Abstract
Context: C1-esterase inhibitor (C1-inh) therapy is currently administered to patients with C1-inh deficiency through intravenous injections. The possibility of subcutaneous administration is currently being explored since this would alleviate need for hospitalization and increase mobility and well-being of patients. Recently, it was observed in pigs that C1-inh indeed can effectively be applied by subcutaneous injection. For studies on the effectiveness of C1-inh therapy for other indications than acquired and hereditary angioedema, rats are commonly used as model animal. For rats, however, subcutaneous C1-inh administration has never been investigated.
Objective: To evaluate the efficacy of subcutaneous C1-inh administration in rats.
Materials and methods: Three boli of 100 U/kg human plasma-derived C1-inh were administered to Wistar rats on three consecutive days through subcutaneous injection or intravenous injection. Blood samples were collected from the tail veins 3, 4.5 or 6 h after C1-inh administration for measurement of C1-inh plasma levels. Antigen and activity levels of C1-inh of each plasma sample were determined by means of a specific ELISA.
Results: For both C1-inh antigen and C1-inh activity, 21- to 119-fold higher plasma levels were measured after intravenous administration compared with subcutaneous administration. Subcutaneous administration also resulted in C1-inh plasma levels that were less stable and with decreased relative activity.
Conclusion: These combined results indicate that in rats, subcutaneous injections in the present formulation are not effective as alternative administration route for C1-inh.
Introduction
C1-esterase inhibitor (C1-inh) is an anti-inflammatory protease inhibitor which inhibits activation of the classical and lectin pathways of the complement system (Sim et al., Citation1979; Kerr et al., Citation2008). Other reported functions include interference with the activation of the kallikrein–kinin, fibrinolytic and coagulation system (Gigli et al., Citation1970; Schreiber et al., Citation1973) and inhibition of leukocyte adhesion to endothelial cells (Cai et al., Citation2005). A detailed review of C1-inh functions has been published elsewhere (Zeerleder, Citation2011). Therapeutic administration of C1-inh is clinically applied in the context of hereditary angioedema (HAE), which is hallmarked by heterozygous C1-inh deficiency (Donaldson & Evans, Citation1963). Randomized controlled trials in patients demonstrated that C1-inh administration effectively decreased the severity of HAE-episodes, without having serious side-effects (Kunschak et al., Citation1998; Craig et al., Citation2009).
Potential alternative therapeutic applications of C1-inh are investigated in animal disease models in which inflammation and especially complement activation play an important pathogenic role. These models include feline (Buerke et al., Citation1995) and rat (Buerke et al., Citation1998; Fu et al., Citation2006a,Citationb) models of ischemia-reperfusion damage, a mouse model of sepsis (Liu et al., Citation2007), a rat model of meningitis (Zwijnenburg et al., Citation2007), rat and mouse models of pancreatitis (Niederau et al., Citation1995), a rat model of burn wounds (Begieneman et al., Citation2012) and a mouse model of vein graft injury (Krijnen et al., Citation2012).
In patients and animal models, C1-inh therapy is applied through intravenous injections. Recently, an in vivo study using a porcine model showed that similar plasma levels of C1-inh were achieved in pigs that received C1-inh subcutaneously, compared with intravenous administration (Jiang et al., Citation2010). Interestingly, the plasma levels were more stable after subcutaneous administration, suggesting that subcutaneous C1-inh might have a depot function. The clinical use of subcutaneous C1-inh administration is of great interest for patients (Zuraw, Citation2010). Subcutaneous administration might result in more stable plasma levels in patients and would be more convenient by increasing mobility and decreasing dependency on professional infusions. Especially patients that require frequent C1-inh injections over a prolonged period (either prophylactic or therapeutic) would strongly benefit from this possibility.
As rats are commonly used as animal model for different C1-inh-related studies, we investigated in this study whether C1-inh plasma levels in rats have a comparable level and stability after subcutaneous administration compared with plasma levels after intravenous administration.
