Abstract
The main aspire of this study was to develop ocular drug delivery system for dual drug glaucoma therapy by timolol maleate–brimonidine tartrate and endeavor the possibility of biocompatibility studies by in ova studies. Matrix type, both hydrophilic and lipophilic polymers, and reservoir-type ocular inserts of timolol maleate were prepared using hydrophilic polymers like polyvinyl alcohol, hydroxyl propyl methyl cellulose K4M and lipophilic polymers like ethylcellulose and eudragit S100 and were optimized. Based on the optimized formulation, triple-layered ocular inserts (reservoir type) of dual drug were prepared by solvent casting technique with an objective of reducing the frequency of administration, obtaining controlled release and greater therapeutic efficacy, preservative free dosage form for the treatment of glaucoma. FTIR spectral studies revealed no pharmaceutical incompatibility and no drug polymer interactions. Maximum drug release (99.18 ± 1.7) was achieved when PVP and HPMC K4M in 1:1 ratio with PEG 400 (0.3 ml) drug reservoir layer was sandwiched between ethyl cellulose as rate control membrane up to 32 h in a controlled fashion. Drug release was by non-Fickian diffusion mechanism for single drug formulation. But in dual drug insert, timolol maleate best fit into zero order and for brimonidine tartrate to Higuchi model and diffusion of drugs from this by non-Fickian diffusion mechanism. In ovo studies suggested that the optimized formulation was found to be sterile, biocompatible and physicochemically stable and support us to claim that the developed formulation was biocompatible.
Introduction
It is a universal knowledge that ophthalmic drugs could be delivered in a controlled fashion ever since the discovery of contemporary drug delivery platforms like ocular inserts, with respect to the traditional ophthalmic drops. Ophthalmic drug delivery and ocular drug bioavailability are the most challenging task because of the drug elimination from the pre-cornial area, tear production, nonproductive absorption, transient residence time and impermeability of corneal epithelium. The goal of ophthalmic drug delivery systems is to maximize the ocular drug absorption and to minimize the systemic absorption (Kulhari et al., Citation2011).
Glaucoma is a group of conditions initiated by elevated pressure greater than 20 mmHg inside the optic chamber (intraocular pressure) characterized by gradual vision field lose due to death of retinal ganglion cells followed by excavation and atrophy of optic nerve head. This unfortunately leads to progressive neuropathy and irreversible blindness even without variation of IOP. Glaucoma which is the second largest contrition to visual disability is affected by 70 to 90 million people worldwide (Munier et al., Citation1998). Most of the conventional dosage forms are ineffective in sustaining the drug release and additionally effective preservatives like benzalkonium chloride (BAC) is toxic to ocular tissue, having the potential to cause adverse effects. Glaucoma being a chronic condition, the medication is preferable to have preservation free and prolong the drug release. Several studies have suggested that switching from a preserved to a preservative-free medication can benefit the tear film, conjunctiva, cornea and eyelids (Fechtner et al., Citation2011). Ophthalmic inserts offer many advantages over conventional dosage forms include accurate dosing, possibility of releasing drug at a slow and constant rate, increase the ocular residence time, exclusion of preservatives and increase the shelf life. We have earlier reported the possible scope of inserts in the treatment of glaucoma (Vinod et al., Citation2011). History of ocular inserts goes back to 19th century when dry square filter paper previously soaked in drug solution (atropine sulfate/pilocarpine hydrochloride) was tried and insert is a trade name filled by ALZA CORPORATION in 1979 (www.trademarkia.com).
Although it is logic to commence glaucoma treatment with a single drug, many patients fail to respond and require combination of drugs. Timolol maleate (C13H24N4O3S.C4H4O4) and brimonidine tartrate (C11H10BrN5.C4H606) are nonspecific β receptor antagonist and highly selective α2-adrenoceptor agonist, respectively, provide a perfect dual drug combination to reduce IOP. Biological half-life of the former and later drug is 2.5–5 h and 2 h, respectively. Although this combination is available in market in ophthalmic solution (COMBIGAN®) (www.rxdrug.com), the need for frequent administration and drug drainage triggered us to report our findings in the development of three designs of ocular inserts, lipophilic and hydrophilic matrix types and triple-layered reservoir type sandwiched between a rate-controlled membrane (Gupta et al., Citation2011).
