Abstract
In this study, topical ethosomal formulation of capsaicin was prepared and evaluated for bio-efficacy in arthritic rats. Physical and biological characterizations of prepared capsaicin-loaded nano vesicular systems were also carried out. Ethosomal capsaicin showed significant reduction of rat paw edema along with promising antinociceptive action. The topical antiarthritic efficacy of prepared formulation of capsaicin was found more than that of Thermagel, a marketed gel of capsaicin. From toxicological study, no predictable signs of toxicity such as skin irritation (of experimental rats) were observed. Based on this finding, ethosomal capsaicin could be proposed as an effective as well as a safe topical delivery system for the long-term treatment of arthritis and associated inflammo-musculoskeletal disorders. Such exciting result would eventually enlighten the analgesic and anti-inflammatory potential of capsaicin for topical remedy.
Introduction
Capsaicin, the active ingredient in hot chili peppers, activates neurons which transmit pain signals to the brain. Capsaicin causes a temporary degradation of causes a temporary degradation of pain-sensing endings and thereby prevents neurons from transmitting pain, which is expected to cause long-lasting pain relief. Capsaicin is a well-known molecule in skeletomuscular disorder, numerous studies have been done over the antiarthritic activity of capsaicin (Backonja et al., Citation2010).
Capsaicin is now currently considered to be a safe and effective topical analgesic that produces antiarthritic, antioxidant and even anticancer properties. Analgesic uses of capsaicin have increased over the last century, where a major breakthrough was made in the treatment of herpes zoster. This breakthrough led to further research into the structure and mechanism of action of capsaicin to determine its method of providing analgesia (Fusco & Giacovazzo, Citation1997). The Osteoarthritis Research International recommends topical agents and capsaicin as effective adjunctive or alternatives to oral analgesic/anti-inflammatory agents, for patients with moderate-to-severe pain and inflammation who do not respond satisfactorily to oral analgesic/anti-inflammatory agents (Zhang et al., Citation2008). On the other hand, adverse event associated with capsaicin may restrict the success of capsaicin as first line choice to manage the skeletomuscular disorders.
Capsaicin binds to nociceptors in the skin, causing an initial excitation of the neurons and a period of enhanced sensitivity to noxious stimuli, usually perceived as itching, pricking or burning sensation. This is followed by a refractory period with reduced sensitivity and, after upon repeated application, persistent desensitization. Injection of capsaicin directly into the limited joint space is expected to yield high local concentrations of capsaicin at the site of action and low systemic distribution, but some investigators reported that oral and intravenous administration of these molecule showed higher degree of first-pass metabolism in rats and mice (Donnerer et al., Citation1990). Moreover, half-life of capsaicin by intravenous administration from rats was also detected very shortly (7.06 min) (Kawada et al., Citation1995).
These two limitations are the basic barrier for oral and intravenous administration of this important therapeutic molecule in arthritic pain. Therefore, topical administration is another possible approach for this molecule, which circumvents the hepatic metabolism and achieves better bioavailability. At a same time dose-dependent irritation effect accompanied by sting, prick and burn is a criteria for selection of the appropriate topical dosage form. In contrast to capsaicin for musculoskeletal pain from simple ligament injuries (e.g. ankle sprains) to intra-articular disorders (e.g. osteoarthritis and rheumatoid arthritis), penetration of capsaicin is necessary for therapeutic action.
Lipoidal carriers are well-accepted carriers for topical administration of drug molecules. From the last four decades a large modification has been done to its composition which altered its properties and added advantages, spatiality on stability and penetration ability. Ethosomes are a member of this lipoidal carrier family which have great ability to deliver molecules in to deeper skin and blood circulation. Previous results are greatly supports for this ability (Sarwa et al., Citation2013b).
Ethosomes are lipid vesicular systems embodying ethanol in relatively high concentrations. These “soft vesicles” represent novel vesicular carrier for enhanced delivery to/through the skin. One main feature of these vesicles is their soft structure which carries the incorporated active agent into the skin lipid bilayers and promotes its delivery. The ethosomes delivery system can be modulated not only for enhanced skin penetration but also for absorption of drugs into the blood (Touitou et al., Citation2000).
Ethosomes provide a dermal delivery system that overcomes the natural skin barrier functions. Drugs encased in ethosomes, when applied to the skin, are able to penetrate even into the deeply skin layers where nerve fibers occur, thereby providing a noninvasive alternative to other NSAIDS which is not accepted by some patients. In this investigation, capsaicin encapsulated inside the ethosomal carrier prevents the initial contact of capsaicin to the upper skin and due to the softness and flexibility, it is squeezed from the pore of the stratum corneum barrier and released into deeper skin.
