing procedure. Subsequently, the organic solvent was evaporated by stirring the dispersion at room temperature using a magnetic stirrer. Finally, nanoparticles were separated from unincorporated drug by ultracentrifugation (Beckman, XL-90) at 40 000 rpm and 4 °C for 1 h. The composition and codes of nanoparticles prepared by nanoprecipitation method with or without chitosan coating are shown in .
For preparation of fluorescently-labeled PLGA nanoparticles, carbodiimide chemistry was adopted whereby fluoresceinamine (FA) was chemically conjugated to PLGA polymer (Weiss et al., Citation2006; Xu et al., Citation2009). Briefly, PLGA was dissolved in dichloromethane at a concentration 1%; then 560 mg of DCC (dicyclohexylcarbodiimide) and 311 mg of NHS (N-hydroxysuccinimide) were added and the mixture was stirred overnight at room temperature followed by filtration to remove any by-product. Fluoresceinamine/DMSO solution (0.0583 g of FA in 20 ml of DMSO) was then added to the filtrate and stirred overnight in the dark. The fluorescent polymer was then purified by dialysis and lyophilized. Fluorescent nanoparticles (FA-NPs) were prepared as previously described using a 3:1 mixture of FA-PLGA and PLGA, respectively. FA-PLGA NPs have a λEXCITATION at 488 nm laser, and its emission was read at λEMISSION 497 to 560 nm (green fluorescence) (Khdair et al., Citation2009).
Morphological examination of nanoparticles
Morphological examination of the nanoparticles was conducted using (TEM) transmission electron microscope JEM-100S (Jeol, Tokyo, Japan). One drop of nanoparticles suspension was placed on a copper grid covered with nitrocellulose membrane and air-dried before staining with phosphotungstic acid solution (1%).
Measurement of nanoparticles size and particle size distribution
The mean particle size of the nanoparticles, the particle size distribution (measured as the polydispersity index (PDI) and zeta potential were determined by Dynamic light scattering method using a Zetasizer Nanoseries ZEN3600 (Malvern Instruments, Worcestershire, UK).
Determination of nanoparticles yield
Nanoparticles yield was determined gravimetrically by Equation (1).
Determination of HCPT loading content and encapsulation efficiency of nanoparticles
The encapsulation efficiency (EE%) and loading efficiency (LC%) were determined as reported previously (Zhang et al., Citation2004, Citation2007a; Wang & Li, Citation2008). Briefly, a predetermined amount of freeze-dried nanoparticles was accurately weighed and completely dissolved in 1 ml of dichloromethane. Then 10 ml of methanol was slowly added to the solution to precipitate the polymer. Drug encapsulation efficiency (EE%) and loading content (LC%) were calculated using Equations 2 and 3, respectively.
The amount was calculated spectrophotometrically at 382 nm (Zhang et al., Citation2004, Citation2007a; Wang & Li, Citation2008). A pre-constructed calibration curve y = 0.061x + 0.044 (R2 = 0.995) was used. The calibration curve was prepared using a stock solution of HCPT (2.6 mg/ml in DMSO; and serial dilutions in 2% Tween 80 in water). The absorbance of the different concentrations (1.3–20.8 μg/ml) was measured at λmax 382 nm.
In vitro release of HCPT-loaded nanoparticles
In vitro release of HCPT from the nanoparticles was evaluated using a dialysis bag diffusion technique on freshly prepared HCPT nanoparticles (Betancourt et al., Citation2007). The nanoparticles (2 mg) were dispersed in 2 ml distilled water; this aliquot of the drug-loaded NPs was then transferred to a glass cylinder having the length of 10 cm and diameter of 2.5 cm fitted with a dialysis membrane with 12000 MWCO (Sigma, St. Louis, MO, USA) presoaked in distilled water. The cylinder was placed in a receiving compartment containing 100 ml PBS containing 2% Tween 80 to maintain sink condition. The donor and receiving compartments were placed in a shaking water bath (70 rpm) maintained at 37 °C. Periodically, 5 ml of the release medium was withdrawn and assayed spectrophotometrically at 382 nm. Samples withdrawn were replaced by the same volume of fresh dissolution medium. The experiments were done in triplicates with control runs using unmedicated nanoparticles.
Mechanism and mathematical modeling for HCPT release from Nanoparticles
The mechanism of drug release from PLGA polymeric nanosystems was determined using Korsmeyer–Peppas model (Equation 2), that correlates drug release to time by a simple exponential equation for the first 60% drug release. This equation is used whenever the drug release mechanism is unknown or when there are more than one release mechanism (Korsmeyer et al., Citation1983; Costa & Lobo, Citation2001)
Mt/M∞ is the proportion of drug released at time t, k is the kinetic constant and the exponent n has been proposed as indicative of the release mechanism (Ritger & Peppas, Citation1987a; Ritger & Peppas, Citation1987b). With the linear form of Equation 2, plotting of log Mt/M∞ against log t, yielded the diffusion exponential (n) from the slope, the correlation coefficient (r2) and the diffusion constant (k) from the intercept. The exponent n has been proposed as indicative of the release mechanism. In this context, n ≤ 0.43 indicates Fickian release and n = 0.85 indicates a purely relaxation controlled delivery. Intermediate values 0.43 < n < 0.85 indicate an anomalous behavior (non-Fickian kinetics corresponding to coupled diffusion/polymer relaxation (Devi Kusum & Bhosale, Citation2009).