Rabbit is the common animal model of choice for ophthalmic researchers who have been involved in the biocompatible studies and pharmacokinetic studies of ophthalmic products. However, it would be rather ethical and rational to avoid pharmacological studies as far as possible. We thus examined the possibilities of in ova modeling in the biocompatibility study of a trilayer ocular insert having dual payload drugs.
Materials and methods
Timolol maleate was obtained as a generous gift from Aurobindo Pharma Ltd., Hyderabad, while brimonidine tartrate from Strides Arco PVT Ltd. (Bangalore, India). Excipients, PVA was obtained from Accord Labs (Secunderabad, India). Other adjuants like HPMC K4M, ethyl cellulose and eudragit S100 were obtained from Yarrow Chem Products (Mumbai, India). Statistical significance of all the data generated was tested by analysis of variance (ANOVA).
Methodology
Initially, single drug formulation of lipophilic and hydrophilic matrix type and triple-layered reservoir-type ocular inserts were prepared. Subsequently, based on the release profile later design was selected for dual drug formulation.
Fabrication of lipophilic and hydrophilic matrix-type ocular inserts
Ocular inserts of timolol maleate (TM) were prepared by solvent casting method by lipophilic and hydrophilic polymers separately on a smooth glass plate as a substrate. Polymers, HPMC K4M and PVA were used to prepare hydrophilic ocular inserts while ethyl cellulose (EC) and eudragit S100 (ES) were used for lipophilic ocular inserts. The required quantity of the polymer was weighed and dissolved in a suitable solvent by gentle stirring. To the above solution, PEG 400 as plasticizer was added and TM was supplemented and stirring was continued for 2 h by a magnetic stirrer at low rpm to acquire a homogenous dispersion. After complete mixing, the casting solution was sonicated (bath sonicator) for 10 min to obtain bubble-free solution. This solution was poured in clean glass mould (area = 25 cm2) and dried at room temperature for 24 h. During drying, glass mould was covered with an inverted funnel and the dried film thus obtained was incised to inserts of 8 mm diameter. All these steps were done strictly under aseptic conditions (Gupta et al., Citation2011) and were UV sterilized and stored (composition available in Table 1).
Preparation of reservoir type of ocular inserts and lamination
Hydrophilic polymer films were used as drug reservoir and lipophilic drug-free polymer films as rate-controlling membranes. The drug reservoir (d = 8 mm) was sandwiched between the two rate-controlling membranes (d = 10 mm) and it was kept on a gauge over beaker which was saturated with ethanol and acetone of 60:40 for 1–2 min. Both sides of this sandwich were exposed (3–4 cycles) to the vapor mixture below until chemically laminated and stored as mentioned earlier (composition available in Table 2).
Preparation of timolol maleate–brimonidine tartrate insert
The optimized formulation variable was selected for dual drug reservoir, TM-BT ocular inserts preparation (F8) by using similar method. Based on the drug concentration of marketed ophthalmic solution, the drug concentrations for TM and BT were fixed at 1.25 mg and 0.48 mg per insert, respectively. Formulation was based on the technique employed for reservoir type as mentioned earlier. The procedure was done under aseptic conditions and the prepared inserts were sterilized and stored.
Calculations
Calculation of drug dose for one mould (area = 25 cm2)
As the diameter of an insert is 0.8 cm, radius will be 0.4 cm and thus the area could be calculated as πr2 (3.14 × 0.4 × 0.4), that is 0.5024 cm2. Because the amount of TM in one insert should be 1.25 mg, total amount of drug to be added in one mould will be 25 × 1.25/0.5024, that is 62.201 mg. Similarly, total amount of BM for one mould was calculated (25 × 0.48/0.5024) as 23.885 mg so as to obtain 0.48 mg of drug per insert.