For the acceptance of this therapeutic molecule in topical dosage form, it is necessary to reduce/circumvent its irritating effect. This irritation is responsible for withdrawal of therapy with most of the currently available topical formulations of capsaicin. Ethosomal capsaicin is expected to reduce this irritation due to the presence of ethanol and phospholipid, which are the main constituents of ethosomal system. The purpose of this study was to investigate the ability of ethosomal capsaicin to reduce skeletomuscular inflammation and pain.
Materials and methods
Chemicals
Phosphatidylcholine (Phospholipon 90G) was kindly provided by Nattermann Phospholipid GmbH (Cologne, Germany) contained 93 ± 3% phosphatidylcholine. Capsaicin (capsaicin 95% USP) was obtained as a gift sample from Chillies Export House, Virudhunagar, India. It contains capsaicin (59.87%), dihydrocapsaicin (34.75%) and nohydrocapsaicin (3.21%). Methanol and acetonitrile were procured from HiMedia, India, and acetone from Merck, India. Absolute ethanol and chloroform were purchased from HiMedia Mumbai, India. All other chemicals were of analytical grade. Triple distilled water was used throughout the study. Freunds Complete Adjuvant (FCA), carrageenan and formaldehyde were purchased from Sigma (St. Louis, MO). Thermagel (Novartis, India) was purchased from a pharmacy which was used as reference.
Preparation of ethosomes
A method for ethosomes development reported by Sarwa et al. Citation(2013) was followed in the development of capsaicin-loaded ethosomes. A specially designed vessel was used, having an inlet for the addition of solvents with less evaporation. A capsaicin (0.05%) and phospholipid (2%) mixture was prepared in 30% ethanol using a sonicator. In lipid drug solution, distilled water (up to 10 ml) was added slowly with a fine stream of a constant stirring rate at 1000 rpm with a magnetic stirrer. Additionally, mixing was continued for 10 min after addition of distilled water. Preparation was carried out at a room temperature and stored in closed vesicles. A similar method was used for the preparation of empty/blank ethosomes without capsaicin. Composition of formulation was previously optimized by 32 factorial designs using the Design-Expert® software (Stat-Ease Corporation, Minneapolis, MN).
Physical characterization
Physical parameter
Prepared vesicles were examined for various physical parameters. Morphological examination was performed by using transmission electron microscope (Hitachi, H-7500, Japan) and scanning electron microscopy (SEM, Zeiss Oxford X Max Zigma VP). Vesicle size and zeta potential were determined by Malvern Zetamaster (MAL, 500962, Malvern, UK). Differential scanning calorimetry (DSC) analysis of vesicular systems was performed using a Mettler STAR SW 9.00 system. Fourier-transform infrared (FTIR) study was performed using FTIR 8400S (Shimadzu). The viscosity of the formulations was determined using an Ostwald Viscometer at room temperature. Details of test procedure were followed as per reported by Sarwa et al. Citation(2013b).
Flexibility test
Flexibility test was done to determine the flexibility of vesicle. A specialized lab-fabricated stainless steel syringe equipped with pressurized system fitted with stainless steel filter junction was prepared. In syringe, a polycarbonate membrane with filter pore size of 200–50–200 nm was packed. Test formulations were passed through filtration assembly to confirm the flexibility of prepared vesicles (Sharma et al., Citation2013).
Entrapment efficiency of ethosomes
The entrapment efficacy in terms of drug loading inside the vesicles was determined by ultracentrifugation method (Sarwa et al., Citation2013b). The drug contain was analyzed by HPLC. The estimation of prepared ethosomes with capsaicin was previously validated for the presence of vesicle matrix; the entire sample was passed through a membrane filter to avoid any interference. The HPLC system used in the study was of CECIL (Cambridge, UK) CE 4200 with UV detector CE 4201; HPLC pump; injector loop Rheodyne (Model No.2767, 20 µL volume loop). Data acquisition was performed by the POWERSYSTEM software (Cambridge, UK). Chromatographic analysis was performed on a C-18 column (250 × 4 × 60 mm, 5 µmm, Luna 5u, Phenomenax). The mobile phase consisted of methanol:acetonitrile (50:50 v/v) and the flow rate was set at 1 ml/min. The mobile phase was filtered through 0.45 µm filter under vacuum and untrasonicated before pumping into HPLC system. The column was maintained at ambient temperature and equilibrated by pumping the mobile phase through the column for at least 30 min prior to the injection of the drug solution. The absorbance of the effluent was monitored by UV detection at 280 nm.
