Abstract
Context: Propolis has traditionally been used in curing infections and healing wounds and burns.
Objective: The aim of this study is to formulate pluronic lecithin organogel of propolis to improve its availability and antimicrobial activity.
Materials and methods: Different organogels were prepared by using soybean lecithin, isopropyl palmitate, pluronic F127 and water. The effect of quantity of lecithin and pluronic F127 and percentage of oil phase was investigated. The organogels were evaluated for appearance, texture, pH, drug content and viscosity. In vitro release studies were carried out using cellophane membrane. Drug permeation through abdominal rat skin from organogels that showed high % drug release was compared to that from propolis suspension in distilled water. Finally, the antimicrobial activity of the selected propolis formulation against different bacterial isolates was compared with that of propolis suspension in water.
Results and discussion: Results showed that all organogel formulations except the formula containing 10% pluronic F127, showed acceptable physical properties. Drug content of organogel formulations was in the range of 97.5–100.2%. The pH of the formulations was in the range of 5.5–6.3 that suits the skin pH, indicating skin compatibility. The viscosity was in the range of 5366–8984 cp. A significant decrease in drug release from formulations was observed with increase in concentration of lecithin and pluronic F127. Decreasing oil phase percentage to 20% w/w led to a decrease in drug release from the formulation.
Conclusion: The formula containing 3% lecithin and 20% pluronic F127 exhibited superior skin permeation and antimicrobial activity over propolis suspension in water.
Introduction
Thermal injury is a major cause of morbidity and impaired quality of life in many areas of the world. It has been estimated that 75% of deaths following burn injuries are related to infection (Nasser et al., Citation2003). The ideal dressing material should be of low cost, safe and relatively painless, it should also discourage infection and promote fast wound healing to minimize morbidity. Furthermore, to increase outpatient compliance, the number of dressing changes should be minimized and be easily performed at home (Berretta et al., Citation2012).
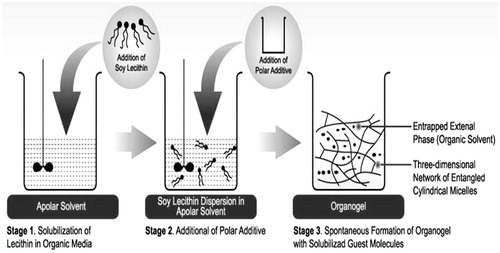
Lecithin organogel, the jelly-like phase, has been shown to provide a promising topical drug delivery system. It consists of a 3-dimensional network of entangled reverse cylindrical micelles, which immobilizes the external organic phase thus, turning a liquid into a gel (Agrawal et al., Citation2010). Lecithin, when being dissolved in non polar media alone, self-assembles into reverse spherical micelles at a concentration of ∼0.01 mM which turns to form elongated tubular micelles with the addition of water (Shchipunov et al., Citation1990; Shchipunov, Citation2001). Transition to polymer-like micelles is accompanied with the formation of hydrogen bonds between the phosphate group of a lecithin molecule and water (Shchipunov & Shumilina, Citation1995, Citation1997). Due to its micellar structure, organogel can contain both water and oil soluble ingredients and shows excellent drug permeability by diffusion through the lipid intracellular matrix and by slight disorganization of skin (Pandey et al., Citation2009). Ease of preparation, thermodynamic stability and enhanced topical performance along with biocompatibility make the organogel a vehicle of choice for topical drug delivery.
Pluronic lecithin organogels were developed in the early 1990s by Marty Jones (Murdan, Citation2005). These gels consist of isopropyl palmitate, soy lecithin, water and pluronic F127. Isopropyl palmitate is a non oleaginous emollient with a high capacity for spreading. Lecithin is a naturally occurring mixture of diglycerides of fatty acids linked to the choline ester of phosphoric acid. It is used as a penetration enhancer in compounding the pluronic lecithin organogel. Water acts as a stabilizing and structure-forming agent in the process of organogel formation. It is also used for solubilizing the pluronic F127 and polar drugs. Pluronic F127 is a thermo reversible polymer and is capable of making the bonds responsible for holding the gel network and act as a stabilizer due to gelling property at room temperature (Escobar-Chávez et al., Citation2006) ().