Simultaneous estimation of timolol maleate, brimonidine tartrate in STF pH 7.4
The drugs were estimated by simultaneous equation method. The concentration of 20 µg/ml of both the drugs were prepared in simulated tear fluid and scanned between the range of 200 and 400 nm and overlain spectra was recorded. The absorptive coefficients were determined for both the drugs at the selected wavelengths by substituting the absorbance value in Equations (1) and (2) (Rathore et al., Citation2010; Sivasubramanian et al., Citation2010; Wagh et al., Citation2011).
where the absorptivities of X at λ1 and λ2, ax1 and ax2, respectively; the absorptivities of Y at λ1 and λ2, ay1 and ay2, respectively. The absorbance of the diluted sample at λ1 and λ2 were A1 and A2, respectively. Cx and Cy are concentrations of X and Y, respectively, in the diluted sample.
Characterization and evaluation of ocular inserts
General appearance of all the three type of ocular inserts was observed. Thickness was measured by using screw gauge at three different points of the insert and the mean value and standard deviation were calculated.
Drug content uniformity
To determine the uniformity of the drug, inserts were cut into small pieces at different points and each insert was placed in a vial containing simulated tear fluid (STF) pH 7.4 and dissolved by triturating with the help of a mortar and pestle. The samples were filtered and diluted suitably and analyzed the drug content by UV spectrophotometer at 294 nm and 247 nm for TM and BM, respectively, with STF serving as blank. The same procedure was adopted for all batches of ocular inserts in triplicate and mean drug content and standard deviation of variance were calculated (Jeganath et al., Citation2011; Upadhyaya et al., Citation2011).
Folding endurance
It is important to check the ability of the sample to withstand folding threshold strain and also gives indication of brittleness. It is performed to evaluate elasticity and plasticity of the ocular inserts and is expressed as the number of folds (number of times) the insert is folded at the same place, either to break the specimen or to develop visible cracks. The specimen was folded in the centre, between the fingers and the thumb and then opened. This was termed as “one folding”. The process was repeated till the insert showed breakage or cracks in centre of insert. The total folding operations were named as folding endurance value.
Surface pH
To keep the ocular inserts surface pH as close to tear fluid, it is mandatory to check the pH. The inserts were allowed to swell in a closed petri plate at room temperature (25 °C) for 30 min in 10 ml of bi-distilled water, slowly shaken and pH was checked with a digital pH meter.
Moisture loss and moisture absorption
It is used to check out the integrity of the formulation in dry conditions. The ocular inserts were weighed accurately and placed in a dessicator containing anhydrous calcium chloride. After three days, the ocular inserts were taken out and reweighed and percentage moisture loss was calculated by the below stated formula:
It is used to check out the physical stability or integrity of the ocular inserts. The ocular inserts were weighed accurately and kept in a dessicator containing 100 ml of saturated solution of aluminum chloride. After three days, the inserts were taken out and reweighed.
The percentage absorption was calculated from the following formula:
Swelling studies
The swelling of the polymer depend upon the concentration of the polymer, ionic strength and presence of water. To determine the swelling index, the initial weight of the insert was weighed and it was placed in beakers containing 4 ml of distilled water. At regular intervals of time (every 10 min), the ocular inserts were taken out and the surface water present on the insert was removed with the help of filter paper, and the insert was reweighed. The procedure was continued till there was no increase in the weight (Jeganath et al., Citation2011). Swelling index (Sw), the equilibrium percent swelling was calculated by:
Wt = Weight of the swollen insert after time “t”
Wo = Original weight of insert at zero time.