Ex vivo permeation study
A human cadaver skin was collected immediately after postmortem from the abdomen. The fatty layers were removed by adopting the method reported by Verma et al. Citation(2004). Skin permeation studies were carried out by using modified Franz-type diffusion cells with an effective surface area of 2 cm2. One milliliter of ethosomal formulation was placed in donor compartment and the dermal side of the skin was immersed in the pH 7.4 phosphate buffer saline (PBS):ethanol (50:50). Pseudo-sink conditions were maintained throughout the 24-hour permeation experiments. The permeation parameters, cumulative percentage drug permeated, flux, permeability coefficient and permeation rate were determined by permeation experiment. For comparative evaluation, permeation study was also performed with Thermagel and 0.05% capsaicin in 50% hydroethanolic solution.
Vesicle penetration study by CLSM
The skin penetration ability of ethosomal vesicles encapsulated with hydrophobic fluorescent probe (Rhodamine red) was observed by confocal laser scanning micrograph (CLSM). The experiment was run in a modified Franz diffusion cell similar to the drug permeation study. The sample skin was fixed in the sample holder with the help of a Tissue frozen medium gel (Guan, Leica, Germany). The perpendicular section of skin was cut with the help of cryomicrotome (Leica, Germany). The treated skin was tested for probe penetration using CLSM. The full skin thickness was optically scanned at 10–20 µm increment through the Z-axis of the Leica DMIRE 2 CLSM, Germany, attached to a Leica TCS SP2 fluorescence microscope. Optical excitation was carried out with a 488-nm argon laser-beam and fluorescence emission was detected at above 555 nm.
Storage-physical stability
Ethosomal system loaded with capsaicin was kept in air light amber color glass bottle. The formulations were evaluated for drug retentive potential at normal room temperature and humidity conditions for a period of 1 year. Samples were withdrawn periodically and analyzed for the drug content, by the method described in the previous section. Samples were also visualized under an electron microscope and analyzed for the vesicle size as well by visual assessment.
Statistical analysis
All the data were expressed as a mean ± standard deviation (SD). In physical characterization, the sample size (n = 3) and unpaired t test was performed. In biological characterization, the sample size was (n = 6) and one-way ANOVA test followed by Bonferroni's Multiple Comparison Test were performed. All the statistical analyses were done with Graphpad prism 6 Demo software (San Diego, CA).
Biological characterization
Animal
Adult male Wistar albino rats (170 ± 20 g) were used in all experiments. All the experimental studies were approved by the Institutional Animal Ethical Committee (1576/GO/a/11/CPCSEA). Animals were acclimated in 12:12 h light dark cycle in a temperature-controlled room. The rats were fed with standard pellet diet and water ad libitum. Food was placed on the floor of the cage to facile access. Body weight and food pad measures were started one week prior to the experiment to familiarize the animal with the recording instrument. Experimental animals were divided into four groups of six in each (n = 6): (1) induce control: receive only inducer; (2) blank ethosomes group; (3) ethosomal capsaicin group; (4) Thermagel-treated group. All the treatments were done topically with (50 µl) blank and capsaicin-loaded ethosomes in their respective groups.
Acute anti-inflammatory activity on carrageenan-induced model
The carrageenan-induced acute inflammatory model reported by Winter et al. Citation(1962) was used in this study. In short, 0.1 ml of 1% carrageenan in 0.9% saline (w/v) was injected in the left hind foot under the plantar aponeurosis. Inflammation was quantified by measuring the volume displacement by the paw, using a Plethysmometer (Orchid, India) at time 0 to 8 h in a 2-h interval. The hind paw immersed in the measurement cell up to the hairline to the ankle to determine the immersed organ volume in ml. Percentage inhibition was calculated by using the following formula:
(1)
where Vcontrol is the volume of the control group and Vtreated is the volume of the treatment group.
Formaldehyde-induced arthritis in rats
Arthritis was induced by injecting 0.1 ml of formaldehyde (2.5 v/v) solution in the subplantar region of the left hind paw of the rats on days 1 and 3 of the experiment (Kaithwas et al., Citation2012). Arthritis was assessed by measuring the paw volume using Plethysmometer (Orchid) and the percentage inhibition was calculated by using EquationEquation 1(1) .