Figure 1. Schematic diagram representing the mechanism of formation of pluronic lecithin organogels (Ophori & Wemabu, Citation2010).
Propolis is a hard, resinous material derived by bees from plant juices and used to seal openings in the hives. The term propolis originates from the Greek words pro – meaning before/in front and polis – meaning town and denotes the fact that bees use propolis to construct the entrance to the beehive. It is also known as “bee glue” (Ivančajić et al., Citation2010). Propolis contains pollen, resins, waxes and large amounts of flavonoids (Avanço & Bruschi, Citation2008). Despite the great differences in the chemical composition of propolis from different geographic locations, all samples exhibited significant antibacterial and antifungal and most of them antiviral activity (Al-Hariri, Citation2011). It has been widely used in traditional medicine for treatment of wounds and burns (Olczyk et al., Citation2013a,Citationb). Phenolic substances, flavonoids and cinnamic acids derivatives compose the major bioactive components of propolis. The antimicrobial properties of propolis are related to the synergistic effect of its components (Sawaya et al., Citation2004).
Propolis had been used in a variety of applications, which include gels, creams and ointments for wound healing and treatment of burns. Berretta et al. (2012) developed a poloxamer 407 gel containing 3.6% propolis for wound healing and suggested that the new formulation can offer an alternative therapeutics to treat skin injuries. Ahmed et al. (2011) investigated the effect of Egyptian propolis extract in cold cream formulation on diabetic burn wound and concluded that propolis alone or in combination with Dermazine is a unique antibacterial cream for the treatment of diabetic burn wound. AL-Hamadaui (Citation2010) reported that a prepared mixture ointment from propolis and black cumin revealed a high efficacy in treatment of wounds.
Considering this information, pluronic lecithin organogels containing 4% the propolis extract, were developed, evaluated and designed to be used for treating wounds, as a product suited for this application is still required.
Materials and methods
Propolis (El Akbr)® was obtained from a honey bee market located in Jeddah, Saudi Arabia (El Maher shop, Wadi El Nahil Co., Taeif, Saudi Arabia). Soy lecithin (Phosphatidylcholine) was purchased from Across Organics, New Jersy, USA. Pluronic F 127 was purchased from Sigma Chemical Co. St. Louis, MO. Isopropyl palmitate and polyethylene glycol 400 were supplied by Loba Chemie, Mumbai, India. All other reagents and solvents used were of reagent grade.
Preparation of propolis extract
Propolis extract (PE), with a propolis/80% ethanol ratio of 1/15 (w/w), was prepared by maceration technique. The sample was left to macerate in the dark for 72 h at room temperature, then filtered with Whatman No. 4 filter paper and the filtrate was evaporated at 50 °C using rotary evaporator (automated 24/7 evaporation, Heidolph, Germany) (Ophori & Wemabu, Citation2010).
Preparation of pluronic lecithin organogel
Pluronic lecithin organogel is a microemulsion-based gel which is made up of an oil phase and an aqueous phase. The oil phase was prepared by mixing lecithin and isopropyl palmitate and allowing the mixture to stand overnight at room temperature to ensure complete dissolution. The aqueous phase was prepared by dispersing weighed amount of pluronic F127 in cold water. The dispersion was stored in refrigerator for effective dissolution of pluronic F127. PE (4% w/w) (Berretta et al., 2012), was dissolved in PEG400 and then incorporated into the lecithin solution. To prepare organogel, oil phase was slowly added to the aqueous phase with stirring at 400 rpm stirrer (Thermolyne, Dubuque, IA) (Pandey et al., Citation2009). The composition of different formulations is summarized in .
Table 1. Formulation of pluronic lecithin oganogels of propolis extract.
Evaluation of organogel formulations
Physical examination
The physical evaluation of formulation is important as it is directly related with patient acceptance. The physical parameters involved color, smoothness/grittiness, ease of application, oiliness/greasiness and phase separation (Pharmedica Enterprise, 2013).
pH determination
The pH of formulated organogels was determined using pH meter (pH/Ion S220, Mettler-Toledo International Inc., Switzerland). The electrode was immersed in organogels and readings were recorded on pH meter.
