Abstract
Context: Single-walled carbon nanotubes (SWCNTs), an important class of artificial nanomaterials with unique physicochemical properties, were used as novel carriers of curcumin.
Objective: Formulation and evaluation of curcumin-loaded SWCNTs systems for utilizing the curcumin’s anticancer potential by circumventing conventional limitations of extremely low aqueous solubility and instability under physiological conditions, and combining SWCNTs photothermal therapy enabled by the strong optical absorbance of SWCNTs in the 0.8–1.4 μm resulting in excessive local heating.
Methods: After functionalized SWCNTs were confirmed, they were conjugated with curcumin (SWCNT-Cur). Subsequently, the formulation was analyzed for size, zeta-potential and morphology. And the solubility, stability and release of curcumin were assessed using spectrofluorometer, and the solid state of the curcumin was determined using X-ray diffraction and UV spectroscopy. Furthermore, in PC-3 cells, photothermal response was further determined by irradiating laser after the antitumor effect of SWCNT-Cur was evaluated.
Results and discussion: SWCNTs were functionalized, and subsequent SWCNT-Cur conjugates were found to possess an average size of 170.4 nm, a zeta potential of −12.5 mV and to significantly enhance the solubility and stability of curcumin, overcoming the barriers to adequate curcumin delivery. Moreover, curcumin in SWCNT-Cur was in an amorphous form and could be rapidly released. In PC-3 cells, improved inhibition efficacy was achieved by SWCNT-Cur compared with native curcumin. Meanwhile, the SWCNTs in SWCNT-Cur served not only as scaffolds but also as thermal ablation agents, further inhibiting PC-3 cell growth.
Conclusion: SWCNT-Cur assemblies may provide a promising delivery system for curcumin for use in cancer therapy.
Introduction
Single-walled carbon nanotubes (SWCNTs) are an important class of artificial nanomaterials with unique physical and chemical properties that have potential novel applications in nanomedicine as pharmaceutical excipients for creating versatile drug delivery systems (Liu et al., Citation2008; Prato et al., Citation2008; Jain, Citation2012). They are of particular interest as vectors in delivering antitumor drugs due to the following properties: (1) they are readily internalized by mammalian cells along with their cargo; (2) their large surface areas (theoretically 1300 m2/g) enable a high drug loading capacity (Meng et al., Citation2012); and (3) due to their strong optical absorbance in the 0.8–1.4 μm near-infrared region (in which tissue is transparent), which results in excessive local heating, SWCNTs may also act as photothermal therapy agents for ablating cancer cells (Moon et al., Citation2009). Moreover, the properties of SWCNTs, such as the length, the content of trace metal contaminants in impure SWCNTs, the degree of aggregation, and the presence of bound functional group(s) and the type of functionalization, can be modified singly or simultaneously to produce CNTs with reduced toxicity based on changes in their hydrophobicity and propensity to form large agglomerates (Lanone et al., Citation2013). Therefore, the SWCNTs used for biomedical purposes are in either dispersed or functionalized forms to minimize their toxicity.
Curcumin, a natural compound that has completed phase II clinical trials, is one of the most promising anti-tumor drugs, demonstrating safety, chemopreventive abilities, multi-target and broad-spectrum activities against malignancy, and the potential to overcome multidrug resistance in cancer cells (Duvoix et al., Citation2005; Anand et al., Citation2008; Shukla et al., Citation2009). However, the extremely low aqueous solubility of curcumin due to its hydrophobic nature (Kurien & Scofield, Citation2009), the instability under physiological conditions due to its degradation at neutral-basic pH’s and light sensitivity (Liang et al., Citation2009), and the poor bioavailability, which hinder its ability to reach the concentrations required for anticancer effects (Anand et al., Citation2007) prevents curcumin from achieving its full potential as an anti-tumor agent. Therefore, the development of better formulations, especially nanoformulations, has become the main effort in overcoming these problems (Cao et al., Citation2011; Gao et al., Citation2011; Rahman et al., Citation2012; Yallapu et al., Citation2012).