Methods
Animals
Ten-week-old male Wistar rats (n = 10) were purchased from Charles River Laboratories (Sulzfeld, Germany), and transported in an air-filtered cage in a climate-controlled van. The rats were specific pathogen free (SPF), as part of the Charles River routine health monitoring programme, following FELASA recommendations. The rats were kept in a closed system at the Laboratory Animal Center at the VU University Medical Center (AARC, Amsterdam, the Netherlands) under constant temperature (21–22 °C), humidity (60–65%) and light–dark periodicity (L:D 12:12 h, lights on from 07:00 to 19:00 with an intensity of 55–60 lux). The rats were housed in groups of five in polycarbonate type 4 cages (1815 cm2 surface area, Bayer, Leverkusen, Germany) with wood chips as bedding and enriched with paper nesting material. Food pellets (2016 Teklad Global 16% Protein Rodent Diet, Harlan Laboratories, Indianapolis, IN) and water (autoclaved filtered tap water) was provided to the rats ad libitum. Experimental procedures started after 1 week of acclimatization, at which point the animals were weighed. Rats in the intravenous administration group had a similar weight (418 ± 7 g) as the rats in the subcutaneous group (414 ± 7 g). All experimental procedures were approved by the local Animal Ethical Committee of the VU University Medical Center, in agreement with EU Directive 2010/63/EU.
Experimental procedures
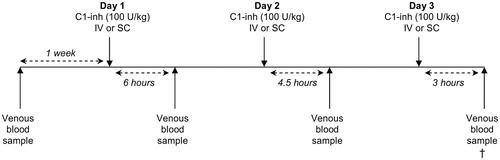
The experiment is displayed schematically in . The rats received boluses of 100 U/kg bodyweight (bw) of human plasma-derived C1-inh (Cetor®, Sanquin, Amsterdam, The Netherlands) on three consecutive days. C1-inh was administrated either through intravenous injection (n = 5) in the lateral tail vein using a mild anesthetic (3% isoflurane), or through subcutaneous injection (n = 5). To measure the changes in C1-inh plasma levels over time after intravenous and subcutaneous administration, venous blood samples were taken through a small cut in the lateral tail vein 6 h after C1-inh administration on day 1, 4.5 h after C1-inh administration on day 2 and 3 h after C1-inh administration on day 3. Blood samples were taken on different time points on different days as withdrawing blood three times from one animal in a 3-h period raises practical and ethical issues. As a control, blood was also taken prior to the first C1-inh injection.
Figure 1. Schematic overview of the experiment. Rats received C1-esterase inhibitor (C1-inh) boluses intravenously (IV) or subcutaneously (SC) on days 1, 2 and 3. Venous blood samples were collected 3, 4.5 or 6 h after C1-inh administration, and 1 week beforehand. Cross symbol: termination of experiment, after last venous blood sample.
C1-esterase inhibitor plasma measurements
To obtain plasma, blood samples were collected in Microvette® heparin-coated vials (Sarstedt, Nümbrecht, Germany) and centrifuged for 10 min at 2250 g immediately after blood withdrawal. Plasma samples were stored at −80 °C. C1-inh antigen levels and C1-inh activity were measured in plasma by an enzyme-linked immunosorbent assay (ELISA) as described before (de Smet et al., Citation1993; Bos et al., Citation2001). 96-Well Maxisorp™ plates (Nunc, Roskilde, Denmark) were coated with a monoclonal antibody against human C1-inh (RII, Sanquin, Amsterdam, the Netherlands, 100 μl per well, 2 μg/ml) for 2 h. Plates were washed five times in an Elx405 plate washer (BioTek®, Winooski, VT) with PBS containing 0.02% Tween. Next, plasma samples were diluted (1:1000, 1:5000 and 1:25 000) in PBS with 0.1% Tween and 0.2% gelatin, and incubated for 1 h (100 μl per well). As a reference, a serial dilution of purified human C1-inh (Cetor®) was used. The plates were washed again and incubated either with 125 ng/ml biotinylated C1s (Calbiochem®, EMD Millipore, Billerica, MA) for C1-inh activity, or with biotinylated polyclonal rabbit α-C1-inh antibody (Sanquin, Amsterdam, The Netherlands) for C1-inh antigen levels, as described previously (Bos et al., Citation2001). After a wash, the plates were incubated (30 min, 100 µl per well) with either Streptavidin-HRP (GE Healthcare, Little Chalfont, UK), diluted 1:1000 in HPE-buffer for the α-C1-inh antibody or poly-HRP (Sanquin, Amsterdam, The Netherlands) diluted 1:10 000 in HPE-buffer for C1s. The plates were washed again, and 0.1 mg/ml TMB, dissolved in 0.11 M NaAc (pH 5.5) +0.003% H2O2, was added (100 μl per well). 2 M H2SO4 was added to stop the reaction (100 μl per well). The plates were then analyzed in a Multiskan Ex plate reader (Thermo Fischer Scientific, Waltham, MA), set to 450/540 nm.