Drug content
Each formulation (0.5 g) was dissolved in 50 ml ethanol. The solution was filtered through Whatman filter paper (No. 41). Same procedure was followed for blank organogel. Blank organogel sample was used as the blank for auto zero the spectrometer at 337 nm (Model: UV-2401 PC, Shimadzu Corporation, Singapore) (Thorat & Rane, Citation2010; Al Msrghita et al., Citation2013).
Viscosity measurement
Viscosities of the formulated organogels were determined using Brookfield Viscometer with Spindle no. 7 (Model: RV-DV-E 230, Brookfield Engineering Lab., Inc., Middleboro, MA) at 25° with the spindle speed of 10 rpm.
In vitro release studies
The developed formulations were subjected to in vitro release through semipermeable cellophane membrane (previously immersed in phosphate buffer, pH 7.4, for 24 h). About 1 g of organogel was applied uniformly on a dialysis membrane then stretched over the lower open end of a dialysis tube (locally fabricated glass cylinder, length 15 cm and internal diameter of 2.9 cm) with the aid of rubber band. The tubes were attached to the dissolution apparatus (Dissolution tester, rotating basket SP6-400, G.B. CALEVA Ltd., Dorset, England) and allowed to stir at 50 rpm. The receptor compartment consisted of 150 ml of saline phosphate buffer (pH 7.4) and methanol (90:10). Methanol was added in medium to maintain sink condition. The whole assembly was maintained at 37 ± 1 °C. Aliquots (3 ml) were withdrawn at regular interval of 1 h for a period of 8 h and replaced with equal volume of fresh medium equilibrated at 37 ± 1 °C and analyzed spectrophotometrically at 337 nm for propolis content. Each experiment was carried out in triplicate.
In order to investigate the mode of drug release from the prepared organogels, the following plots were made: cumulative % drug release versus time (zero order kinetic model); log cumulative of % drug remaining versus time (first order kinetic model) and cumulative % drug release per surface area of membrane versus square root of time (Higuchi model). The correlation was used as an indicator of goodness-of-fit (Ramanauskienė et al., Citation2011).
Comparative skin permeation study
To investigate the effect of organogel formulation on drug permeation, a comparative drug permeation study from optimized pluronic lecithin organogels (high % drug release) and PE suspension in dist. water (control) was carried out through excised abdominal rat skin. The rat skin was mounted between the donor and receptor compartment keeping the stratum corneum facing towards the donor side and the experiment run as previously mentioned. The mean cumulative amount of drug permeated per unit surface area was plotted versus time. The slope of the linear portion of the plot was calculated as flux J (μg/cm2/h) and the permeability coefficient was calculated using equation, Kp = J/Cd, where, Kp is the permeability coefficient and Cd is the initial drug concentration in drug compartment. Moreover, enhancement ratio (Er) was calculated as (Idrees et al., Citation2011): Er = J (formulation)/J (control).
Antimicrobial activity
Antimicrobial activity of the selected propolis organogel (F2), that exhibited better release and skin permeation properties, compared with that of PE was studied according to the method described by Jerwood and Cohen (2008) and AL-Waili et al. (2012). Briefly, 20 bacterial isolates (Klebsiela sp., methicillin resistant Staphylococcus aureus, E. coli, Pseudomonas aeruginosa and Proteus mirabilis) were recovered from frozen or ambient saves by spreading on blood agar plates and inspected for contamination after overnight incubation. The above-mentioned microorganisms were chosen on the basis that they are the most frequently isolated from injuries and burn wounds (Wojtyczka et al., Citation2013). The test organisms from these plates were made up to a turbidity equivalent to that of a 0.5 McFarland standard then diluted 1:100 in the Mueller–Hinton broth. These inocula were prepared immediately prior to each experiment. The MIC was determined based on the classical microtitre broth dilution according to CLSI (formerly the NCCLS). A suspension in water was made for PE, organogel base and the selected propolis organogel (F2) to give an initial concentration of 1 g/l. The first test well therefore contained a concentration of 500 mg/l after the addition of isolate broth. There were six further wells tested, each with half the concentration of the previous and the concentration in the final well tested was 7.8 mg/l. The trays were read after 18–24 h of incubation at 37 °C in air. Positive growth and negative sterility controls were included on each microtitre tray. The MIC was defined as the lowest concentration that restricted the bacterial growth (no visible growth).