The delivery of curcumin using functionalized SWCNTs by phosphatidylcholine (PC) and polyvinylpyrrolidone (PVP) is proposed in this investigation. The formulation developed was evaluated for its ability to load and release curcumin, to protect curcumin from degradation and to increase its solubility. The in vitro cytotoxicity of the formulation and the synergistic enhancement of its anticancer effect by the photothermal ablation of SWCNTs in PC-3 tumor cells were studied to confirm the applicability of the delivery system in cancer therapies.
Materials and methods
Materials
SWCNTs were purchased from Chengdu Organic Chemicals Co. Ltd. (Chengdu, Sichuan Province, China, produced by chemical vapor deposition, purity >90%, Catalog No. 1109). A human prostate cancer PC-3 cell line (Catalog No. TCHu158) was obtained from the Chinese Academy of Sciences Cell Bank. Curcumin was extracted and purified by our laboratory (purity >98%). PC, PVP, sodium dodecyl sulfate (SDS) and sulforhodamine B (SRB) were purchased from Sigma-Aldrich, Inc. (St Louis, MO).
Methods
Purification, cutting and oxidation of SWCNTs
Similar to the steps described previously (Villa et al., Citation2008), SWCNTs were processed overnight with 1 M HNO3 at room temperature. They were then filtered through a membrane with a pore size of 100 nm, washed with purified water until a pH of 7 was reached, and vacuum dried. Subsequently, the following procedures were performed to obtain the purification, cutting and oxidation of the SWCNTs: the SWCNTs were allowed to react in an ultrasonic bath at 45 °C in a strong acid solution (3:1 mixture of 18.4 M H2SO4 and 4 M HNO3) for 4 h, filtered, washed with purified water until a pH of 7 was reached and finally vacuum dried.
Preparation of SWCNT-curcumin (SWCNT-Cur)
Curcumin (20 mg) was mixed with SWCNTs (10 mg) and PVP K30 (110 mg) in methanol (5 mL) using an ultrasonic bath for 10 min then rotary evaporated to dryness to obtain SWCNTs complexed with curcumin. The complex was suspended with 10 mL of an aqueous solution of PC (150 mg) and PVP K30 (10 mg) by sonication for 30 min, and the suspension was subsequently ultrasonicated (100 w, 10 times) using a JV92-ΙΙ ultrasonic cell disruption system (Ninbo Scientz Biotechnology CO., Ltd., Zhejiang, China). The resulting suspension was centrifuged at 5000 rpm for 10 min to remove the excess curcumin and insoluble SWCNTs, then the supernatants were lyophilized to produce the final formulation of SWCNT-Cur and diluted to 10 mL prior to use, unless stated otherwise.
Characterization of SWCNT-Cur
Size distribution and zeta potential determination
A 100 μL aliquot of SWCNT-Cur was diluted in 1.9 mL ultra-pure H2O. The size and zeta potential of the SWCNT-Cur were determined in triplicate using a Zetasizer Nano ZS-90 (Malvern, UK) based on quasi-elastic light scattering.
Determination of loading efficiency of curcumin
A 100 μL volume of SWCNT-Cur was diluted with 900 μL of methanol, sonicated for 1 h, and then centrifuged at 10 000 rpm for 10 min to remove the SWCNTs in the formulation. The supernatant solution was used to determine the curcumin concentration using an RF5301pc spectrofluorometer (Shimadzu, Kyoto, Japan) with an excitation wavelength at 443 nm and an emission wavelength at 543 nm.
In vitro release experiment
A 5 mg sample of the freeze-dried powder of the SWCNT-Cur assemblies was re-dispersed in a 100 mL phosphate-buffered saline (PBS) solution (0.01 M, pH = 7.4) containing 0.5% SDS, and the solution was placed in a thermostatic oscillator at a temperature of 37 °C and a shaking speed of 120 rpm. A 2 mL volume of the samples was collected at designated time points, and the same amount of medium was supplemented each time. The curcumin was further extracted in methanol after filtering 2 mL of the solution through a membrane filter with a pore size of 100 nm to separate the released curcumin from the SWCNT-Cur. Subsequently, according to the standard curve of curcumin, which was constructed from the fluorescence intensity of the curcumin as a function of its concentration, the quantities of released curcumin were determined by a spectrofluorometer to calculate the cumulative release rate.