Statistical analysis
Data were analyzed using a one-way ANOVA with a Bonferroni’s multiple comparison test. A p value <0.05 was considered statistically significant. Prism v4.0 (GraphPad Software, La Jolla, CA) was used for the statistical analysis. Data values are displayed as Mean ± Standard Error of the Mean (SEM).
Results
Intravenous administration of human C1-inh results in higher plasma levels, compared with subcutaneous administration
Rats received C1-inh boluses on three consecutive days. As it is known that administered human C1-inh in rats has a half-life of roughly 4.5 h (de Smet et al., Citation1993), C1-inh present in plasma from boluses administered the day before is expected to have had a negligible influence on plasma measurements. Human C1-inh antigen plasma levels of each rat measured before C1-inh administration (control) and after 6 h on day 1, 4.5 h on day 2 and 3 h on day 3, are displayed in .
Figure 2. C1-esterase inhibitor (C1-inh) antigen levels in plasma of rats 0 (control), 3, 4.5 and 6 h after C1-inh after intravenous (IV, closed symbols) or subcutaneous (SC, open symbols) administration. Each point in the graph represents the value of one individual plasma sample. Horizontal lines between the points represents the average value. ***p < 0.001.
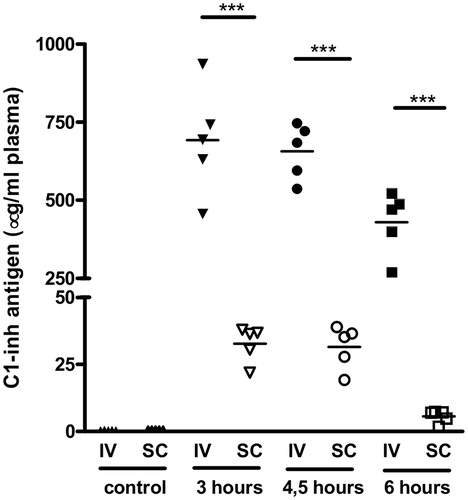
No C1-inh was detected in control samples, indicating that the antibodies used for the detection of human C1-inh were specific and did not cross-react with rat C1-inh. In the rats from the intravenous group, antigen plasma levels at 3, 4.5 and 6 h after C1-inh administration were 692 ± 78, 657 ± 40 and 430 ± 45 µg/ml, respectively. In the subcutaneous group, the C1-inh antigen plasma levels at 3, 4.5 and 6 h after administration were significantly lower, namely 33 ± 3, 32 ± 4 and 5.6 ± 1.1 µg/ml, respectively (p < 0.001). As such, plasma levels after intravenous administration were 21-fold higher compared with subcutaneous administration 3 h (day 3) and 4.5 h (day 2) after C1-inh administration. C1-inh plasma levels at 6 h (day 1) were even 77-fold higher after intravenous compared with subcutaneous administration.