Statistical analysis
All values were expressed as mean ± SEM. The statistical analysis was performed using one-way analysis of variance (ANOVA). The value of p < 5% (p < 0.05) was considered statistically significant.
Results
Evaluation of organogel formulations
Physical examination
The results of physical examination are illustrated in . All the developed formulations exhibited gel like consistency except F5 (contained 10% w/w pluronic F127). So, F5 was excluded from the evaluation. The obtained organogels were brownish, non-greasy, smooth in feel and free from grittiness. All the organogels showed no phase separation.
Table 2. Evaluation of pluronic lecithin oganogels of propolis extract.
pH determination
The pH was in the range of 5.5–6.3 ().
Drug content
The drug content was in the range of 97.5–100.2% ().
Viscosity measurement
The organogels showed viscosities in the range of 5366–8984 cps ().
In vitro release studies
The cumulative percentage of drug release of various formulations except F5 through cellophane membrane over a period of 8 h is shown in . The results revealed that maximum percent drug release of propolis in 8 h was observed from F2 and F3 formulations. A significant (p < 0.05) increase in propolis release was obtained as lecithin concentration was increased to 3–4% w/w in formulations. Further increase in the concentration of lecithin decreased percent drug release. In addition, further increase in concentration of pluronic to 30% rather than 20% decreased percent drug release. The data revealed that decreasing the oil phase % of organogel from 30% to 20% w/w resulted in a decrease in propolis release. The kinetic values obtained from different plots are listed in . When data were plotted according to zero-order kinetics, linear plots were obtained for all formulations except F2 with regression coefficient values ranging from 0.924 to 0.979. Regarding F2, the best linearity was according first order kinetics with regression coefficient 0.995.
Figure 2. In vitro release profile of propolis from pluronic lecithin organogels through cellophane membrane in saline phosphate buffer pH 7.4.
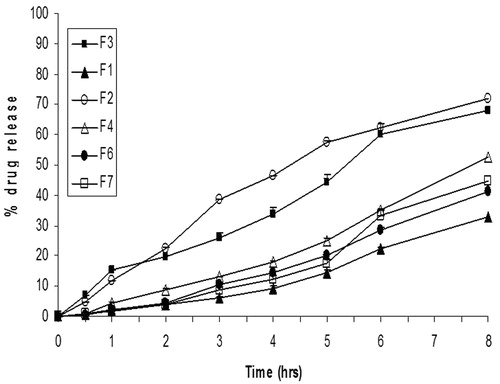
Table 3. Kinetic data of the release studies.
Comparative skin permeation study
The results of skin permeation study are illustrated in and . It is clear that skin permeation of propolis was significantly increased (p < 0.05) from its organogel formulations F2 and F3 in comparison to the control (PE in water). Since F2 formulation produced the highest flux (434.6 ± 4.6 μg/cm2/h), it was selected for the antimicrobial study.
Figure 3. Permeation profiles of propolis through excised rat skin from organogels compared with PE suspension in water.
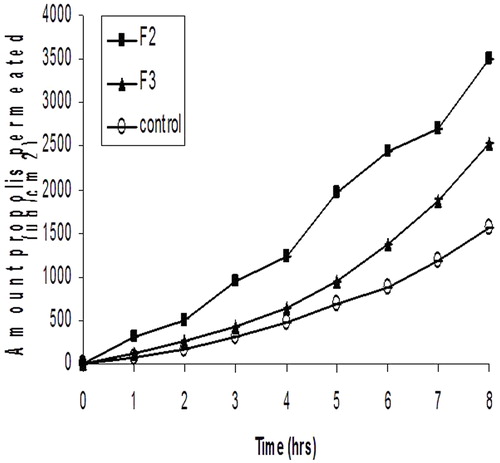
Table 4. Permeation parameters of optimized organogel formulations compared with PE suspension in water.