In vitro diffusion studies
The in vitro diffusion of drug from the different ophthalmic inserts was studied by slightly modifying the earlier reported students diffusion cell (SDC-1T09) which was indigenously fabricated and validated (Vinod et al., Citation2012) and is an analogy of the conventional Franz diffusion cell. Briefly, this consists of a donor unit I, a receiving unit II and an outer unit III for maintaining the temperature. The unit I (donor chamber) connects unit II (receiving chamber, 60 ml) by a circular platform having a sampling port and another for thermometer. Donor chamber consist of 60 ml of simulated tear fluid (STF) composing of 0.67 g sodium chloride, 0.2 g sodium bicarbonate, 8 mg calcium chloride dehydrate in 1000 ml double-distilled pyrogen-free water (Vodithala et al., Citation2010). One side of the donor chamber was fixed with an egg membrane on which the insert was attached. Egg membrane is commonly used as a semipermeable membrane which is prepared by dissolving the egg shell in concentrated HCl and thus obtaining the inner shell membrane (ISM) (Vinod et al., Citation2010). Unit I was placed in such a way that the lower surface of the insert just breaches the diffusion medium (ph 7.4 STF at 37 ± 0.5 °C) of unit II which was stirred at 20 rpm. At specified time intervals, 4 ml of aliquot sample solution was withdrawn and transferred to amber-colored ampoules for drug quantitative analysis using UV spectrophotometer at the respective λmax. Fresh STF solution was immediately replaced to maintain sink conditions. The experiments were continued for 24 h.
Drug release kinetics
To analyze the mechanism of the drug release rate kinetics of the insert, the obtained in vitro diffusion data of all the formulations were subjected to goodness to fit test by linear regression analysis according to zero-order, first-order, Higuchi and Korsmeyer–Peppas release model. The predominant mechanism of drug release from the formulations was determined by comparing their respective regression coefficients. Release exponent “n” was determined to know the drug transport mechanism (Ritger et al., Citation1987; Rajasekaran et al., Citation2010; Shivhare et al., Citation2012).
Sterility test
Soya bean casein digestive culture media was used to confirm the sterilized ophthalmic device was free from microbes. For this Bacillus subtilis was inoculated by streak method and referred as positive control while negative control contains the media excluding the inserts. Test petri dish contained the formulation without inoculation. All the three petri dishes were incubated under aseptic conditions at 35 ± 0.5 °C as the test and growth of the organisms in the negative, positive control and in the test after 48 h was observed.
In ovo studies
Ocular irritation and biocompatibility test of the optimized formulation was performed by chick embryo’s chorio allentonic membrane (CAM) test. The eggs (viable) for the experiment were incubated at 37 °C and on 12th day of incubation 1 cm2 of egg shell was carefully line cracked and peeled away from the polar region. Once the opaque dual shell layer “chorioallontoic membrane-inner shell membrane” (CAM-ISM) was exposed, irrigate with 0.9% sodium chloride solution and carefully peel away the ISM so the CAM was exposed. Slight bleeding due to breakdown of capillaries was observed which was rinsed away with fresh 7.4 pH solution. Samples were placed on the CAM and observed for any erythrema, capillary bleeding, angiogenesis, abnormal growth and color change after 48 h as biocompatible study (Leng et al., Citation2004).
Stability studies
The ocular inserts were stored in amber-colored glass bottles at three different temperatures at 4 °C, controlled room temperature (25 °C) and 50 °C for a period of 1 month. The samples were withdrawn after 7, 14, 21 and 30 days and assessed for physical and chemical stability of the ocular inserts (Krosmeyer et al., Citation1983).
Results
Digital photographs of matrix type lipophilic, hydrophilic (F1–F5) and reservoir (F6–F12) ocular inserts prepared by solvent casting method are displayed in and . Hydrophilic matrix-type ocular inserts were clear and transparent while lipophilic matrix ocular inserts were slightly translucent in nature and reservoir type insert was more translucent with a yellowish tint.
Figure 2. Optimized triple-layered formulations having TM-BT insert (a) and laminated preparation held by a forceps exposing the layers (b).