Freund’s adjuvant-induced arthritis in rats
Experimental immunological arthritis was induced in rats according to the method of Newbould (Newbould, Citation1963). In short, 0.1 ml of FCA [Difco: 0.05% (w/v) Mycobacterium butyricum in mineral oil] was injected into subplantar surface of the hind paw by using a 26-gauge needle. Subplantar injection of Freund’s adjuvant produced a defined edema within a few hours with progressive arthritis by the 8th day after inoculation. All the treatments were initialized from this day and continued till the 28th day. To follow the course of the disease, swelling of the adjuvant-injected hind paw was determined with a plethysmograph. A percentage inhibition was calculated by using EquationEquation 1(1) .
Evaluation of paw volume
The paw volume was measured from 1st day to 28th day thereafter, to confirm the reduction of joint swelling and edema. The paw volume of the hind paws was measured in triplicate using a water displacement plethysmometer (Orchid) every day for 28 days after adjuvant injection and the mean values were recorded. Paw volume measured just prior to adjuvant injection was used as the control (day 0). A percentage inhibition was calculated by using EquationEquation 1(1) .
Ankle evoked vocalization test
Arthritis-induced hyperalgesia and their suppression by various formulations was evaluated by quantifying the total number of vocalizations evoked by ankle flexion or ankle extension test as reported method by Lee et al. Citation(2009). A vocalization was rated as 0 = no vocalization, or 1 = vocalization on each flexion extension stimuli, depending on whether or not the animal vocalized. Thus, for each animal the vocalization rating ranged from 0 to 10 at each hind limb.
Thermal hyperalgesia test
Thermal hyperalgesia was evaluated by the paw withdrawal test using a thermal Plantar Test (Orchid, India) model previously described by Lee et al. Citation(2009). The apparatus automatically displayed the paw withdrawal latency to the nearest 0.1 s. The cutoff time was 20 s to avoid damaging dermal tissue. The average of the three trials with 3 min interval was determined. The test was duplicated in 10 min intervals in each hind paw.
The mechanical hyperalgesia test
The Randall–Selitto test was applied to evaluate mechanical hyperalgesia in arthritic animals. A graded mechanical force (g) was delivered through an analgesia meter (Orchid, India) onto the convex surface of the paw. Rats withdrew their hind paw or vocalized when the applied force reached the nociceptive threshold. The test was duplicated at 10 min intervals in each hind paw. The threshold force in normal animals ranged from 150 to 160 g. The animals were acclimatized for 1 h before behavioral testing and the mechanical hypersensitivity was evaluated at several time points.
Measurement of body weight and temperature in arthritic rats
The change in body weight measured as difference between weight of animals before and after inoculation. Body temperature, as an index of inflammation, was monitored for rats, before and daily after disease induction using a rectal thermometer.
Skin tolerability
The skin tolerance of prepared formulations was determined by the method described by Jain et al. Citation(2005) in normal rats. Hairs were removed from the back (area 2.54 cm2) of each animal using commercial hair remover 24 h before commencement of the experiment. The first group received 30% hydroalcoholic solution, the second and third groups received blank and drug-loaded ethosomes vesicles, respectively, and the fourth group received Thermagel as a standard reference drug. Data were recorded depending on the degree of erythema and skin color as follows: no erythema/ no any changes – 0, very slight erythema, barely perceptible light pink – 1, well-defined dark pink/red – 2, moderate-to-serve erythema deep red – 3. Behavioral assessment was also done (liking and biting of applied area as well as their momentary behavior) and count on a visual scale.
Results
Physical characterization
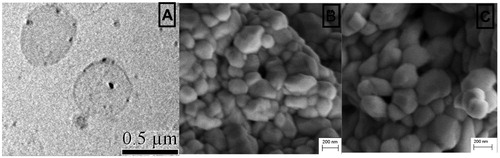
The ethosomal vesicle carrier is a novel flexible nanolipid vesicle that has various merits in the case of topical as well as transdermal administration of biomarkers. In this study, composition of the formulation was previously optimized for concentration of lipid and ethanol with respect to the vesicle size as well as entrapment efficacy by factorial design approaches. The applied software suggested a used composition with a desirability of 0.967. The classical dispersion method produced a nanosize vesicle, a vesicle size of blank and capsaicin-loaded vesicles which were 295 and 271 nm, respectively, with 61.31 ± 3.45% drug loading. Electron microscopic images confirm the preparation of homogenous, soft and spherical vesicles loaded with capsaicin (). Uniformity index of capsaicin-loaded ethosomes and empty were 0.115 ± 0.024 and 0.112 ± 0.019, respectively, which agreed with the applicability of dispersion method for preparation of homogenous lipid vesicles.