Antimicrobial activity
The results of antimicrobial activity are illustrated in . It is clear that organogel base (without propolis) was not effective against the tested microorganisms. The results revealed that PE suspension in water displayed varying degrees of activity against different bacterial isolates with MIC values ranging from >32 to >500 mg/l. For propolis organogel (F2), the MIC values were ranging from 15.62 to 125 mg/l comprising 2–4-folds increase in antibacterial activity. The results of MIC showed that the most sensitive bacteria were Klebsiela sp. (15.62 and >32 mg/l for propolis organogel and propolis suspension, respectively). While, the least sensitive bacteria were Staphylococcus aureus and Pseudomonas aeruginosa (125 mg/l and 250 to >500 mg/l for propolis organogel and propolis suspension, respectively).
Table 5. Minimum inhibitory concentration (MIC) of propolis organogel (F2) compared with PE in water against different bacterial isolates.
Discussion
Evaluation of organogel formulations
Physical examination
The results of physical examination indicated that the formulated organogels had good texture. Freedom from grittiness reflects the degree of acceptability of formulation by the patients.
pH determination
The pH of all the formulations was compatible with the skin pH reflecting no risk of skin irritation (Mantry et al., Citation2013).
Drug content
The results were found in the acceptable range, indicating uniform distribution of drug throughout the base and no interaction of drug with component of base.
Viscosity measurement
A significant increase in viscosity was observed with increase in lecithin concentration due to the fact that long flexible and cylindrical giant micelles were formed (Parsaee et al., Citation2002). Moreover, increase in pluronic concentration resulted in viscosity increase of the formulated organogel. This result may be ascribed to formation of complex network, as in the case of gel, the consistency depends on percentage of solids in relation to liquid (Agrawal et al., Citation2010). Decreasing oil phase % from 30% w/w to 20% w/w resulted in a significant increase (p < 0.05) in the viscosity of the organogel. As mentioned before, the mechanism of organogel formation depends on the transformation of spherical reverse micelles into cylindrical micelles by the addition of water and then into long tubular micelles which entangle and build up a three-dimensional network. So, by decreasing the oil phase to 20% w/w and hence increasing the aqueous phase to 80% w/w resulted in extensive entanglement of long tubular micelles into three-dimensional network with a high viscosity. Similar results were obtained by Attar et al. (2003).
In vitro release studies
The results revealed that, there was a significant decrease (p < 0.05) in propolis release as the lecithin concentration increased from 4% to 6% w/w in formulation. As mentioned before, at higher lecithin concentration, there is more extensive entanglement of long cylindrical micelles, forming a network-like structure with a very high viscosity and hence less amount of free drug is available for release which leads to decrease in the drug release rate (Surjyanarayan et al., Citation2010). In addition, further increase in concentration of pluronic to 30% rather than 20% decreased significantly the percent drug release which ascribed to extensive formation of network like structure with very high viscosity (Stationwala et al., Citation2011). The data revealed that decreasing the oil phase % of organogel from 30% to 20% w/w resulted in a decrease in propolis release. As previously mentioned, by decreasing the oil phase % and hence increasing the aqueous phase %, there will be an increase in the entanglement of long tubular micelles forming a three-dimensional network with a very high viscosity which affects the release rate of system. Moreover, higher quantity of pluronic would be present which potentiates the increase in viscosity resulting in much lower drug release. An inverse correlation existed between the release rate and the gel viscosity values. These data are confirmed by Santoyo et al. (Citation1996) who reported that drug release rate is inversely related to the viscosity of the continuous phase.
The results of kinetic study suggested that the mechanism of release from all the formulations except F2 followed zero-order kinetics, while, the release from F2 obeyed first-order kinetics.
Comparative skin permeation study
The improved skin permeation of propolis from its organogel formulation (F2) may be attributed to the modification of skin barrier function by the organogel components. Lecithin and pluronic (from the organogel) was thought to penetrate into the skin, interact and disorganize the lipid layers of the stratum corneum (Agrawal et al., Citation2010).