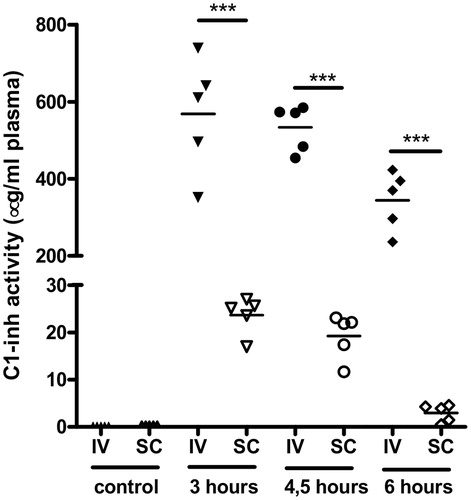
Next, the level of C1-inh activity in the same plasma samples were measured, as displayed in . In the rats from the intravenous group, active C1-inh plasma levels 3, 4.5 and 6 h after C1-inh administration were 568 ± 67, 533 ± 27 and 344 ± 34 µg/ml, respectively. In the rats in the subcutaneous group, the active C1-inh plasma levels 3, 4.5 and 6 h after C1-inh administration were significantly lower (p < 0.001), namely 24 ± 2, 19 ± 2 and 2.9 ± 0.8 µg/ml, respectively. After 3 h, intravenous administration resulted in 24-fold higher level of C1-inh activity in plasma compared with subcutaneous administration. This difference increased to 28-fold after 4.5 h and 119-fold after 6 h. In summary, both C1-inh antigen and C1-inh activity plasma levels were significantly lower after subcutaneous compared with intravenous administration.
Figure 3. C1-esterase inhibitor (C1-inh) activity in plasma of rats 0 (control), 3, 4.5 and 6 h after C1-inh after intravenous (IV, closed symbols) or subcutaneous (SC, open symbols) administration. Each point in the graph represents the value of one individual plasma sample. Horizontal lines between the points represents the average value. ***p < 0.001.
Subcutaneous administration results in C1-inh plasma levels that are less stable and less active compared with intravenous administration
To determine the stability of C1-inh plasma levels after intravenous and subcutaneous administration, we investigated the decrease in plasma levels between 3 and 4.5 h after administration, and between 4.5 and 6 h after administration. The plasma levels measured 4.5 h after intravenous administration (day 2) for C1-inh antigen and C1-inh activity were 5% and 6% lower, respectively, compared to 3 h (day 3), and 3% and 21% lower, respectively, after subcutaneous administration. When comparing plasma levels between 4.5 (day 2) and 6 h (day 1) after C1-inh administration, the antigen levels and activity at 6 h were both 35% lower after intravenous administration, and respectively 82% and 85% lower after subcutaneous administration. This difference between administration routes indicates that plasma levels of human C1-inh are more stable after intravenous administration, compared to subcutaneous administration.
C1-inh activity was lower compared with the C1-inh antigen levels of corresponding time points for the two administration route. The amount of C1-inh antigen that can actively bind C1s 3, 4.5 and 6 h after intravenous administration remains equal, namely 82%, 81% and 80%, respectively. After subcutaneous administration however, the relative amount of C1-inh activity is lower and decreases in time, with 73%, 59% and 52% of C1-inh antigen levels, respectively, 3, 4.5 and 6 h after administration. This indicates that subcutaneous administration of C1-inh not only inhibits transport of C1-inh to the systemic circulation, but disrupts the activity of the protein as well.
In summary, subcutaneous C1-inh administration does not only result in lower C1-inh antigen and activity plasma levels, but also in plasma levels that are less stable and with a decreased relative activity.
Discussion
Subcutaneous administration of C1-inh in a clinical setting is a topic of interest (Zuraw, Citation2010). Jiang et al. (Citation2010) investigated subcutaneous administration of human C1-inh in a porcine model, in which C1-inh plasma levels were similar after subcutaneous and intravenous administration. In the current study, we investigated the efficacy of subcutaneous administration of human C1-inh in rats and observed that, in contrast to observations made in the porcine model, plasma levels were considerably lower, less stable and with a lower relative activity after subcutaneous administration, compared with intravenous administration.
In our study, plasma concentrations of C1-inh were between 21- and 119-fold lower after subcutaneous administration, which indicates that subcutaneous injection is a less effective method of C1-inh administration in rat models. Jiang et al. (Citation2010) also observed that C1-inh plasma levels were more stable after subcutaneous administration, compared with intravenous administration. In contrast, we observed that the plasma levels were declining more rapidly after subcutaneous administration, which indicates that the lower plasma levels after subcutaneous administration is not likely caused by delayed transfer of C1-inh from the subcutaneous compartment.