Figure 1. Electron microscope images. (A) TEM photograph of spherical capsaicin-loaded ethosomes vesicles. (B) SEM photograph of fresh prepared ethosomal capsaicin vesicles, homogeneous in size and shape with dense population. (C) SEM photograph of 1 year room temperature stored ethosomal capsaicin vesicles.
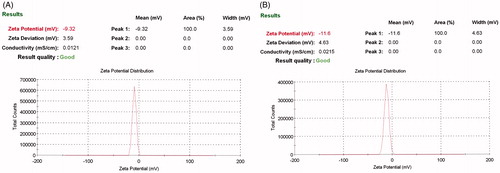
Zeta potential of drug-loaded vesicle was found to be higher than an empty ethosomes vesicle. Zeta potential value of capsaicin loaded ethosomes and empty ethosomes was –11.6 mV and –9.32 mV, respectively (). Higher zeta potential reflects the better stability as well as better skin interaction. The phase transition temperatures of blank and capsaicin-loaded ethosomes were –8.11°C and –11.46°C. Reduction of temperature peaks in capsaicin-loaded ethosomes confirmed the drug loading inside the lipid vesicles as well as to the core. Results of physical characterization parameters are summarized in .
Table 1. Physical evaluation of empty and capsaicin-loaded ethosomes.
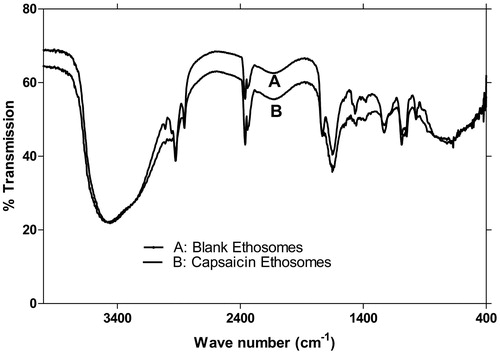
FTIR spectra of blank and drug-loaded vesicles are presented in . In lipid membrane, functional group absorption bands for NH2 stretching at 3450–3500 cm–1, C–H stretching at 2800–2950 cm–1, C = O stretching at 1600–1650 cm–1 and PO–2 antisymmetric double bond stretching bond at 1200–1250°cm–1 were studied by FTIR spectroscopy. In the tested formulations, NH2 stretching at 3458.37 cm–1 and 3475.73 cm–1, C–H stretching at 2924.09 cm–1 and 2906.01 cm–1, C = O stretching at 1647.21 cm–1 and 1643.35 cm–1, PO-2 stretching band at 1228.66 cm–1 were recorded for blank and capsaicin-loaded ethosomes, respectively.
Figure 3. FTIR spectra of empty and capsaicin-loaded ethosomes. Similarity of spectra confirming the drug loading without any significant structural changes in vesicles.
Skin penetration and permeation studies are the simplest and most cost-effective methods for characterizing a drug’s skin absorption and penetration profiles. In the permeation study, aqueous receptor solutions are the most commonly used media for hydrophilic and moderately lipophilic permeates. Buffered solutions such as phosphate, around pH 7.4, in combination with solubility enhancer are often used in case of insoluble drugs. Capsaicin is insoluble in the buffer and water, so PBS (pH 7.4):ethanol (50:50) were selected on the basis of solubility study. Investigated ethosomal capsaicin vesicles demonstrated 72.98 ± 2.84% capsaicin permeated with a flux of 15.20 ± 1.7 cm/h×10–3 in modified diffusion cell in 24 h study that was higher than both Thermagel and hydroethanolic solution of capsaicin. Summary of permeation study is presented in and . A CLSM graph further confirms the passing of the capsaicin-loaded vesicles throughout the epidermal barrier ().
Figure 4. Permeation study of various formulations. Ethosomal capsaicin shows a better permeation profile than other formulations in 24 hours study.
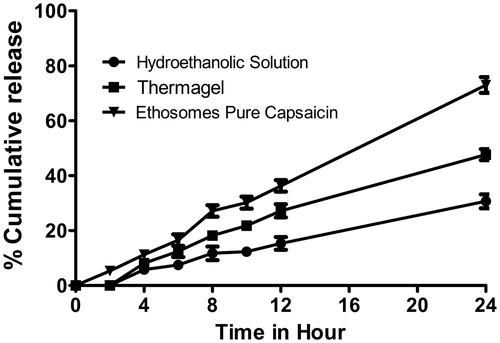
Figure 5. A CLSM graph reveals that the penetration ability of capsaicin-loaded ethosomes in skin membrane. Vesicles easily cross the stratum corneum barrier as well as entire thickness of skin membrane (700 µm). X-axis represents death from upper epidermis and Y-axis, intensity of probe.