X-ray diffraction (XRD)
The XRD patterns of vacuum drying the mixture of curcumin, SWCNTs and PVP K30 in methanol; the solid curcumin; and the physical mixture of curcumin, PVP K30 and SWCNTs, were determined using a Siemens D-500 diffractometer with Cu Kα radiation (k = 1.5405 A˚). The scanning angle was set from 5° to 40° of 2θ, the current was 30 mA, and the voltage was 35 kV.
UV–Vis spectroscopy
The UV–Vis spectrum of curcumin, the SWCNTs and the SWCNT-Cur conjugates were measured with a UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan) to provide direct evidence of the attachment of curcumin to the SWCNTs. The absorbance at 808 nm was used to determine the SWCNTs concentration in the suspension of SWCNT-Cur using a visible spectrophotometer (Shimadzu, Kyoto, Japan) according to the method described by Wang et al. (Citation2011).
Transmission electron microscopy (TEM)
TEM was performed to identify the presence of SWCNT aggregates. After a drop of the sample suspension was placed on the TEM grids and dried, the TEM analysis was performed with a JEM-1400 TEM (JEOL, Japan) operating at 80 kV.
IR spectroscopy
After being mixed and milled with potassium bromide (KBr), the samples were compressed into a thin pellet for characterization by FT-IR (Nicolet iS10 spectrometer, Thermo Scientific, Waltham, MA).
Stability experiment
The stabilities of the SWCNT-Cur and of native curcumin were evaluated by fluorescence spectrophotometry according to the method of Mohanty et al. with minor modifications (Mohanty & Sahoo, Citation2010). Briefly, native curcumin with the aid of 5% (v/v) methanol and SWCNT-Cur were dissolved in PBS (0.01 M, pH 7.4) to a final concentration of 50 μg/mL and incubated at 37 °C under gentle agitation for 8 h. At designated time points, 200 μL of the samples was collected, diluted with 1.8 mL methanol, and quantified spectrophotometrically to confirm whether the SWCNT-Cur assemblies prevented curcumin from degrading. The concentration of curcumin was calculated according to the standard curve of curcumin dissolved in methanol.
Cell inhibition experiment
PC-3 cells were cultured in RPMI-1640 medium that contained 10% fetal bovine serum, 1 × 105 μg · L−1 streptomycin and 1 × 105 U · L−1 penicillin at 37 °C with 5% CO2 in a humidified incubator. The experiments were performed 24 h after the PC-3 cells were seeded in 96-well plates with a density of 8 × 103. Various concentrations of SWCNT-Cur and curcumin, as well as the highest concentration of SWCNTs in SWCNT-Cur, were added separately for 24 h, 48 h or 72 h. The cell inhibition was subsequently assessed using the sulforhodamin B (SRB) assay (Huang et al., Citation2006).
To explore the photothermal ablation induced by the SWCNTs under 808 nm laser irradiation, we evaluated the effect of different irradiation times on cell proliferation 24 h after irradiation. An examination of the synergistic inhibition obtained by combining curcumin with the photothermal ablation of SWCNTs was conducted as follows: after the PC-3 cells had been treated with SWCNT-Cur assemblies, SWCNTs or curcumin for 4 h followed by laser irradiation for 1.5 min, the cells were further incubated for 24 h before the cell inhibition rate was determined by the SRB assay.
Statistical analysis
Quantitative data were expressed as the mean ± standard error of mean (SEM). Differences between the groups were analyzed using an analysis of variance (ANOVA) followed by the Dunnett post test using SPSS17.0 statistical software, and p values of <0.05 were considered statistically significant.
Results and discussion
Influence of SWCNT-Cur on solubility of curcumin
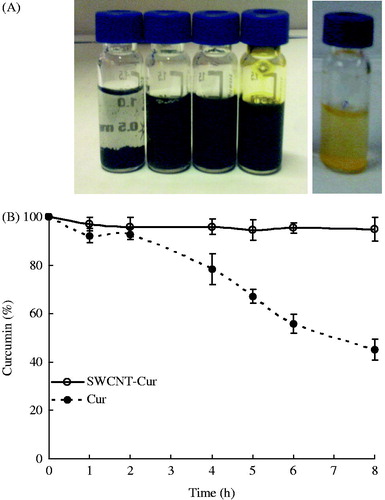
reveals that the native curcumin was nearly insoluble in aqueous media, with a solubility of only 0.006–0.007 mg/mL (Xu et al., Citation2008). However, after being formulated by functionalized SWCNTs, the concentration of curcumin measured by a spectrophotometer increased to 1.88 mg/mL and a stable aqueous suspension with the natural color of curcumin was obtained, suggesting that the curcumin solubility was significantly enhanced.