Cytotoxicity of nanoparticles
For measurement of cytotoxicity on HCPT-NPs, the MTT colorimetric assay was used (Mosmann, Citation1983). Briefly, A549 and HT29 cells were diluted with culture medium to a count of 1 × 105 cells/ml and seeded in 96-well micro-titer plate at density of 10 000 cells/well. At the time of each experiment, the culture medium was replaced with 100 μl HCPT solution (free drug dissolved in DMSO), NPs-loaded HCPT dispersions (equivalent to HCPT dose 10–1200 ng/ml). Cells incubated with culture medium served as negative control) while cells incubated with plain NPs (unmedicated) were used to determine cytotoxicity due to NPs formulation per se. The cells were incubated for 48 h at 37 °C in a humid atmosphere with 5% CO2, then the test dispersion was removed by gentle aspiration and the cells were rinsed three times with pre-warmed pH 7.4 PBS. Subsequently, a 100 μl of 0.5 mg/ml of MTT reagent in DMEM (3-[4,5-dimethylthiazol-2-yl]-3,5-diphenyl tetrazolium bromide dye) was added to each well, and the plates were incubated for 4 h after which the stain was removed. To solubilize the formazan crystals, a 100 μl of sterile DMSO was added to each well, and the plates were shaken for 5 min (Mosmann, Citation1983). Finally, the absorbance (A) was measured at 550 nm in a micro-titer plate reader (TECAN Safire, Tecan Austria GmbH, Grödig, Austria). The cell cytotoxicity was calculated using the following equation (Zaki & Tirelli, Citation2011):
Where Atest is the absorbance of the cells incubated with the NPs dispersions and Acontrol is the absorbance of the cells incubated with the culture medium only (negative control). IC50, the drug concentration at which reduction of 50% cell viability occurs in comparison with that of the control sample, is calculated by the curve fitting of the cell viability data (Zaki & Tirelli, Citation2011).
Cellular uptake studies
Visualization by Confocal Microscopy
The cellular uptake was performed using A549 and HT29 cells. The visualization study was done using confocal laser scanning microscopy as previously described (Zaki et al., Citation2011). Briefly, the cells were seeded on sterile poly-L-lysine-coated coverslips placed in six-well plates and placed in the incubator at 37 °C to allow cell attachment. To each well, 250 μl of nanoparticles dispersion was added, and the plates were incubated for 30 min in a humid atmosphere at 37 °C in a CO2 incubator. Immediately after removal of the colloidal dispersion by gentle aspiration, 1 ml trypan blue solution was incubated with the cells for 1 min after which the cells were rinsed at least four times with pre-warmed PBS pH 7.4. Cell fixation and permeabilization was achieved by dipping in 4% formaldehyde solution for 20 min at room temperature followed by 0.05% Triton X-100 in PBS for 5 min to allow actin staining using Texas-red phalloidin (Molecular probes, Leiden, The Netherlands). At the same time, the cells were incubated for 3 min with 200 nM 4′, 6-diamidino-2- phenylindole (DAPI) solution in PBS for nuclear staining followed by rinsing. The coverslips were carefully inverted onto a drop of mounting liquid on a microscopic slide and stored in the dark at 4 °C. For visualization using confocal microscopy (CLSM, Zeiss Axiovert 200M, Oberkochen, Germany) equipped with a LSM 5 Image Browser (Carl Zeiss, Oberkochen, Germany), Fl-NPs were excited using a 488-nm laser, and its emission was read from 497 to 560 nm (green emission) while excitation at 358 nm induces DAPI fluorescence and its emission was read from 450 to 468 nm (blue emission). For further image processing, ImageJ® Software (in the public domain) was used.
Quantitative uptake by microfluorimetry
For quantitative study, A549 and HT29 cells were seeded into 96-well plates (black wall with transparent bottom, Costar, IL) at 1.3 × 104 cell/well, and after the cells reached 80% confluence, the medium was changed with that containing 2 mg/ml of different FA-PLGA-labeled nanoparticles. At 12 h post-incubation, the NPs suspension was removed and the wells were washed with 100 μl of PBS to eliminate traces of nanoparticles left in the wells. Fifty microliters of 0.5% Triton X-100 in 0.2 N NaOH was added to the sample wells at room temperature to lyse the cells. The amount of fluorescence of the cell lysate in each well was then measured using a fluorescence plate reader (TECAN Safire, Tecan Austria GmbH, Grödig, Austria) with excitation wavelength at 485 nm and emission wavelength at 520 nm for FA. Cell-bound and internalized nanoparticles are quantified by making use of a calibration curve obtained with FA-labeled nanoparticles in a cell lysate solution (106 untreated cells dissolved in 1 ml of the Triton X-100 solution). The protein content of the cell lysate in each well was determined using the QuantiPro micro BCA protein assay kit (Sigma, St. Louis, MO, USA). Uptake was expressed as the amount (micrograms) of nanoparticles associated with unit weight (milligrams) of cellular protein.
Statistical analysis
All tests were conducted in triplicate, and the results were expressed as the mean ± standard deviation. Statistical analysis of the data was performed using one-way analysis of variance (ANOVA) followed by the Bonferroni test for multiple comparison at p < 0.05 using Instat-ANOVA software (CA, USA).
Results and discussion
PLGA nanoparticles of HCPT was prepared using TPGS, Pluronic and chitosan as pharmaceutical excipients to investigate and compare their chemosensitizing effect and to attempt to elucidate the underlying mechanism. PLGA was chosen for (i) stabilization of the therapeutically active lactone form of the anticancer drug due to the acidic microclimate it affords, (ii) passive targeting of cancer tissues by virtue of nanometric size range, (iii) bypassing the efflux transporters responsible for cell resistance of chemotherapeutic drugs by encapsulating HCPT in nanocarriers. The excipients may afford, (iv) additional circumvention of drug efflux (TPGS and Pluronics), (v) minimal burst drug release and (vi) maximal cell membrane interaction (chitosan-coat). To assess the previous hypothesis, different nanoparticles were evaluated in cancer cell lines and compared with free drug solution. Two cell lines were used human lung carcinoma cell line A549 and human colon adenocarcinoma cell line HT29. The latter cell line is a p53-null line representing one of the most common indications for currently FDA-approved camptothecins (Morgan et al., Citation2006). HCPT has proven to be an effective anti-proliferative agent against A549 (Song & Hu, Citation2012) and HT29 (Morgan et al., Citation2006) so the effect of nanoparticles form of the drug remains to be elucidated.