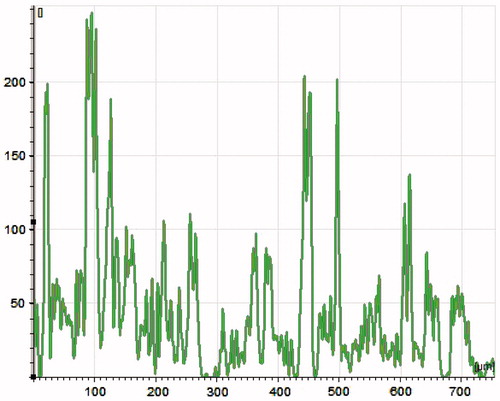
Table 2. Ex vivo permeation data of various formulations.
Stability of the lipid vesicles was confirmed by testing of various parameters reported in . In 1 year stability study, capsaicin-loaded ethosomal carrier showed no significant change in their structure (represented in ) at room temperature. There were no significant changes in vesicle size, uniformity index, zeta potential, viscosity, percentage drug entrapment and percentage permeation reported. Stored vesicle also was passed for the flexibility test to confirm the retentions of soft structure.
Biological characterization
Carrageenan-induced acute rat paw edema model, by evaluating the paw thickness, discriminates between different formulations (). Capsaicin-loaded ethosomes showed a significant inhibition with respect to control group (p < 0.001) and Thermagel group (p < 0.05). Thermagel group did not produce the significant inhibition from the control group. The highest inhibition was observed on the 4th hour of study (27.64%), on the same day Thermagel confirmed 10.63% inhibition. Thermagel showed highest inhibition on the 6th hour of study but lower than capsaicin ethosomes (12.00% and 19.81%, respectively).
Figure 6. (A) Percentage inhibition of carrageenan-induced edema by various formulations; inhibition ability of ethosomes was higher than Thermagel in all time points (p < 0.05). (B) Percentage inhibition of formaldehyde-induced arthritis by various formulations; inhibition ability of ethosomes was higher than other formulation. Thermagel also shows inhibitor effect but its response was late. The inhibitory effect of capsaicin loaded ethosomes was significant than Thermagel (p < 0.05).
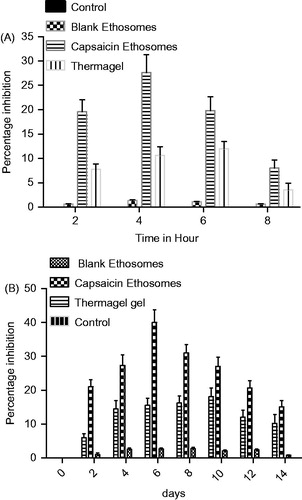
In an investigation, capsaicin ethosomes on the proliferative phase of inflammation of formaldehyde-induced arthritis rat model provoking an anti-inflammatory effect (). Change in paw volume was statistically significant in ethosomal capsaicin treatment group (p < 0.001) as compared to control. Thermagel also showed a significant inhibition from control but inferior (p < 0.05). The potential of capsaicin ethosomes was higher than Thermagel (p < 0.05). While blank ethosomes did not produce a significant effect compared to control group in both carrageenan and formaldehyde inflammation model.
Arthritis was induced in all animals after injection of FCA. The first manifestation of disease was erythema of ankle joints, followed by the involvement of metatarsal and interphalangeal joints (considered as a primary response observed just after 3 rd day of FCA induction). A secondary response was evident in 8–10 days post-injection. Change in the paws volume and joint was increased gradually and reached to peak on 12th day of study in an induced control group (data not shown). The inhibition effect of ethosomal capsaicin was significantly from induced control (p < 0.001), inhibition rate was gradually increased and attained a near constant value on 16th day. The highest inhibition of capsaicin-loaded ethosomes was 40.320%, on the same day Thermagel confirmed only 15.30% inhibition. The highest inhibition value of Thermagel was 25.45% on the 24th day of study ().