Influence of SWCNT-Cur on stability of curcumin
Because curcumin is an easily degraded compound, the stabilities of native curcumin and the curcumin in the SWCNT-Cur assemblies were studied. As displayed in , 55% of the native curcumin was degraded after 8 h of incubation in PBS; however, the curcumin in the SWCNT-Cur was quite stable and remained intact under the same conditions, with only 5% of the curcumin being hydrolyzed and biotransformed. This result demonstrates that our formulation can increase the stability of curcumin by reducing its biodegradation.
Determination of functionalized SWCNTs in SWCNT-Cur
Pristine SWCNTs are insoluble in aqueous media (), limiting their dispersibility in biosystems and contributing to their toxicity. After being processed with acids (H2SO4 and HNO3) and sonicated, carboxylated SWCNTs were obtained through oxidative modification, as confirmed by the characteristic carboxyl peaks (1713.4 cm−1) in the infrared spectrum (). The dispersibility of the oxidated SWCNTs increased slightly relative to the pristine SWCNTs (). Following further non-covalent functionalization with PC and PVP K30, the SWCNTs were easily dispersed in aqueous media (). The concentration of the SWCNTs in the SWCNT-Cur assemblies was measured by a spectrophotometer to be 626.7 μg/mL. High-magnification TEM images revealed that the outer diameter of the SWCNTs increased in size and the surfaces of the SWCNTs became non-uniform after the SWCNTs were non-covalently functionalized with PC and PVP K30 (), confirming the non-covalent functionalization of the SWCNTs. These results suggest that the increase in the dispersibility of the SWCNTs might be partially due to the PC and PVP K30 surrounding the SWCNTs (), which help prevent aggregation. And, these results are in agreement with previous reports that PVP’s long polymeric chains can wrap around the nanotube (Didenko et al., Citation2005).
Figure 2. IR spectra of the purified SWCNTs (A) and the carboxylated SWCNTs (B). The characteristic peaks (1713.4 cm−1) of the carboxylated SWCNTs were not found in purified SWCNTs, suggesting that carboxyl groups were bound to the nanotubes.
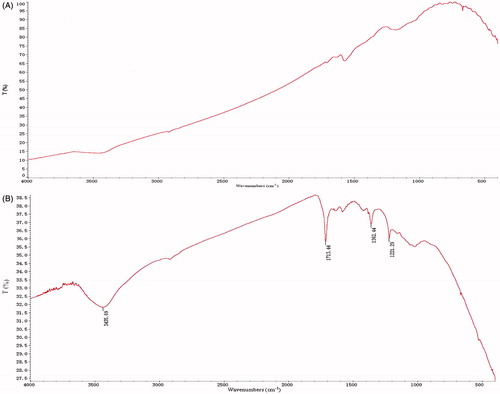
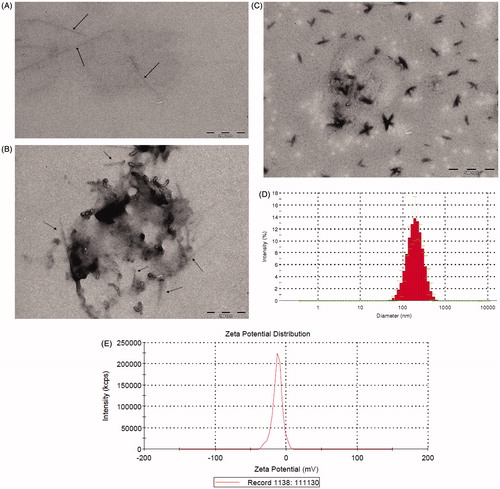
Figure 3. Characterization of SWCNTs-Cur. (A–C) TEM images of SWCNTs only and SWCNTs-Cur. The scale bars are 200 nm (A, B) and 500 nm (C). Arrows indicate the SWCNTs. (D, E) Particle size and Zeta-potential of the SWCNTs-Cur.