Physicochemical characterization of HCPT nanoparticles
Regarding nanoparticles sizes, charge (zeta potential), encapsulation efficiency and loading capacity, results are shown in .
Table 2. The composition and codes of nanoparticles prepared by nanoprecipitation method with or without chitosan coating.
Table 3. Particle Size, zeta potential, EE and LC of HCPT-PLGA nanoparticles.
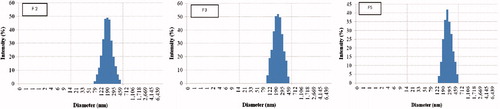
The method and conditions of nanoparticles preparation affected their characteristics; shows that NPs prepared by emulsification-solvent evaporation using PVA as emulsifier had particle size 242 nm but with large size variation as indicated by PDI value 0.39 whereas the encapsulation efficiency was 50.4%. These results resemble those previously reported (Kunii et al., Citation2007, Citation2008). On the other hand, nanoparticles prepared using 0.1% TPGS 1000 as emulsifier (F2) afforded the smallest size and the highest encapsulation efficiency followed by those prepared using Pluronic P85 (F3) indicating the impact of emulsifier type on the properties of nanoparticles. These results are in accordance with those previously reported (Mu & Feng, Citation2003; Xie & Wang, Citation2005; Feng et al., Citation2007). Indeed, TPGS 1000 being a potent emulsifier by virtue of its bulky structure and large surface area, has demonstrated higher emulsification effect, higher drug encapsulation efficiency and better therapeutic effects of anticancer drugs in nanoparticles formulations (Pan & Feng, Citation2008). By comparing the both methods of preparation, it is noted that uncoated nanoparticles prepared by nanoprecipitation method (F4 and F6) exhibited a smaller size and higher encapsulation efficiency than those prepared by emulsification-solvent evaporation method (F1–F3). These results agree with those previously reported (Cohen-Sela et al., Citation2009). On the other hand, data revealed that CS-coated nanoparticles (F5 and F7) displayed larger size but higher encapsulation efficiency than the corresponding uncoated NPs prepared by nanoprecipitation method. This might be attributed to the electrostatic anchorage of the coating onto the PLGA. Similar results were previously reported (Calvo et al., Citation1997; De Campos et al., Citation2003). However, a recent study by Chronopoulou et al. (Chronopoulou et al., Citation2013) reported null effect of chitosan coat on size and drug encapsulation. This discrepancy may be attributed to the different method of fabrication where the authors used an osmosis-based method rather than nanoprecipitation; the formed provided better control on size.
A narrow particle size distribution is a key aspect in passive targeting of nanoparticles and for stability issues (Feng et al., Citation2004). Regarding the size distribution of the developed nanoparticles, this parameter is revealed by the particle size distribution (PDI) data in and . Results show that the size was narrow and mono-modal with PDI values 0.17, 0.21 and 0.24 for formulas F2, F3 and F5, respectively. These are the nanoparticles that possess the smallest particle size together with the highest encapsulation efficiency. Our chief target was to optimize nanoparticles preparative conditions to achieve passive targeting for tumor cells. Regarding the nanoparticles’ surface charge, reveals that PLGA NPs prepared with PVA initially possessed a negative charge as indicated by a negative zeta potential value (ζ range from −17.5 mV). The NPs additionally containing TPGS 1000 (F2) or Pluronic P85 (F3) emulsifiers maintained this negative zeta potential. This might be attributed to the carboxylate end group on the acid terminated PLGA polymer (Feng et al., Citation2004; Zhang & Feng, Citation2006a; Betancourt et al., Citation2007). On the other hand, CS-coated nanoparticles switched ζ from negative to positive (+25.5 and +28.5 mV for F5 and F7, respectively), thus confirming the chitosan coating of these NPs by electrostatic interaction between the negatively charged carboxylate groups on the PLGA polymer and the positively charged amine groups on the chitosan. These results are confirmed by those previously reported (Chung et al., Citation2008; Yang et al., Citation2009b; Parveen & Sahoo, Citation2011; Chronopoulou et al., Citation2013)
The morphology of the prepared nanoparticles was revealed by Transmission Electron Microscopic (TEM) images for formulations F2, F3 and F5 containing TPGS, Pluronic P85 and chitosan-coated ones, respectively (). From the images, it is obvious that nanoparticles were nanometer-sized and spherical, with no obvious drug crystals. The extensive drying before TEM imaging resulted in smaller size particles than DLS data. Images indicate no significant difference in the external morphology of PLGA-TPGS and PLGA-Pluronic P85 nanoparticles (F2 and F3, respectively). On the other hand, the electrostatic interaction between PLGA and chitosan that resulted in coating with a chitosan shell was revealed in the TEM images where a lighter region surrounded by a dark coating of chitosan was revealed (F5). Similar results are reported by Parveen & Sahao (Citation2011) and Chung et al. (Citation2008) and others (Prego et al., Citation2006; Nafee et al., Citation2007; Bilensoy et al., Citation2009).