Antimicrobial activity
Different mechanisms were reported to explain propolis antibacterial activity, they include: inhibition of protein synthesis and bacterial growth, disorganization of the cytoplasmic membrane and cell wall and inhibition of bacterial enzymes (Wojtyczka et al., Citation2013). The results of antimicrobial study revealed that there was a significant increase in the antimicrobial activity of propolis by its incorporation in pluronic lecithin organogel. This result may be ascribed to increase in propolis penetration into the bacterial membrane by the presence of lecithin and pluronic in organogel. As mentioned previously, lecithin and pluronic have penetration enhancing properties.
The results of antimicrobial study were in disagreement to that reported by Berretta and coworkers who reported that gram positive bacteria (Staphylococci) were more sensitive to propolis standardized extracts ( EPP-AF®, Brazil) than Gram negative bacteria (P. aeruginosa, K. pneumoniae and E. coli) (Berretta et al., 2012). This result may be attributed to difference in the composition of propolis according to the geographical and plant sources, and the collection season. Burdock mentioned that, the applied studies on the antimicrobial activity of propolis show different results (Burdock, 1998). Kashi and coworkers reported that reported that the propolis samples collected from different geographic origin with different climates and vegetation show different antibacterial activities (Kashi et al., 2011). Moreover, seasonal effect on Brazilian propolis antibacterial activity was investigated by (Sforcin et al., 2000).
Conclusion
The topical organogel formulation of propolis containing 3% lecithin and 20% pluronic (F2) is an effective formulation for topical delivery of propolis as it showed higher drug release, higher skin permeation and higher antimicrobial activity as compared to propolis suspension in water. So, it can offer an alternative therapeutics to treat skin injuries.
Declaration of interest
The authors report no declarations of interest.
References
- Agrawal V, Gupta V, Ramteke S, Trivedi P. (2010). Preparation and evaluation of tubular micelles of pluronic lecithin organogel for transdermal delivery of sumatriptan. AAPS Pharm Sci Tech 11:1718–25
- Ahmed ET, Abo-Salem OM , Osman A. (2011). The influence of Egyptian propolis on induced burn wound healing in diabetic rats; antibacterial mechanism. Sci J Med Clin Trails. Article ID: 2011--2317, 8 pages
- AL-Hamadaui AHU. (2010). The use of propolis and black cumin in treatment of some wounds and burn infections. QMJ 6:210--22
- Al-Hariri MT. (2011). Propolis and its direct and indirect hypoglycemic effect. J Fam Commun Med 18:152–4
- Al Msrghita GL, Dezmirean DS, BobiG O. (2013). Important developments in Romanian propolis research. Evid Based Complement Alternat Med Article ID 159392: 9 pages
- AL-Waili N, Al-Ghamdi A, Ansari MJ, et al. (2012). Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus Aureus, Escherichia Coli and Candida Albicans isolates in single and polymicrobial cultures. Int J Med Sci 9:793--800
- Avanço GB, Bruschi ML. (2008). Preparation and characterisation of ethylcellulose microparticles containing propolis. Rev Ciênc Farm Básica Apl 29:129–34
- Berretta AA, Nascimento AP, Bueno PC, et al. (2012). Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int J Biol Sci 8:512–21
- Burdock G. (1998). Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 36:347--63
- Escobar-Chávez JJ, López-Cervantes M, Naïk A, et al. (2006). Applications of thermoreversible pluronic f-127 gels in pharmaceutical formulations. J Pharm Pharmaceut Sci 9:339–58
- Idrees MA, Rahman NU, Ahmad S, et al. (2011). Enhance transdermal delivery of flurbiprofen via microemulsions: effects of different types of surfactants and cosurfactants. DARU 19:433–9
- Ivančajić S, Mileusnić I, Cenić-milošević D. (2010). In vitro antibacterial activity of propolis extracts on 12 different bacteria in conditions of 3 various ph values. Arch Biol Sci 62:915–34