Recent studies have shown that, although the physicochemical characteristics of SWCNTs, including their highly hydrophobic surface, insolubility in aqueous solutions and contamination with metals used in the manufacturing process lead to their toxicity and poor biocompatibility, carefully optimizing these physicochemical parameters to minimize the toxicity of CNTs is highly advantageous and can be used in biomedical applications (Lanone et al., Citation2013). Based on literature reports that processing pristine SWCNTs in strong acids (3:1 mixture of H2SO4 and HNO3) in conjunction with sonication can remove residual metallic catalysts or amorphous carbon impurities and shorten the SWNTs (Villa et al., Citation2008; Gaillard et al., Citation2009), as well as the nature of PC and PVP K30 as non-toxic lipids and dispersants, respectively, we chose to use acid, PC, PVP K30 and ultrasonication to process the SWCNTs. Following the oxidative modification () and non-covalent functionalization () of the SWCNTs, their dispersibility significantly increased (). Thus, the biocompatibility of the SWCNTs can be tremendously enhanced.
Biophysical characteristics of SWCNT-Cur
Size and zeta-potential measurements
The TEM results in reveal that the SWCNT-Cur conjugates can be effectively assembled into nanostructures. The size of the SWCNT-Curs was further characterized by a laser particle size analyzer. presents the average diameter and zeta potential of the SWCNT-Cur formulation of 170.4 ± 8.5 nm and −12.5 ± 1.1 mV, respectively. The nanoscale size and strong interparticle repulsions produced by the negative surface charges on the SWCNT-Cur suggest that the formulation may be suitable for in vivo administration.
Cationic liposomes ensure efficient endocytotic cellular uptake by negatively charged biological cell surfaces. Unfortunately, such cationic liposomes also exhibit non-specific electrostatic interactions with negatively charged hydrophobic serum albumin proteins and cellular components, such as low density lipoproteins, macroglobulins, and myriads of other negatively charged systemic molecules (Karmali & Chaudhuri, Citation2007). Compared with cationic liposomes, slightly anionic liposomes possess the advantage of considerable physicochemical stability even in serum as well as of long blood circulation times following intravascular application (Lakkaraju et al., Citation2001; Sedlacek, Citation2001). In the present study, the slightly negative charge of the SWCNT-Cur could enable the drug to arrive at the “front of door” of every cell in target organs through the rich capillary network of the body following intravascular administration.
Loading efficiency of curcumin
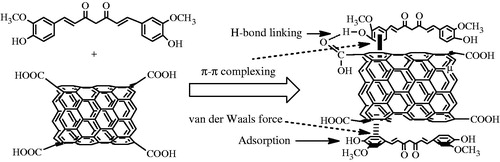
Based on the measured concentration of curcumin in the SWCNT-Cur conjugates of 1.88 mg/mL, the loading efficiency of curcumin in the SWCNT-Cur was estimated to be as high as 94.0%. This high loading efficiency can be attributed to the following factors (). First, in methanol, curcumin exists in a molecular state that fully exposes it to the surface of the SWCNTs. Further, due to the large surface areas of the SWCNTs and the molecular structure of curcumin, which contains two benzene rings and a conjugated ethylenic linkage, the energy of the ultrasonic bath and the ultrasonic probe allowed the drug to easily adsorb onto the SWCNTs or attach to the SWCNTs through π–π complexing with the benzene ring and the formation of hydrogen bonds between the carboxyl groups of the functionalized SWCNTs and the phenolic hydroxyl group of curcumin. In addition, the van der Waals forces between curcumin and the SWCNTs enhanced the loading of curcumin because both curcumin and the SWCNTs are hydrophobic. During the preparation of the conjugates, we observed that the frequency, power and time of the ultrasonication exerted a great impact on the drug-loading efficiency, which may validate the proposed mechanism.
Curcumin release from SWCNT-Cur and the underlying mechanism
Drug release is critical to its activity, and, therefore, we determined the release of curcumin from our formulation in vitro. Our results demonstrated that a high curcumin concentration could be achieved within a relatively short time period and maintained for hours; nearly 95% of the curcumin was released from the SWCNT-Cur into the medium after 1 h. The cumulative release rate of curcumin exhibited a slightly downward trend at 12 h (), which can be explained by the degradation of curcumin.