In vitro release of HCPT from developed nanoparticles
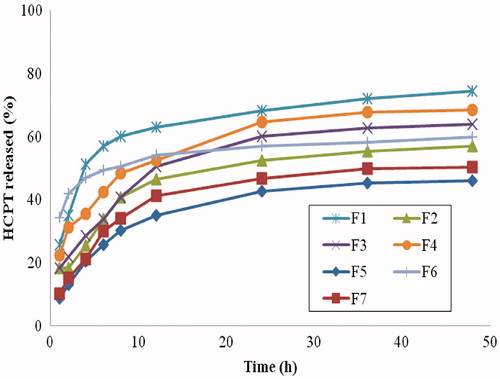
The release rate of drugs from nanoparticles has been proposed to arise from any/all of the following: i) desorption of the surface-bound/adsorbed drug; ii) diffusion through the nanopaticle matrix; iii) nanoparticle matrix erosion; and iv) a combined erosion/diffusion process (Devi Kusum & Bhosale, Citation2009; Devi et al., Citation2012). The release profiles of HCPT from all formulated PLGA nanoparticles (F1–F7) are shown in . It was revealed that all nanoparticles initially released 13–42% of drug was released over a period of 2 h, that is, burst drug release, followed by a more sustained release up to 48 h. This release behavior is characteristic of PLGA nanoparticles and has been extensively reported (Basarkar et al., Citation2007; Pillai et al., Citation2008; Danhier et al., Citation2009; Shu et al., Citation2009) and is considered a limitation of PLGA nanoparticles. One attempt to decrease the burst drug release from PLGA NPs is coating with chitosan. As shown in , the drug release in F5 and F7 exhibited reduced burst effect, these formulas have chitosan coating (as revealed by zeta potential and TEM images) which corroborated previously reported results on the decreased burst drug release as a consequence of chitosan coating (Grabovac & Bernkop-Schnurch, Citation2007; Bilensoy et al., Citation2009; Taetz et al., Citation2009).
Figure 3. In vitro release of HCPT from PLGA nanoparticles (F1–F7) in PBS containing 1% Tween 80 at 37 oC.
Kinetic analysis of complex systems like nanoparticles was studied using Equation (4) where factors like penetration rate of liquid into the system; hydration, swelling, relaxation, erosion and dissolution of polymer (Barzegar-Jalali, Citation2008) can be stated. shows the kinetic analysis of release data. The data revealed that HCPT from the prepared nanoparticles followed Fickian diffusion (Higuchi model) and revealed by the value of n in Eq. 4 (Ritger & Peppas, Citation1987a; Ritger & Peppas, Citation1987b). Similar results were reported on PLGA nanoparticles of acyclovir (Devi Kusum & Bhosale, Citation2009)
Table 4. Release kinetics data of HCPT release from different PLGA nanoparticles.
Cytotoxicity of HCPT nanoparticles to cancer cell lines
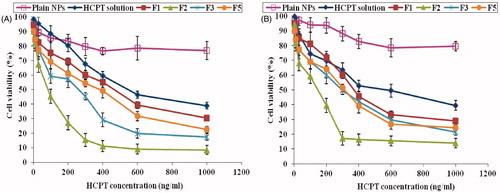
The in vitro cytotoxicity of different HCPT-loaded PLGA nanoparticles was investigated on human lung cancer cells A549 and human colon cancer cells HT-29 using the MTT colorimetric assay for the dehydrogenase activity of viable cell. The results were compared with that of the free drug solution in DMSO at two days. depicts that in A549 and HT29 cell lines, HCPT encapsulated NPs demonstrated a significantly superior cytotoxicity compared to that of the free drug. The cytotoxicity was highest with F2 followed by F3, F5 then F1.
Figure 4. Cytotoxicity of different HCPT NPs formulations as a function of HCPT concentration in A549 (A) and HT29 (B) cells for 48 h at 37 oC.
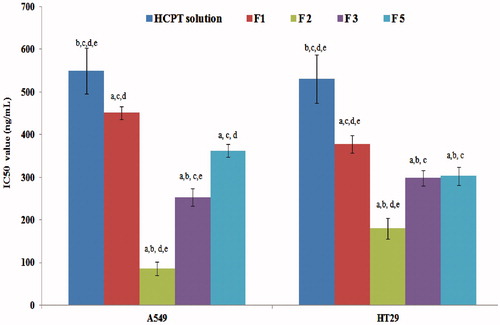
To evaluate and compare the different formulations regarding their cytotoxicity, the IC50 was determined in each case. Results illustrated in reveal that the IC50 values toward A549 cells were 549 ± 53.4 ng/ml for HCPT solution in DMSO whereas the different NPs lowered the IC50 to 451, 362, 253 and 86 ng/ml for HCPT-loaded NPs F1, F5, F3 and F2, respectively. Statistical analysis revealed that these values were significantly different (p < 0.05). Toward HT29 cells, the IC50 values were 542.3 ± 56.1 ng/ml for HCPT solution and 378, 336, 297 and 180 ng/ml for HCPT-loaded NPs F1, F5, F3 and F2, respectively. Statistical analysis revealed that these values were significantly different (p < 0.05) except between F3 and F5 where results were not significantly different. The augmented cytotoxicity of F1 over free HCPT solution might be attributed to the nanoparticles-mediated intracellular delivery of the anticancer drug (Misra et al., Citation2010), bypass of the efflux mechanism experienced by free drug solution (Acharya & Sahoo, Citation2011) and increased stability of the cytotoxic lactone form of HCPT inside the PLGA NPs core due to its acidic microclimate as previously reported (Shenderova et al., Citation1997, Citation1999).
Figure 5. The IC50 values of different HCPT NPs formulations in A549 and HT29 cells. a, b, c, d or e significantly different from HCPT solution, F1, F2, F3 or F5, respectively, at p < 0.05 using one-way ANOVA followed by Bonferroni test for multiple comparison.