It remains unclear why in our study, subcutaneous C1-inh is not transported to the systemic circulation as effectively as compared to the observations made in the porcine model (Jiang et al., Citation2010). There are some differences in methodology between both studies, though this provides no evincive explanation. Jiang et al. used a different brand of purified plasma-derived human C1-inh (Cinryze®, ViroPharma Incorporated, Exton, PA) than we did (Cetor®), though the only difference between both brands lies in the human donor populations from which the compound is isolated (Cinryze® originates from American donors and Cetor® from European donors). In addition, the C1-inh dosage differed between both studies, though this provides no likely explanation as well as the animals in our study received a higher dose (100 U/kg bw in a single bolus) compared to the porcine model (50 U/kg bw over a 30-min infusion).
It is more likely that human C1-inh is either degraded rapidly in the subcutaneous compartment of the rat, or is otherwise prevented from entering the systemic circulation from the subcutaneous compartment. Similar pharmacokinetic studies in rats for other proteins found comparable plasma levels after subcutaneous and intravenous administration, with higher initial levels after intravenous administration, but more stable levels after subcutaneous administration (Woo et al., Citation2006; Gedulin et al., Citation2008). This indicates that the inefficacy of subcutaneous administration is specific for C1-inh and not a general problem of rats.
It is known that C1-inh binds extracellular matrix proteins (Patston & Schapira, Citation1997). It may therefore be possible that human C1-inh cross-reacts with extracellular matrix proteins in the subcutaneous compartment of rats, thereby inhibiting effective transport to the systemic circulation. As the remaining C1-inh that reaches the systemic circulation after subcutaneous administration in our study has become less active in the subcutaneous compartment, components that actively inactivate C1-inh may also play a role. In contrast, Liu et al. (Citation2003) observed that intraperitoneal C1-inh administration in a mouse model of endotoxin shock increases survival rates similar to intravenous C1-inh administration, suggesting successful transfer of C1-inh to the systemic circulation, even though plasma levels were not measured in this study. Whether this difference is due to dissimilarities in subcutaneous and intraperitoneal compartments, blood vessel permeability changes induced by the endotoxemia or differences between mouse and rat (or a combination of these factors) remains to be determined.
Subcutaneous administration of C1-inh would be especially beneficial for patients with frequent HAE episodes, who receive C1-inh injections usually as often as twice a week as prophylaxis (Zuraw & Kalfus, Citation2012). However, clinical trials on subcutaneous C1-inh administration have not been published thus far. Subcutaneous C1-inh administration in rats would be preferable as well, as besides the homogeneity between animal models and the clinical situation, subcutaneous administration also has practical benefits for these animal models. As C1-inh is a compound with a short half-life in animal models, C1-inh administration in murine models often occurs at least once a day (Niederau et al., Citation1995; Buerke et al., Citation1998; Liu et al., Citation2003, Citation2007; Croner et al., Citation2004; Fu et al., Citation2006a,Citationb; Begieneman et al., Citation2012). Prolonged treatment periods using intravenous injections contain a risk of damage to the lateral tail veins due to excessive puncturing. Furthermore, intravenous injections are more burdensome for the animal compared with subcutaneous injections.
Conclusion
We observed that in rats compared with the traditional intravenous injections C1-inh administration through subcutaneous injection results in much lower and less stable C1-inh plasma levels, with a lower relative activity. This indicates that subcutaneous injections are not suitable to replace intravenous injections in studies where C1-inh is administered to rats.
Declaration of interest
The authors report no declarations of interest. This work was supported by an unrestricted grant from ViroPharma Incorporated.