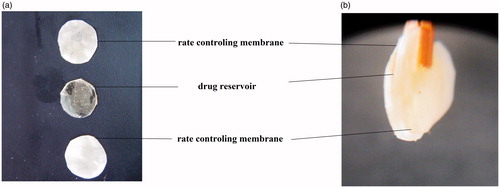
Laminated reservoir ocular inserts were thicker than matrix type. From the overlain spectrum of BT-TM in STF pH 7.4, it was observed that BT showed an absorbance peak at 247 nm, whereas TM at 294 nm. The surface pH of the all prepared insert varied between 6.5 and 7.5. Other parameters are provided in . It was observed that the average weight of nonlaminated ocular inserts were ranging from 4.830 mg (F1) to 5.030 mg (F4) and for laminated (reservoir) was 14.066 mg (F9) to 14.633 mg (F7) while the thickness was ranging from 0.096 mm (F1) to 0.120 mm (F4) and 0.326 mm (F9) to 0.350 mm (F7). With the exception of F1, F6 and F9, all other formulations were found to have above 90% drug content. Folding endurance of laminated ocular inserts (maximum 242, F3) was more than the nonlaminated type (maximum value 254, F9). Moisture loss for nonlaminated and laminated ocular inserts ranged from 2.400 to 9.366 and 6.700 to 9.000 respectively. It was observed that swelling index was more in nonlaminated (49.33, F1) than laminated reservoir type (39.53, F9). Moisture absorption was more for nonlaminated (7.830%, F2) than laminated (3.700%, F10). Insert having PVA was having more swelling index than HPMC K4M. It was interesting to note that both moisture absorption and lose were swelling index depended.
Table 1. Composition of matrix type of ocuserts.
Table 2. Composition of reservoir type of ocuserts.
Table 3. Physicochemical properties of timolol maleate ocuserts.
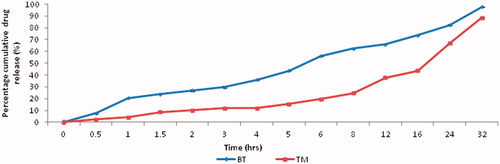
From the results of in vitro diffusion studies of nonlaminated ocular inserts, F8 gave maximum drug release (99.18% ± 1.7) in 32 h. F1 and F2 studies showed maximum release in 5th hour (98.03% ± 2.4) and 8th hour (81.2% ± 1.4), respectively, while F3 extended the release up to 95.56% ± 0.9. In the reservoir type, that is, laminated ocular inserts maximum release was found to be for 99.18% ± 1.7 (F8). Among laminated reservoir type, F6, F10 and F11 formulations showed 87.97%, 85.34% and 96.37% at the end of 24 h, respectively. In dual combination F12, as it is shown in , BT had more release (97.86% ± 0.5) than TM (88.63% ± 0.3) (). In the sterility test, the growth of the microorganisms (B. subtilis) was observed in the positive control and no growth in the negative control and in the test. During the in ovo studies, neither occluded seepage capillaries (A capillary zone accompanied by leakage) nor circulating micro thrombi, erythrema and thrombosis of arteries were observed after 48 h. Different development stages of CAM studies are shown in . Stability assessment of ocular inserts (F12) conducted at 4 °C, 37 °C and 50 °C showed that at the end of the 4th week the intact TM/BT concentrations (%) were 92.01 ± 0.135/93.91 ± 1.109, 92.91 ± 1.016/92.89 ± 1.573 and 85.16 ± 0.634/86.23 ± 0.574, respectively. Physical examination of ocular inserts stored at 4 °C were found to be slightly brittle in nature, 37 °C was unchanged and 50 °C was soft and distorted.
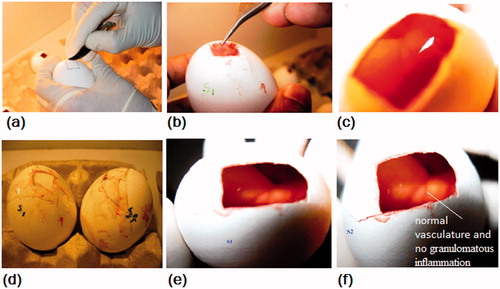
Figure 4. Different stages of in ovo studies (a) incising the shell on the marked line to create a window, (b) removal of ISM, (c) insert placed on the exposed CAM, (d) image showing incubation of sealed eggs for two days, (e) and (f) images showing the test and reference embryo. It may be noticed that there was absence of erythrema and color change after 48 h in the test.