The further augmented cytotoxicity in formulations F2, F3 and F5 over drug solution and F1 points at the chemopotentiator effect of the pharmaceutical additives. In this context, the highest cytotoxicity (revealed by the lowest IC50) observed with F2 could be explained by the cytotoxic effect of TPGS 1000 specifically against cancer cells where it acts as a pro-apoptotic agent against cancer cells only (Yu et al., Citation1999, Citation2008; Neuzil et al., Citation2001a,Citationb, Citation2002, Citation2006). In addition, TPGS 1000 has been shown to enhance cellular uptake of NPs and thus increase the cancer cell mortality (Mu & Feng, Citation2003). Camptothecin analogues including HCPT were reported to suffer multidrug resistance by various resistance proteins (Morgan et al., Citation2006; Yamazaki et al., Citation2011). Hence, the co-administration of TPGS 1000 could potentiate the cytotoxicity by inhibition of P-glycoprotein-mediated multidrug resistance, and increase the oral absorption and bioavailability of anticancer drugs (Johnson et al., Citation2002; Feng et al., Citation2004, Citation2009; Zhang & Feng, Citation2006a; Zhang et al., Citation2007b). The influence of the length of the alkyl-chain of various TPGS 1000 derivatives on their efflux pump inhibitory effect was assessed using 10 different TPGS derivatives ranging from 200 to 6000; data revealed that the most potent efflux pump inhibitor was TPGS 1000 (Collnot et al., Citation2006).
On the other hand, F3 (containing Pluronic P85 as emulsifier) displayed significant reduction of IC50, that is, higher cytotoxicity compared to F1 (containing PVA) and free HCPT in solution. These results are in good agreement with those previously reported by Shah et al. who demonstrated significant increase in cytotoxicity of paclitaxel loaded in PLGA-NPs coated with pluronic P85 as compared to uncoated PLGA nanoparticles (Shah et al., Citation2009). The enhanced cytotoxicity of F3 might be attributed to the inhibitory effect of Pluronic P85 on multidrug resistant proteins (MRP) responsible of efflux in cancer cells (Batrakova et al., Citation1999, Citation2001, Citation2004; Miller et al., Citation1999; Johnson et al., Citation2002; Yamagata et al., Citation2007). Mechanistically, cancer cells which attain their drug resistance through energy-dependent transporters are less resistant to chemotherapy by depletion of ATP (Kabanov et al., Citation2002). Batrakova and Kabanov demonstrated that the underlying mechanism of pluronics-inhibited anticancer efflux is through ATPase inhibition and subsequent ATP depletion (Batrakova et al., Citation2004; Batrakova & Kabanov, Citation2008).
The significantly enhanced cytotoxicity of F5 (chitosan-coated) as compared to F1 is corroborated by previous studies on the improved association and interaction of chitosan-coated PLGA nanoparticles with tumor cells (Zhang et al., Citation2006; Yang et al., Citation2009a; Chakravarthi & Robinson, Citation2011). The interactions of nanocarriers with cells and their eventual internalization can be mediated by surface adsorption (Zaki et al., Citation2011). This could be explained by the enhanced cellular uptake of nanoparticles by adsorptive endocytosis due to chitosan coat on nanoparticles (Zhang et al., Citation2006; Kim et al., Citation2008; Nasti et al., Citation2009). Earlier studies have correlated the uptake of chitosan or chitosan-coated nanoparticles to their Zeta potential due to the electrostatic interactions between the positively charged nanoparticles and the negatively charged cell membranes (Huang et al., Citation2004; Harush-Frenkel et al., Citation2008; Nasti et al., Citation2009).
Internalization of nanoparticles into the cells
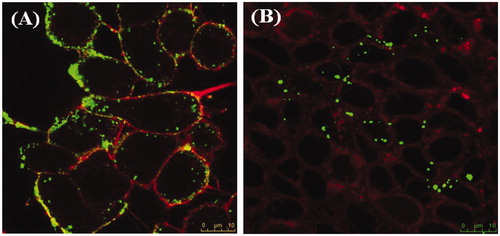
To confirm the uptake of nanoparticles by the cells and their internalization, fluorescent PLGA nanoparticles were incubated with A 549 and HT29 cells. shows the intracellular localization of nanoparticles formulation F2 (green fluorescent spots) inside the cells (stained red for actin filaments existing just below cell membrane). These results suggest that cytotoxic effect is mediated by internalization of nanoparticles and subsequent release of the anticancer drug intracellularly and not the simple diffusion of HCPT through cell membrane. These results are in agreement with those previously reported on camptothecin analogues in different nanocarriers (Arias et al., Citation2009; Zhang et al., Citation2010) and other anticancer drugs (Zhang & Feng, Citation2006b; Dong & Feng, Citation2007; You et al., Citation2007)
Figure 6. Confocal laser scanning microscope images showing internalization and cellular uptake of FA-PLGA nanoparticles formula F2 (green fluorescence) by A549 (A) and HT29 (B) stained with for actin red fluorescence) following incubation of nanoparticles with the cells for 12 h.
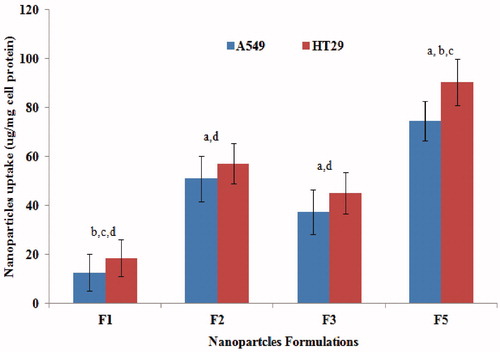
In attempt to evaluate whether the cytotoxic effect correlates with cellular uptake of nanoparticles per se or cellular uptake as a consequence of inhibition of efflux mechanism, the microfluoremetry assay was used. shows that at 1 mg/ml nanoparticles concentration, F5 displayed the highest uptake by A549 and HT29 cells followed by F2, F3 then F1. Except for F5, this ranking correlates with that observed with IC50 values where the greatest reduction was observed with F2 followed by F3, F5 then F1. The discrepancy observed for F5 might be due to overestimation due to bound as well as internalized chitosan-coated NPs (electrostatic interactions between positively charged chitosan and negatively charged cell membrane). The microfluoremetry assay which measures both bound and internalized nanoparticles (Zaki et al., Citation2011; Zaki & Hafez, Citation2012) would thus give an overestimation of the cellular uptake for F5 where the sum of bound and internalized chitosan-coated NPs would then overweigh the actually internalized NPs (F2, F3 and F1). More importantly, the augmented cytotoxic effect of HCPT NPs cannot be attributed solely by increased interaction with the cells but rather by the inhibition of the efflux transporters by TPGS 1000 and Pluronic P85. Hence, a strong correlation might not exist between IC50 reduction and cellular uptake of NPs because the latter is not the only factor contributing to the cytotoxicity enhancement.