References
- Begieneman MP, Kubat B, Ulrich MM, et al. (2012). Prolonged C1 inhibitor administration improves local healing of burn wounds and reduces myocardial inflammation in a rat burn wound model. J Burn Care Res 33:544–51
- Bos IG, van Mierlo GJ, Bleeker WK, et al. (2001). The potentiation of human C1-inhibitor by dextran sulphate is transient in vivo: studies in a rat model. Int Immunopharmacol 1:1583–95
- Buerke M, Murohara T, Lefer AM. (1995). Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation 91:393–402
- Buerke M, Prufer D, Dahm M, et al. (1998). Blocking of classical complement pathway inhibits endothelial adhesion molecule expression and preserves ischemic myocardium from reperfusion injury. J Pharmacol Exp Ther 286:429–38
- Cai S, Dole VS, Bergmeier W, et al. (2005). A direct role for C1 inhibitor in regulation of leukocyte adhesion. J Immunol 174:6462–6
- Craig TJ, Levy RJ, Wasserman RL, et al. (2009). Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 124:801–8
- Croner RS, Lehmann TG, Fallsehr C, et al. (2004). C1-inhibitor reduces hepatic leukocyte-endothelial interaction and the expression of VCAM-1 in LPS-induced sepsis in the rat. Microvasc Res 67:182–91
- Donaldson VH, Evans RR. (1963). A biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C′ 1-esterase. Am J Med 35:37–44
- Fu J, Lin G, Wu Z, et al. (2006a). Anti-apoptotic role for C1 inhibitor in ischemia/reperfusion-induced myocardial cell injury. Biochem Biophys Res Commun 349:504–12
- Fu J, Lin G, Zeng B, et al. (2006b). Anti-ischemia/reperfusion of C1 inhibitor in myocardial cell injury via regulation of local myocardial C3 activity. Biochem Biophys Res Commun 350:162–8
- Gedulin BR, Smith PA, Jodka CM, et al. (2008). Pharmacokinetics and pharmacodynamics of exenatide following alternate routes of administration. Int J Pharm 356:231–8
- Gigli I, Mason JW, Colman RW, Austen KF. (1970). Interaction of plasma kallikrein with the C1 inhibitor. J Immunol 104:574–81
- Jiang H, Zhang HM, Frank MM. (2010). Subcutaneous infusion of human C1 inhibitor in swine. Clin Immunol 136:323–8
- Kerr FK, Thomas AR, Wijeyewickrema LC, et al. (2008). Elucidation of the substrate specificity of the MASP-2 protease of the lectin complement pathway and identification of the enzyme as a major physiological target of the serpin, C1-inhibitor. Mol Immunol 45:670–7
- Krijnen PA, Kupreishvili K, de Vries MR, et al. (2012). C1-esterase inhibitor protects against early vein graft remodeling under arterial blood pressure. Atherosclerosis 220:86–92
- Kunschak M, Engl W, Maritsch F, et al. (1998). A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion 38:540–9
- Liu D, Cai S, Gu X, et al. (2003). C1 inhibitor prevents endotoxin shock via a direct interaction with lipopolysaccharide. J Immunol 171:2594–601
- Liu D, Lu F, Qin G, et al. (2007). C1 inhibitor-mediated protection from sepsis. J Immunol 179:3966–72
- Niederau C, Brinsa R, Niederau M, et al. (1995). Effects of C1-esterase inhibitor in three models of acute pancreatitis. Int J Pancreatol 17:189–96
- Patston PA, Schapira M. (1997). Regulation of C1-inhibitor function by binding to type IV collagen and heparin. Biochem Biophys Res Commun 230:597–601
- Schreiber AD, Kaplan AP, Austen KF. (1973). Inhibition by C1INH of Hagemann factor fragment activation of coagulation, fibrinolysis, and kinin generation. J Clin Invest 52:1402–9
- Sim RB, Arlaud GJ, Colomb MG. (1979). C1 inhibitor-dependent dissociation of human complement component C1 bound to immune complexes. Biochem J 179:449–57
- de Smet BJ, de Boer JP, Agterberg J, et al. (1993). Clearance of human native, proteinase-complexed, and proteolytically inactivated C1-inhibitor in rats. Blood 81:56–61
- Woo S, Krzyzanski W, Jusko WJ. (2006). Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther 319:1297–306
- Zeerleder S. (2011). C1-inhibitor: more than a serine protease inhibitor. Semin Thromb Hemost 37:362–74
- Zuraw BL. (2010). HAE therapies: past present and future. Allergy Asthma Clin Immunol 6:23
- Zuraw BL, Kalfus I. (2012). Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am J Med 125:938 e1–7
- Zwijnenburg PJ, van der Poll T, Florquin S, et al. (2007). C1 inhibitor treatment improves host defense in pneumococcal meningitis in rats and mice. J Infect Dis 196:115–23