Discussion
The core issue of this work was aimed to optimize a suitable fabrication for an ocular insert in pursuit of glaucoma therapy, and to explore the possibility of a dual drug regiment in a single controlled release insert. Ocular inserts of timolol maleate were prepared and the polymers were chosen according to biocompatibility, film forming properties and retardant to biodegradability to provide controlled release pattern, and PEG-400 as a plasticizer was used as it is extremely miscible with water and acetone solutions. The addition of plasticizer leads to a decrease in intermolecular forces along the polymer chains and will produce the improvement in flexibility which improves ocular tolerance especially when the eyeball is non static. The mechanism of formation of films occurs in three stages: (a) Evaporation of the casting solvent and subsequent concentration of polymer particles. (b) Deformation and coalescence of polymer particles and (c) further fusion by inter diffusion of polymeric molecules of adjacent polymer particles (Krosmeyer et al., Citation1983).
All the formulations showed good film forming property, thin, smooth and uniform in appearance. Slow and uniform evaporation of solvent was obtained by inverted funnel technique. The F1, F2 and F3 formulations were transparent and all other formulations were translucent. This indicates the light transmittance of PVP and HPMCK4M polymers whether alone or in combination. Transparency need not be a deciding factor for selection because the site of application is not at the pupil but it is at the cornea or cul-de-sac and thus will not perturb the vision.
There were no marked variations in the thickness of three-layered ocular inserts within each formulation indicating uniform behavior of film throughout the sealing process. The dimensions of the first marketed commercially available ocular insert, “insert” by ALZA Corporation, Palo Alto, CA: the PILO-40 system is 5.5 × 13 mm on its axes and 0.5 mm thick (Gilhotra et al., Citation2011). Thickness of the formulations was comparable to commercially available ocular inserts, indicating their physiologic suitability.
Weight variation and drug content uniformity indicates uniform distribution of drug in the polymer and plasticizer, which in turn confirms the suitability of the process and gave reproducible results and thus occupying less space in cul-de-sac.
Surface pH of films is important because the films are to be placed in the sensitive region of the eye. The formulations had pH near to the neutral pH and hence they would not alter the pH of the tear fluid in the eye and the lachrymal fluid pH is 7.4 and will not create any difficulty or irritation while placing in the cul-de-sac of the eye.
The folding endurance test reflects the flexibility of the films which ensure ocular inserts can be detached from the substrate as well as apply at the site of administration (eye) without breaking or tearing and have the good film forming property for all the polymers. PVA film showed good folding endurance than other polymers, because the polymer forms hydrogen bond with the plasticizer molecule there by imparting flexibility to the film. This test is suitable for large-scale manufacture to produce long continuous film without breaking.
Ocular inserts prepared from hydrophilic polymers (F1, F2, F3) showed less moisture loss compared to that prepared by lipophilic polymers (F4, F5). Hydrophilic polymers have high moisture absorption than lipophilic polymers. Among PVA and HPMC K4M, former has the moisture barrier property and is hygroscopic, the concerned formulations exhibited less moisture loss and absorption. In case of lipophilic polymers, the ES100 have permeable nature so its moisture loss and moisture absorption is higher than EC formulations. From this, it was also observed that there was no change in physical appearance and integrity of the formulation.
The swelling increases as the time passes because the polymers gradually imbibe water. Swelling index is important because of its influence in mucoadhesion which is obligatory for ocular inserts. Inserts containing hydrophilic polymers had higher swelling index compared to hydrophobic inserts, because the hydrophilic inserts decreases contact angle which is invariably inversely proportional to surface wettability and, consequently, water penetrates within the matrix. In the hydrophilic polymers, the PVA inserts having more swelling tendency compared to the HPMC films. These differences in swelling were may be due to the difference in resistance of matrix network structure (hydrogen bond) toward the movement of water molecule. The ocular inserts intended for local therapy, the contact area should be as large as possible; a requirement that must be balanced with patient compliance, excessive swelling index might cause discomfort and dislodgement of the swollen films.
The in vitro diffusion studies of TM ocular inserts were carried out using semi permeable membrane (egg membrane) in STF pH 7.4 solutions. The diffusion membrane acts as a corneal epithelium and 20 rpm simulates the normal human blinking rate. Formulation F1 showed 98.03% at the end of 5 h, whereas formulation F2 and F3 showed 81.2% and 95.56% at the end of 8 h, respectively. Formulations F4 and F5 showed 87.35% and 97.45% at the end of 12 h. The results suggest that, hydrophilic polymers release the drug faster than lipophilic polymers.