Figure 7. Cellular uptake of different NPs formulations 12 h post-incubation with A549 and HT29 cells at nanoparticles dose of 1 mg/ml at 37 °C as measured by the microfluorimetry technique a, b, c or d significantly different from F1, F2, F3 or F5, respectively, in both A549 and HT29 at p < 0.05 using one-way ANOVA followed by Bonferroni test for multiple comparison.
Conclusion
PLGA nanoparticles containing some pharmaceutical excipients were designed for passive targeting and enhancement of cytotoxic effect of HCPT anticancer agent. Factors like particle size, surface charge and surface modification were optimized during nanoparticles preparation. The optimized NPs containing TPGS 1000, pluronic P85 or chitosan-coat proved more cytotoxic to cancer cells than simple HCPT-PLGA nanoparticles or free HCPT solution. The enhanced cytotoxicity could be a result of better internalization of HCPT-containing NPs into cancer cells as a result of inhibition of efflux mechanism in cancer cells by TPGS 1000 or Pluronic P85 or by adsorptive endocytosis due to chitosan coat on nanoparticles. Hence, PLGA nanoparticles with chemosensitizers and/or chitosan coat can be used as a chemotherapeutic delivery system of HCPT to combat lung and colon cancer cells.
Declaration of interest
The author reports no declarations of interest
References
- Acharya S, Dilnawaz F, Sahoo SK. (2009). Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 30:5737–50
- Acharya S, Sahoo SK. (2011). PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Delivery Rev 63:170–83
- Arias JL, Reddy LH, Couvreur P. (2009). Polymeric nanoparticulate system augmented the anticancer therapeutic efficacy of gemcitabine. J Drug Target 17:586–98
- Barzegar-Jalali M. (2008). Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 11:167–77
- Basarkar A, Devineni D, Palaniappan R, Singh J. (2007). Preparation, characterization, cytotoxicity and transfection efficiency of poly(DL-lactide-co-glycolide) and poly(DL-lactic acid) cationic nanoparticles for controlled delivery of plasmid DNA. Int J Pharm 343:247–54
- Batrakova EV, Kabanov AV. (2008). Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release 130:98–106
- Batrakova EV, Li S, Li Y, et al. (2004). Effect of pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res 21:2226–33
- Batrakova EV, Li S, Miller DW, Kabanov AV. (1999). Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res 16:1366–72
- Batrakova EV, Miller DW, Li S, et al. (2001). Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther 296:551–7
- Betancourt T, Brown B, Brannon-Peppas L. (2007). Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation. Nanomed 2:219–32
- Betancourt T, Byrne JD, Sunaryo N, et al. (2009). PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J Biomed Mater Res A 91:263–76
- Bilensoy E, Sarisozen C, Esendagli G, et al. (2009). Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. Int J Pharm 371:170–6
- Calvo P, Remunan C, Vila Jato JL, Alonso MJ. (1997). Development of positively charged colloidal drug carriers: chitosan-coated polyester nanocapsules and submicron emulsions. Colloid Polym Sci 275:46–53
- Chakravarthi SS, Robinson DH. (2011). Enhanced cellular association of paclitaxel delivered in chitosan-PLGA particles. Int J Pharm 409:111–20
- Chronopoulou L, Massimi M, Giardi MF, et al. (2013). Chitosan-coated PLGA nanoparticles: a sustained drug release strategy for cell cultures. Colloids Surf B Biointerfaces 103:310–17
- Chung TW, Wang SS, Tsai WJ. (2008). Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials 29:228–37
- Cohen-Sela E, Teitlboim S, Chorny M, et al. (2009). Single and double emulsion manufacturing techniques of an amphiphilic drug in PLGA nanoparticles: formulations of mithramycin and bioactivity. J Pharm Sci 98:1452–62
- Collnot E-M, Baldes C, Wempe MF, et al. (2006). Influence of vitamin E TPGS poly (ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release 111:35–40
- Costa P, Lobo JM. (2001). Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–33
- Danhier F, Lecouturier N, Vroman B, et al. (2009). Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Control Release 133:11–17
- Davaran S, Rashidi MR, Pourabbas B, et al. (2006). Adriamycin release from poly(lactide-coglycolide)-polyethylene glycol nanoparticles: synthesis, and in vitro characterization. Int J Nanomedicine 1:535–9
- De Campos AM, Sanchez A, Gref R, et al. (2003). The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci 20:73–81
- Derakhshandeh K, Erfan M, Dadashzadeh S. (2007). Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: factorial design, characterization and release kinetics. Eur J Pharm Biopharm 66:34–41
- Devi JS, Bhimba BV, Ratnam K. (2012). In vitro anticancer activity of silver nanoparticles synthesized using the extract of Gelidiella Sp. Int J Pharm Pharm Sci 4:710–15
- Devi Kusum V, Bhosale UV. (2009). Formulation and optimization of polymeric nano drug delivery system of acyclovir using 32 full factorial design. Int J Pharm Tech Res 1:644–53
- Dong Y, Feng SS. (2007). In vitro and in vivo evaluation of methoxy polyethylene glycol-polylactide (MPEG-PLA) nanoparticles for small-molecule drug chemotherapy. Biomaterials 28:4154–60
- Feng SS, Mei L, Anitha P, et al. (2009). Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials 30:3297–306