Figure 5. Curcumin release from the SWCNTs-Cur and the underlying mechanism. (A) In vitro release. (B) X-ray diffraction patterns of different forms of curcumin: ① native curcumin, ② SWCNTs-Cur and ③ physical mixture of curcumin and void vector. Curcumin existed in an amorphous form. (C) UV-Vis spectra of curcumin, SWCNTs and SWCNTs-Cur.
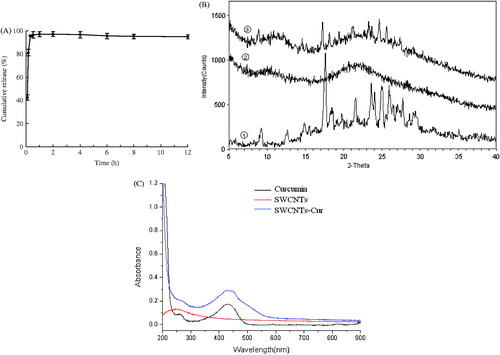
Various studies have shown that drugs present in an amorphous molecular state in medicinal formulations exhibit enhanced release and increased bioavailability (Vasconcelos et al., Citation2007; Kim et al., Citation2011). To determine whether the rapid release of curcumin from SWCNT-Cur was related to its molecular form, XRD patterns of the formulation were measured. The result in demonstrates that the powder of native curcumin exhibited characteristic high-intensity diffraction peaks at 2θ values of 9.20°, 14.84°, 17.60°, 18.44°, 19.76°, 21.57°, 23.61°, 24.92°, 25.03°, 25.92° and 27.04°, in accordance with a previous report (Mohanty & Sahoo, Citation2010), indicating that the native curcumin was present in a highly crystalline form. The physical mixture of curcumin, SWCNTs and PVP K30 exhibited most of the characteristic peaks of native curcumin, indicating that the highly crystalline structure of the curcumin molecules in the physical mixture and the vector did not obscure its traits. However, none of the characteristic peaks of curcumin were detected in the conjugates of the SWCNTs and Cur, which contained the same proportion of curcumin, SWCNTs and PVP K30 as the physical mixture. The absence of the crystalline curcumin peaks in the SWCNT-Cur demonstrated the phase transition of curcumin from a crystalline structure to an amorphous form. Meanwhile, the UV-vis spectra of the SWCNT-Cur retained the characteristic absorption peaks of native curcumin, further verifying the presence of curcumin molecules in the SWCNT-Cur and providing direct evidence that curcumin was in an amorphous form (). Therefore, the rapid release of the curcumin in the SWCNT-Cur can be attributed to the following factors. First, the SWCNT-Cur were in a well-dispersed state after being dissolved (). Then, the single SWCNTs rather than the bundles () attached to curcumin quickly filled with the solution. Furthermore, the amorphous form of curcumin resulted in enhanced release by facilitating the diffusion of the drug molecules from the substrate (). This principle is similar to solid dispersion formulations.
Influence of SWCNT-Cur on cell inhibition in PC-3 cells
Enhancement of curcumin anti-tumor activity
presents the in vitro cell inhibition of curcumin. The results revealed that treatment with the void vector SWCNTs did not cause any significant changes in cell viability (p > 0.05), and the low concentrations of SWCNT-Cur (5 μM) and curcumin (15 μM) exhibited no significant cytotoxicity toward the PC-3 cells (p > 0.05). However, exposure of the PC-3 cells to high concentrations of SWCNT-Cur (15, 40 μM) led to a dose-dependent and time-dependent increase in the cell inhibition rate, and the 40 μM curcumin-treated group also exhibited a significant increase in the cell inhibition rate compared with the blank group (p < 0.05). In addition, the 15 and 40 μM SWCNT-Cur groups displayed better inhibition potency than the respective curcumin group with significant differences between them (p < 0.05). These results suggested that with the help of the delivery system we developed, the SWCNT-Cur enabled low concentrations of curcumin, which were unable to inhibit the PC-3 cells, to become effective and further increased the cytotoxicity of high concentrations of curcumin.