PVA is more soluble than HPMC K4M, reasoning for faster drug release. In case of EC (F4) and EC: ES100 (F5), F5 showed high permeability nature and less lipophilic. F7 and F8 showed 95.68% and 99.18% at the end of 32 h, respectively, suggesting that release from F7 could be further extended were as F8 already reached its maximum release (99.18%) in 32 h. Triple-layered laminated ocular inserts showed sustained and prolonged release of drug than single-layered ocular inserts. From this, the F7 was selected as optimized formulation because of sustained drug releasing capacity. From the dual drug, TM-BT insert drug release of BT (97.86%) was more than TM (88.63%) at the end of the 32 h. Ocular insert of combined drugs, the TM best fit to zero order and for BT to Higuchi model, and diffusion of drugs from this by non-Fickian diffusion mechanism.
There was no growth of the microorganisms in the test which confirms the sterility of the insert and its suitability for use (Sivasubramanian et al., Citation2010). It is well known that TM-BT fixed combination may induce allergy and granulomatous inflammation of the eye at higher concentrations. Thus, it is prudent to consider ocular irritation of the developed formulation was checked by hen’s egg CAM test as a preliminary test which is rapid, sensitive and inexpensive. Testing with incubated egg is a border line case between ethical and legal obligations. The CAM of the chick embryo is a complete tissue, including veins, arteries and capillaries and is technically ever easy to study. It is a suitable alternative to animal testing and it is based on the direct application onto the CAM of the sample and reactions, such as hemorrhage, intravascular coagulation or lysis of blood vessels which can be assessed on a time course basis. It responds to injury with an inflammatory process similar to what one would observe in the conjunctival tissue of a rabbit eye and chick embryo moments analog the nonstatic cornea. Normal CAM physiology and anatomy, in physical contact with the ocular insert for 48 h, supports us to claim that the developed formulation was biocompatible (Colombo et al., Citation1996; Rajasekaran et al., Citation2010). This suggests that the release of the drug from the device occurred in a controlled level without “sudden leakage phenomenon” and at a concentration below which there could be no inflammation and perhaps the folding endurance data supports up to certain extend that the ocular insert does not cause any physical friction to the nonstatic biological substrate.
Stability assessment showed the formulation was stable physically and chemically at 37 °C. Rigidization at 4 °C could be due to polymer crystallization and softy distorted nature of ocular inserts at 50 °C could be of glassy transition of the polymers. Thus, we recommend that the storage conditions must be at cool dry conditions. It is desirable to keep in dark place to avoid the effect of external UV radiations.
Conclusions
Hydrophilic and lipophilic ocular inserts were prepared alone and in combination and reservoir-type laminated ocular inserts were also prepared by timolol maleate. Triple-layered laminated ocular inserts showed sustained and prolonged release of drug than single-layered ocular inserts. All the formulations showed good film forming property, thin, smooth and uniform in appearance. PVA film showed good folding endurance and more swelling tendency than HPMC K4M. In vitro drug release from HPMC K4M suggested that release could be further extended from 95.68% after 32 h. From the dual drug ocular insert, drug release of brimonidine tartrate was more than timolol maleate. The inserts were found to be sterile, stable and biocompatible complying to lachrymal fluid, thus physiological suitable in the cul-de-sac of the eye. The preservative-free inserts were shown to have prolonged drug release, making it potential to be used in the treatment of glaucoma. Normal CAM physiology and anatomy and immune irresponsiveness, in physical contact with the insert for 48 h, suggested this procedure can be considered as an alternative to biocompatibility test on rabbit eyes.
Declaration of interest
All authors report no conflict of interest and are responsible for the content and writing of the manuscript.
Acknowledgements
Authors express their heartfelt gratitude to Nalanda College of Pharmacy, Dept. of Pharmaceutics and Novel Drug Delivery where this project work was carried out. Timolol maleate and brimonidine tartarate were generously gifted from Aurobindo Pharma Ltd., Hyderabad, and Strides Arco PVT Ltd., Bangalore, respectively, and authors eloquent a heartening acknowledgement.