- Feng SS, Mu L, Win KY, Huang G. (2004). Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem 11:413–24
- Feng SS, Zeng W, Teng Lim Y, et al. (2007). Vitamin E TPGS-emulsified poly(lactic-co-glycolic acid) nanoparticles for cardiovascular restenosis treatment. Nanomed 2:333–44
- Gindy ME, Prud'homme RK. (2009). Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv 6:865–78
- GLOBOCAN. (2008). Estimated cancer incidence, mortality, prevalence and disability-adjusted life years (DALYs) worldwide in 2008. Available from: http://globocan.iarc.fr/factsheet.asp [last accessed 10 March 2013]
- Grabovac V, Bernkop-Schnurch A. (2007). Development and in vitro evaluation of surface modified poly(lactide-co-glycolide) nanoparticles with chitosan-4-thiobutylamidine. Drug Dev Ind Pharm 33:767–74
- Gu F, Langer R, Farokhzad OC. (2009). Formulation/preparation of functionalized nanoparticles for in vivo targeted drug delivery. Methods Mol Biol 544:589–98
- Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. (2008). Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules 9:435–43
- Hatefi A, Amsden B. (2002). Camptothecin delivery methods. Pharm Res 19:1389–99
- Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. (2005). Liposomal encapsulated anti-cancer drugs. Anticancer Drugs 16:691–707
- Hu CM, Zhang L. (2009). Therapeutic nanoparticles to combat cancer drug resistance. Curr Drug Metab 10:836–41
- Huang M, Khor E, Lim LY. (2004). Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res 21:344–53
- Johnson BM, Charman WN, Porter CJ. (2002). An in vitro examination of the impact of polyethylene glycol 400, Pluronic P85, and vitamin E d-alpha-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSci 4:193--205
- Kabanov AV, Batrakova EV, Alakhov VY. (2002). Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release 82:189–212
- Khdair A, Handa H, Mao G, Panyam J. (2009). Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. Eur J Pharm Biopharm 71:214–22
- Kim BS, Kim CS, Lee KM. (2008). The intracellular uptake ability of chitosan-coated Poly (D,L-lactide-co-glycolide) nanoparticles. Arch Pharm Res 31:1050–4
- Korsmeyer RW, Gurny R, Doelker E, et al. (1983). Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
- Kumari A, Yadav SK, Yadav SC. (2009). Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 75:1--18
- Kunii R, Onishi H, Machida Y. (2007). Preparation and antitumor characteristics of PLA/(PEG-PPG-PEG) nanoparticles loaded with camptothecin. Eur J Pharm Biopharm 67:9–17
- Kunii R, Onishi H, Ueki K, et al. (2008). Particle characteristics and biodistribution of camptothecin-loaded PLA/(PEG-PPG-PEG) nanoparticles. Drug Deliv 15:3–10
- Mahor A, Alok S, Gupta Y, Jain SK. (2010). Body distribution and stability studies on mitoxantrone loaded solid lipid nanoparticles conjugated with concanavalin-A. Int J Pharm Pharm Sci 2:39–42
- Miller DW, Batrakova EV, Kabanov AV. (1999). Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res 16:396–401
- Misra R, Acharya S, Sahoo SK. (2010). Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today 15:842–50
- Morgan MT, Nakanishi Y, Kroll DJ, et al. (2006). Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res 66:11913–21
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Mu L, Feng SS. (2003). A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J Control Release 86:33–48
- Nafee N, Taetz S, Schneider M, et al. (2007). Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine 3:173–83
- Nasti A, Zaki NM, de Leonardis P, et al. (2009). Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: systematic optimisation of the preparative process and preliminary biological evaluation. Pharm Res 26:1918–30
- Neuzil J, Dong LF, Wang XF, Zingg JM. (2006). Tocopherol-associated protein-1 accelerates apoptosis induced by alpha-tocopheryl succinate in mesothelioma cells. Biochem Biophys Res Commun 343:1113–17
- Neuzil J, Weber T, Schroder A, et al. (2001a). Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. Faseb J 15:403–15
- Neuzil J, Weber T, Terman A, et al. (2001b). Vitamin E analogues as inducers of apoptosis: implications for their potential antineoplastic role. Redox Rep 6:143–51
- Neuzil J, Zhao M, Ostermann G, et al. (2002). Alpha-tocopheryl succinate, an agent with in vivo anti-tumour activity, induces apoptosis by causing lysosomal instability. Biochem J 362:709–15
- Pan J, Feng SS. (2008). Targeted delivery of paclitaxel using folate-decorated poly(lactide)-vitamin E TPGS nanoparticles. Biomaterials 29:2663–72
- Parveen S, Sahoo SK. (2011). Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol 670:372–83
- Pillai RR, Somayaji SN, Rabinovich M, et al. (2008). Nafcillin-loaded PLGA nanoparticles for treatment of osteomyelitis. Biomed Mater 3:034114, 1--7
- Prego C, Torres D, Fernandez-Megia E, et al. (2006). Chitosan–PEG nanocapsules as new carriers for oral peptide delivery. Effect of chitosan pegylation degree. J Control Release 111:299–308
- Ritger PL, Peppas NA. (1987a). A simple equation for description of solute release. I. Fickian and non-Fickian release from nonswellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 5:23–36
- Ritger PL, Peppas NA. (1987b). A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
- Shah N, Chaudhari K, Dantuluri P, et al. (2009). Paclitaxel-loaded PLGA nanoparticles surface modified with transferrin and Pluronic((R))P85, an in vitro cell line and in vivo biodistribution studies on rat model. J Drug Target 17:533–42