Figure 6. SWCNTs-Cur enhanced the growth inhibition of curcumin in PC-3 cells. (A) Dose-dependent inhibition for various concentrations and times. **p < 0.01 versus control group, #p < 0.05, ##p < 0.01 versus respective curcumin concentration group. (B) Laser-induced enhancement of cell growth inhibition for 40 μM SWCNTs-Cur toward PC-3 cells. The inset depicts the effect of the SWCNTs on cell growth inhibition under 808 nm laser irradiation for different time periods. *p < 0.05, **p < 0.01 versus control group without laser irradiation, ▵p < 0.05, ▵▵p < 0.01 versus control group with laser irradiation, #p < 0.05 versus respective group without laser irradiation.
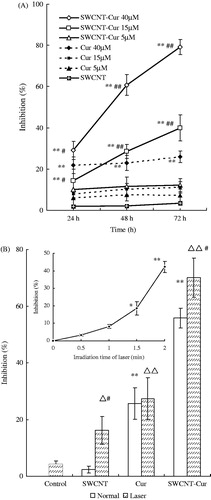
The non-covalent functionalization of the SWCNTs with PC and PVP, i.e., the void vector SWCNTs, exhibited greater biocompatibility and lacked any obvious toxicity in the PC-3 cells (), in agreement with our earlier results from the functionalized SWCNTs with biocompatible PC and PVP surface groups () and higher dispersity ().
The mechanism underlying the more efficient anti-tumor effect of the SWCNT-Cur compared with curcumin alone can be ascribed to the increased solubility and dispersibility of the conjugates as well as the decreased degradation of curcumin in the formulation. Furthermore, the unique ability of SWCNTs to be readily internalized by mammalian cells via endocytosis (Kam et al., Citation2006), phagocytosis (Cherukuri et al., Citation2004) and/or diffusion (Pantarotto et al., Citation2004) has been demonstrated by an increasing number of studies. During SWCNT internalization, their cargo is also internalized. In addition, PC can easily insert into the phospholipid bilayer that comprises mammalian cell membranes, enhancing the delivery of the curcumin in the SWCNT-Cur from across the cell membrane to the inside of the cell. In addition, after the SWCNT-Cur are internalized in the cell, the rapid release of curcumin may be beneficial in quickly achieving a high concentration of the drug.
Further photothermal ablation
Because the curcumin and the SWCNT-Cur at 40 μM exhibited a significant increase in the cell inhibition rate compared with the blank group, the 40 μM curcumin concentration was selected for further evaluation of the effect of irradiation on cell inhibition. The inhibition rate for PC-3 cells irradiated under 808 nm light was found to be time-dependent. At an irradiation time of 1.5 min, the SWCNTs induced a marked decrease in survival compared with the un-irradiated cells (p < 0.05, , inset). A radiation time of 1.5 min and a SWCNT-Cur treatment of 4 h were therefore used for further cell studies. Under 808 nm laser irradiation, the SWCNT-Cur induced a significantly higher cell inhibition rate compared with that of the SWCNT-Cur without irradiation (p < 0.05), with inhibition rates of 70.1% and 55.8%, respectively. At the same time, there was no significant difference in the cell inhibition rate between the curcumin with and without irradiation groups (). These results suggest that the SWCNT-Cur conjugates can further improve the antitumor effects of curcumin in vitro by harnessing the photothermal effect of SWCNTs.
Conclusion
A curcumin delivery system using SWCNTs non-covalently functionalized with PC and PVP was formulated as SWCNT-Cur. The nanoscale SWCNT-Cur not only increased the solubility of curcumin in aqueous media but also protected curcumin from degradation and rapidly released curcumin by presenting it in an amorphous form in the formulation. In human prostate cancer PC-3 cells, the SWCNT-Cur enhanced the anti-tumor activity of curcumin. Moreover, thermal therapy mediated by the SWCNTs further improved the ability of SWCNT-Cur to ablate tumor cell growth. These findings suggest that SWCNT-Cur may be a promising curcumin delivery system in cancer therapies.
Declaration of interest
The authors declare no conflicts of interest.
Supplementary Material
Download PDF (284 KB)Acknowledgements
The study was supported by National Natural Science Foundation of China No. 30973482 and No. 30973660.