Figure 7. (A) Percentage inhibition of FCA-induced arthritis by various formulations; inhibition ability of ethosomes was higher than Thermagel. Ethosomal capsaicin, despite better results, is statistically significant compared to control group (p < 0.001). (B) Effect of Ethosomal capsaicin on the ankle flexion-extension score in adjuvant-induced arthritic animals. Ethosomal capsaicin showed better results compared to Thermagel. Performance of ethosomal capsaicin was statically significant at 16, 24 and 28 day at level of p < 0.01 compare to Thermagel. (C) Effect of Ethosomal capsaicin on paw withdrawal latency noxious heat stimuli produced in adjuvant-induced arthritic animals. In Thermagel group, a near constant value was obtained but paw withdrawal latency was statistically increased up to the termination of therapy (p < 0.01). (D) Effect of Ethosomal capsaicin on mechanical threshold produced in adjuvant-induced arthritic animals. Ethosomal capsaicin showed better results compared to control, statistically significant at level p < 0.001. Performance of ethosomal capsaicin was statically significant on 12, 16, 20 and 24 day at level of p < 0.001 compared to Thermagel. (E) Effect of Ethosomal capsaicin on body weight in adjuvant-induced arthritic animals), Ethosomal capsaicin showed better results compared to control, statistically significant at level p < 0.001. Performance of ethosomal capsaicin was statically significant on 20, 21and 28 day at level of p < 0.001 compared to Thermagel. (F) Effect of Ethosomal capsaicin on body temperature in adjuvant-induced arthritic animals, Ethosomal capsaicin showed better results compared to control, statistically significant at level p < 0.001. Performance of ethosomal capsaicin was statically significant on 16 and 28 day at level of p < 0.001 compared to Thermagel.
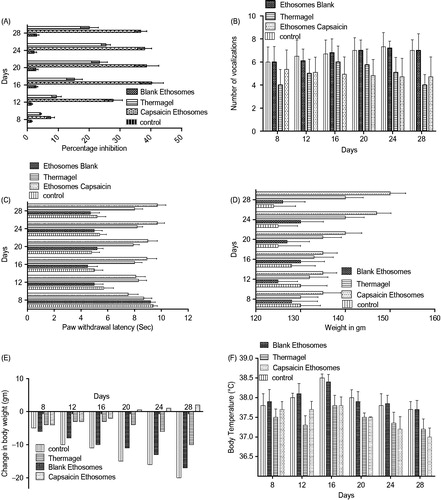
Ankle flexion test
In the arthritic control group, the vocalizations caused by flexion or extension of the hind limb increased gradually from 8th day post-adjuvant injection. The number of vocalizations caused by hind limb flexion or extension was decreased in the ethosomal capsaicin group (p < 0.001). Concern results were shown in (). In reduction in hyperalgesia, ethosomal capsaicin was more effective than Thermagel especially significant difference occurred on 20th, 24th and 28th day (p < 0.01).
Thermal hyperalgesia test
There was a significant decrease in the paw withdrawal latency of the hind paw observed in all the treated groups (). The paw withdrawal latency of the ethosomal capsaicin group showed a significantly higher thermal threshold as compared to those of the arthritic-induced groups (p < 0.001). Increment in thermal threshold in terms of paw withdrawal latency was observed in Thermagel group, parallel to capsaicin-loaded ethosomes, but inferior (p < 0.01).
Mechanical hyperalgesia test
In mechanical hyperalgesia test, the threshold of the empty ethosomes group was not statistically different from that of the arthritic control group. In contrast, the weight-bearing capacity was significantly increased in the ethosomal capsaicin group as compared to the arthritic control group (p < 0.01). There were no differences recorded in arthritic control and Thermagel-treated group ().
Body weight changes
Body weight of the adjuvant control group was markedly decreased starting from day 1 and up to termination of the study (). The ethosomal capsaicin group recovered the weight loss (p < 0.001). In Thermagel-treated group, weight loss was also recovered than arthritic control up to the 16th day of study, after that further decrease was reported which was not recovered till termination of study.
Temperature changes
Inoculation of FCA-induced humoral cellular immunological response had produced acute and chronic inflammation characterized by changes in body temperature. In this study two peaks were observed during the course of study in arthritic control rats (temperature difference from normal rates). First peak was on the 1st day of inoculation which returned to normal level on the 4th day, and the second peak was recorded on the 16th day. Treatment of adjuvant arthritic rats with ethosomal capsaicin and Thermagel abolished the second peak as well as the temperature was returning toward normal temperature (p < 0.01) (). In abolition, ethosomal capsaicin showed better potential than Thermagel, but the difference between these groups were not significant.
Tolerance study
In capsaicin-treated group, severe skin color changes were recorded, 100% animals showed dark pink. The leaking and biting were also recorded in all animals. On the second application, animals were violent and their momentary activity was also high. On first 24-hour study, their skin color did not return to normal. On this basis, abnormal behavior treatment with capsaicin solution was stopped. Capsaicin-loaded ethosomes did not produce any change and animal’s behavior was also normal, similar to blank ethosomes group. No licking and biting of the treated area was recorded with both ethosomal capsaicin and blank ethosomes. After the application of Thermagel, licking and biting were recorded in all animals, especially higher in 1st hour of application. About 40% animals’ skin became perceptible-light pink colored and 20% animal’s skin became dark pink after treatment of Thermagel.