- Jerwood S, Cohen J. (2008). Unexpected antimicrobial effect of statins. J Antimicrob Chemother 61:362–4
- Kashi TSJ, Kermanshahi RK, Dastjerdi MEEV, et al. (2011). Evaluating the in-vitro antibacterial effect of Iranian propolis on oral microorganisms. Iranian J Pharm Res 10:363--68
- Mantry S, Patnaik A, Sriram N, Bharath Raju V. (2013). Formulation and evaluation of bifonazole organogel as a novel topical drug delivery system. Int J Pharm 3:1–8
- Murdan S. (2005). A review of pluronic lecithin organogel as a topical and transdermal drug delivery system. Hosp Pharm 12:267–70
- Nasser S, Mabrouk A, Maher A. (2003). Colonization of burn wounds in Shams University Burn Unit. Burns 29:229–33
- Ophori EA, Wemabu EC. (2010). Antimicrobial activity of propolis extract on bacteria isolated from nasopharynx of patients with upper respiratory tract infection admitted to Central Hospital, Benin City, Nigeria. African J Micro Res 4:1719–23
- Olczyk P, Komosinska-Vassev K, Winsz-Szczotka K, et al. (2013a). Propolis induces chondroitin/dermatan sulphate and hyaluronic acid accumulation in the skin of burned wound. Evid Based Complement Alternat Med, Article ID 290675: 8 pages
- Olczyk P, Wisowski G, Komosinska-Vassev K, et al. (2013b). Propolis modifies collagen types I and III accumulation in the matrix of burnt tissue. Evid Based Complement Alternat Med, Article ID 423809: 10 pages
- Pandey MS, Belgamwar VS, Surana SJ. (2009). Topical delivery of flurbiprofen from pluronic lecithin organogel. Ind J Pharm Sci 71:87–90
- Parsaee S, Sarbolouki MN, Parniapour M. (2002). In vitro release of diclofenac iethylammnium from lipid based formulations. Int J Pharm 241:185–90
- Pharmedica Enterprise. (2013). Pluronic Lecithin Organogel. Available from: www.plo-gel.com
- Ramanauskienė K, Zilius M, Briedis V. (2011). A study on release of propolis extract components from emulsion-type dispersions. Medicina (Kaunas) 47:354–9
- Santoyo S, Arellano A, Ygartua P, Martin C. (1996). In vitro percutaneous absorption of piroxicam through synthetic membranes and abdominal rat skin. Pharm Acta Helv 71:141–6
- Sawaya ACHF, Souza KS, Marcucci MC, et al. (2004). Analysis of the composition of Brazilian propolis extracts by chromatography and evaluation of their in vitro activity against gram-positive bacteria. Brazilian J Micro 35:104–9
- Sforcin J, Fernandes Jr A, Lopes C, et al. (2000). Seasonal effect on Brazilian propolis antibacterial activity. J Ethnopharmacol 73:243--9
- Shchipunov YA. (2001). Lecithin organogel: a micellar system with unique properties. Colloids surf A Physicochem Eng Asp 183–185:541–54
- Shchipunov YA, Duressschmidt T, Hoffmann H. (1990). Electrorheological effects in lecithin organogels with water and glycerol. J Colloid Interface Sci 212:390–401
- Shchipunov YA, Shumilina EV. (1995). Lecithin bridging by hydrogen bonds in the organogel. Mater Sci Eng C 3:43
- Shchipunov YA, Shumilina EV. (1997). Molecular model for the lecithin self-organization into polymer-like micelles. Prog Colloid Polym Sci 106:228
- Stationwala R, Patidar A, Main P, et al. (2011). Transdermal delivery of lornoxicam from pluronic lecithin organogel. Int J Chem Pharm Sci 2:32–7
- Surjyanarayan M, Snigdha SM, Krutika KS. (2010). Lecithin stabilized organogel: design and development for topical application of clobetasol propionate. Int J Pharm Tech Res 2:1133–8
- Thorat SP, Rane SI. (2010). Formulation and in vitro evaluation of lecithin (soya and egg) based aceclofenac organogels. J Pharm Res 3:1438–41
- Trusheva B, Trunkova D, Bankova V. (2007). Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J 1:13–6
- Wojtyczka RD, Kwpa M, Idzik D, et al. (2013). In vitro antimicrobial activity of ethanolic extract of polish propolis against biofilm forming staphylococcus epidermidis strains. Evid Based Complement Alternat Med, Article ID 590703: 11 pages