References
- Anand P, Kunnumakkara AB, Newman RA, et al. (2007). Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–18
- Anand P, Sundaram C, Jhurani S, et al. (2008). Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 267:133–64
- Cao F, Ding B, Sun M, et al. (2011). Lung-targeted delivery system of curcumin loaded gelatin microspheres. Drug Deliv 18:545–54
- Cherukuri P, Bachilo SM, Litovsky SH, et al. (2004). Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J Am Chem Soc 126:15638–9
- Didenko VV, Moore VC, Baskin DS, et al. (2005). Visualization of individual single-walled carbon nanotubes by fluorescent polymer wrapping. Nano Lett 5:1563–7
- Duvoix A, Blasius R, Delhalle S, et al. (2005). Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223:181–90
- Gaillard C, Duval M, Dumortier H, et al. (2009). Carbon nanotube-coupled cell adhesion peptides are non-immunogenic: a promising step toward new biomedical devices. J Peptide Sci 17:139–42
- Gao Y, Li Z, Sun M, et al. (2011). Preparation and characterization of intravenously injectable curcumin nanosuspension. Drug Deliv 18:131–42
- Huang D, Ding Y, Li Y, et al. (2006). Anti-tumor activity of a 3-oxo derivative of oleanolic acid. Cancer Lett 233:289–96
- Jain KK. (2012). Advances in use of functionalized carbon nanotubes for drug design and discovery. Expert Opin Drug Discov 7:1029–37
- Kam NW, Liu Z, Dai H. (2006). Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem Int Ed Engl 45:577–81
- Karmali PP, Chaudhuri A. (2007). Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med Res Rev 27:696–722
- Kim MS, Kim JS, Park HJ, et al. (2011). Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int J Nanomed 6:2997–3009
- Kurien BT, Scofield RH. (2009). Heat-solubilized curcumin should be considered in clinical trials for increasing bioavailability. Clin Cancer Res 15:747
- Lakkaraju A, Dubinsky JM, Low WC, et al. (2001). Neurons are protected from excitotoxic death by p53 antisense oligonucleotides delivered in anionic liposomes. J Biol Chem 276:32000–7
- Lanone S, Andujar P, Kermanizadeh A, et al. (2013). Determinants of carbon nanotube toxicity. Adv Drug Deliv Rev. http://dx.doi.org/10.1016/j.addr. 2013.07.019
- Liang G, Shao L, Wang Y, et al. (2009). Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem 17:2623–31
- Liu Z, Chen K, Davis C, et al. (2008). Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68:6652–60
- Meng L, Zhang X, Lu Q, et al. (2012). Single walled carbon nanotubes as drug delivery vehicles: targeting doxorubicin to tumors. Biomaterials 33:1689–98
- Mohanty C, Sahoo SK. (2010). The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 31:6597–611
- Moon HK, Lee SH, Choi HC. (2009). In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 3:3707–13
- Pantarotto D, Briand JP, Prato M, et al. (2004). Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) (1):16–7
- Prato M, Kostarelos K, Bianco A. (2008). Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res 41:60–8
- Rahman S, Cao S, Steadman KJ, et al. (2012). Native and beta-cyclodextrin-enclosed curcumin: entrapment within liposomes and their in vitro cytotoxicity in lung and colon cancer. Drug Deliv 19:346–53
- Sedlacek HH. (2001). Pharmacological aspects of targeting cancer gene therapy to endothelial cells. Crit Rev Oncol Hematol 37:169–215
- Shukla S, Zaher H, Hartz A, et al. (2009). Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res 26:480–7
- Vasconcelos T, Sarmento B, Costa P. (2007). Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today 12:1068–75
- Villa CH, Mcdevitt MR, Escorcia FE, et al. (2008). Synthesis and biodistribution of oligonucleotide-functionalized, tumor-targetable carbon nanotubes. Nano Lett 8:4221–8
- Wang L, Zhang M, Zhang N, et al. (2011). Synergistic enhancement of cancer therapy using a combination of docetaxel and photothermal ablation induced by single-walled carbon nanotubes. Int J Nanomed 6:2641–52
- Xu DH, Wang S, Mei XT, et al. (2008). Studies on solubility enhancement of curcumin by Polyvinylpyrrolidione K30. Zhong Yao Cai 31:438–42
- Yallapu MM, Jaggi M, Chauhan SC. (2012). Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today 17:71–80