- Shaji J, Jain V. (2010). Solid lipid nanoparticles: a novel carrier for chemtherapy. Int J Pharm Pharm Sci 2:8–17
- Shenderova A, Burke TG, Schwendeman SP. (1997). Stabilization of 10-hydroxycamptothecin in poly(lactide-co-glycolide) microsphere delivery vehicles. Pharm Res 14:1406–14
- Shenderova A, Burke TG, Schwendeman SP. (1999). The acidic microclimate in poly(lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm Res 16:241–8
- Shu S, Zhang X, Teng D, et al. (2009). Polyelectrolyte nanoparticles based on water-soluble chitosan-poly(L-aspartic acid)-polyethylene glycol for controlled protein release. Carbohydr Res 344:1197–204
- Song H, Hu H. (2012). Hydroxycamptothecin inhibits proliferation of human lung carcinoma cell line A549 and down-regulates its Bcl-2 gene expression in vitro. Nan Fang Yi Ke Da Xue Xue Bao 32:1341–5
- Taetz S, Nafee N, Beisner J, et al. (2009). The influence of chitosan content in cationic chitosan/PLGA nanoparticles on the delivery efficiency of antisense 2'-O-methyl-RNA directed against telomerase in lung cancer cells. Eur J Pharm Biopharm 72:358–69
- Wang A, Li S. (2008). Hydroxycamptothecin-loaded nanoparticles enhance target drug delivery and anticancer effect. BMC Biotechnol 8:46
- Weiss B, Schaefer UF, Zapp J, et al. (2006). Nanoparticles made of fluorescence-labelled Poly(L-lactide-co-glycolide): preparation, stability, and biocompatibility. J Nanosci Nanotechnol 6:3048–56
- Xie J, Wang CH. (2005). Self-assembled biodegradable nanoparticles developed by direct dialysis for the delivery of paclitaxel. Pharm Res 22:2079–90
- Xu P, Gullotti E, Tong L, et al. (2009). Intracellular drug delivery by poly(lactic-co-glycolic acid) nanoparticles, revisited. Mol Pharm 6:190–1
- Yamagata T, Kusuhara H, Morishita M, et al. (2007). Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos 35:1142–8
- Yamazaki R, Nishiyama Y, Furuta T, et al. (2011). Novel acrylonitrile derivatives, YHO-13177 and YHO-13351, reverse BCRP/ABCG2-mediated drug resistance in vitro and in vivo. Mol Cancer Ther 10:1252–63
- Yang R, Shim WS, Cui FD, et al. (2009a). Enhanced electrostatic interaction between chitosan-modified PLGA nanoparticle and tumor. Int J Pharm 371:142–7
- Yang R, Yang SG, Shim WS, et al. (2009b). Lung-specific delivery of paclitaxel by chitosan-modified PLGA nanoparticles via transient formation of microaggregates. J Pharm Sci 98:970–84
- You J, Hu FQ, Du YZ, et al. (2007). High cytotoxicity and resistant-cell reversal of novel paclitaxel loaded micelles by enhancing the molecular-target delivery of the drug. Nanotechnology 18:495101, 1--7
- Yu JM, Li YJ, Qiu LY, Jin Y. (2009). Polymeric nanoparticles of cholesterol-modified glycol chitosan for doxorubicin delivery: preparation and in vitro and in vivo characterization. J Pharm Pharmacol 61:713–19
- Yu W, Israel K, Liao QY, et al. (1999). Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res 59:953–61
- Yu W, Jia L, Wang P, et al. (2008). In vitro and in vivo evaluation of anticancer actions of natural and synthetic vitamin E forms. Mol Nutr Food Res 52:447–56
- Zaki NM, Al-Barraq A, Hafez MM. (2013). Development of a pharmaceutically optimized nanometric system of a chemotherapeutic drug. Int J Pharm Pharm Sci Vol 5:161–8
- Zaki NM, Hafez MM. (2012). Enhanced antibacterial effect of ceftriaxone sodium-loaded chitosan nanoparticles against intracellular Salmonella typhimurium. AAPS PharmSciTech 13:411–21
- Zaki NM, Nasti A, Tirelli N. (2011). Nanocarriers for cytoplasmic delivery: cellular uptake and intracellular fate of chitosan and hyaluronic acid-coated chitosan nanoparticles in a phagocytic cell model. Macromol Biosci 11:1747–60
- Zaki NM, Tirelli N. (2010). Gateways for the intracellular access of nanocarriers: a review of receptor-mediated endocytosis mechanisms and of strategies in receptor targeting. Expert Opin Drug Deliv 7:895–913
- Zaki NM, Tirelli N. (2011). Assessment of nanomaterials cytotoxicity and internalization. Methods Mol Biol 695:243–59
- Zhang K, Wang Y, Yu A, et al. (2010). Cholic acid-modified dendritic multimolecular micelles and enhancement of anticancer drug therapeutic efficacy. Bioconjug Chem 21:1596–601
- Zhang L, Hu Y, Jiang X, et al. (2004). Camptothecin derivative-loaded poly(caprolactone-co-lactide)-b-PEG-b-poly(caprolactone-co-lactide) nanoparticles and their biodistribution in mice. J Control Release 96:135–48
- Zhang L, Sun M, Guo R, et al. (2006). Chitosan surface-modified hydroxycamptothecin loaded nanoparticles with enhanced transport across Caco-2 cell monolayer. J Nanosci Nanotechnol 6:2912–20
- Zhang L, Yang M, Wang Q, et al. (2007a). 10-Hydroxycamptothecin loaded nanoparticles: preparation and antitumor activity in mice. J Control Release 119:153–62
- Zhang Z, Feng SS. (2006a). Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials 27:262–70
- Zhang Z, Feng SS. (2006b). Self-assembled nanoparticles of poly(lactide)–Vitamin E TPGS copolymers for oral chemotherapy. Int J Pharm 324:191–8
- Zhang Z, Huey Lee S, Feng SS. (2007b). Folate-decorated poly(lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials 28:1889–99
- Zhou Y, Hopper-Borge E, Shen T, et al. (2009). Cepharanthine is a potent reversal agent for MRP7(ABCC10)-mediated multidrug resistance. Biochem Pharmacol 77:993–1001