References
- Colombo P, Bettini R, Santi P, et al. (1996). Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J Cont Rel 39:231–7
- Kulhari H, Pooja D, Narayan H, et al. (2011). Design and evaluation of insert for controlled delivery of flurbiprofen sodium. Curr Eye Res 36:436–41
- Jeganath S, Ashlin Viji A, Sheeja Devi K, et al. (2011). Design and evaluation of controlled release ocuserts of indomethacin. Int J Pharm Sci Res 2:80–6
- Rathore KS, Nema RK, Sisodia SS. (2010). Timolol maleate a gold standard drug in glaucoma used as ocular films and inserts: an overview. Int J Pharm Sci Revi Res 3:1512–22
- Krosmeyer RW, Deolkar DEP, Peppas NA. (1983). Mechanisms of release potassium chloride from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J Pharm Sci 72:1189–91
- Sivasubramanian L, Lakshmi KS, Tintu T. (2010). Simultaneous spectrophotometric estimation of paracetamol and lornoxicam in tablet dosage form. Int J Pharm Pharm Sci 4:166–8
- Munier A, Gunning T, Kenny D, O’Keefe M. (1998). Causes of blindness in the adult population of the Republic of Ireland. Br J Ophthalmol 82:603–33
- Rajasekaran A, Sivakumar V, Karthika K, et al. (2010). Design and evaluation of polymeric controlled release Natamycin ocular inserts. Kathmandu Uty J Science Eng Tech 6:108–15
- Ritger PL, Peppas NA. (1987). A simple equation for description of solute release: fickian and non fickian release from non-swellable devices in the form of slabs, spheres, cylinder or discs. J Cont Rel 5:23–6
- Gilhotra RM, Nagpal K, Mishra DN. (2011). Azithromycin novel drug delivery system for ocular application. Int J Pharm Inv 1:22–8
- Fechtner RD. (2011). Reducing the preservative load in glaucoma therapy, therapeutic update. Glaucoma Today:64–5
- Wagh RS, Hajare RA, Tated A, Anil Chandewar V. (2011). Absorption correction method and simultaneous equation method for the simultaneous estimation of ebastine and phenylephrine hydrochloride in bulk and in combined tablet dosage form. Int J Res Pharm Che 4:813–19
- Shivhare UD, Chavan MA, Bhusari KP, et al. (2012). Formulation development and evaluation of controlled release ocular insert. Int J Biol Pharm Res 3:66–74
- Vodithala S, Khatry S, Shastri N, Sadanandam M. (2010). Formulation and evaluation of ion activated ocular gels of ketorolac tromethamine. Int J Current Pharm Res 2:33–8
- Gupta S, Rajendra Songara KR, Lokwani P. (2011). Ocular inserts: an overview. Int J Pharm Res Dev 6:141–8
- Leng T, Miller JM, Kalayaan BS, Palanker V. (2004). The chick chorioallantoic membrane as a model tissue for surgical retinal research and simulation. The J Retinal Vitreous Dis 24:427–34
- Vinod KR, Anbazhagan S, Suneel Kumar M, et al. (2012). Developing ultra deformable vesicular transportation of a bioactive alkaloid in pursuit of vitiligo therapy. Asian Pacific J Trop Biomed:301–6
- Vinod KR, Sandhya S, Banji D, Santhosha D. (2010). Development of a herbal topical formulation for vitiligo. Indian drugs 47:17–21
- Vinod KR, Swathi R, Sandhya S, Banji D. (2011). Scope of ocuserts in glaucoma therapy. Inventi Rapid: Novel Drug Del Sys 2:20–4
- Upadhyaya N, Patidar A, Agrawal S, Gupta D. (2011). Development and evaluation of polymeric sustained release levofloxacin ocuserts. Res J Pharm Biol Chem Sci 2:411–20
- Available at http://www.trademarkia.com/insert-73206260.html [last accessed 03 Oct 2013]
- Available at http://www.rxlist.com/combigan-drug.htm [last accessed 03 Oct 2013]