Discussion
Prepared capsaicin-loaded ethosomes vesicles were spherical and of nanostructure, previous report clearly confirmed the ability of flexible lipid carriers to cross the barrier with pores significantly smaller than the average vesicles size of vesicles (Sarwa et al., Citation2013a,b). Loading of capsaicin in lipid vesicle decreases the transition temperature of vesicles which was favorable for liquid state vesicle (Aranda et al., Citation1995), which was further confirmed by decreasing the wavenumber of the CH3 asymmetric stretching in the FTIR spectra (Severcan et al., Citation2005).
Homogeny, flexibility and viscosity were maintained initially as well as during long-term storage at room temperature. There was no significant difference in vesicle size of empty and capsaicin-loaded ethosomes with acceptable uniformity index and other physical characterization parameter. Viscosity of drug ethosomes was less than empty vesicle, indicating enhancement of flexibility by synergistic effect of ethanol as well as capsaicin on the lipid membrane, composed of phospholipid (Lodzki et al., Citation2003). On the other hand, both ethanol and capsaicin may also affect the fluidity of epidermis lipid; it would induce membrane fluidization effect which further enhances the drug permeation and penetration ability. Despite the suggestion of the potent influence on membrane fluidity, little information is available regarding their mechanism (Tsuchiya, Citation2001). CLSM fluoresce intensity graph also supports this effect by confirming the florescence intensity from top to bottom of skin (full thickness of tested skin membrane, 700 µm). The pattern of intensity graph suggested partition of entire vesicles which would be possible only when a rapid partition from applied area to stratum corneum barrier (Kuijk-Meuwissen et al., Citation1998). Negatively charged vesicles were favorable for better skin–vesicle interaction as well as for long-term stability study which reflects the drug–vesicle interaction as well as the ionic strength of the environment. Probably, zeta potential of drug-loaded vesicles had –11.6 mV strength, sufficient enough to prevent the instability of the system which could be responsible for success of 1 year stability testing.
In the carrageen and formaldehyde inflammation model, inhibition pattern of ethosomal capsaicin and Thermagel was similar (). The most interesting fact is that the highest inhibition of edema level was obtained in both inflammation models on the time period (4th hour and 6th day in carrageenan and formaldehyde model, respectively) when edema level was high in the control group. This observation indicates its suitability of capsaicin-loaded ethosomes for various skeletomuscular disorders. The difference of potential in the test and reference compound could be suggested by the consistency differences and permeation ability. In the course of inflammation, vasopermeability of the cells has increased and opened the gaps between adjacent endothelial cells at the level of post capillary venules. These gaps are large enough for penetration of nanosized ethosomes (Simionescus, Citation1980).
The effect of higher penetration and permeation ability of ethosomal capsaicin could be responsible for higher inhibition of edema level in FCA-induced model to achieve thrice the inhibition value than Thermagel on 12th day. On that day ethosomes inhibition was around 27.85%, twice than Thermagel inhibition (9.45%) difference of response was significant (p < 0.001). Antiarthritic potential was maintained until the experiment was terminated on day 28 especially with the constant value with the ethosomal capsaicin.
The inflammatory hypernociception induced by FCA is related to a number of events that include the sensitization of primary afferent neurons and also regarding the excitability of central nervous system (CNS) neurons. Both events are believed to be associated with at least three main pathways. First, degranulation of mast cells with release of histamine and serotonin that have pronociceptive effects. Second, activation of glial cells and consequent overproduction and/or release of cytokines both at the periphery and at the CNS (Raghavendra et al., Citation2003). These cytokines are in turn responsible for the hypersensibilization of peripheral and central neurons. In the third pathway, there is upregulation and activation of various proteins including channels such as sodium and calcium (Porreca et al., Citation1999). The first and second pathways are believed to be involved in the acute hyperalgesia, whereas the second and third are responsible for the subacute and chronic maintenance of pain (Watkins et al., Citation2001). Ethosomes capsaicin exerted a markable effect on both the phase by inhibiting the release of various mediators which are involved in this process, especially substance P and other trachykinins that are considered to be the putative mediators of neurogenic inflammation. Ethosomal capsaicin significantly attenuates the nociception by better penetration ability of the carrier as well as by binding of capsaicin over vanilloid receptors, which could be responsible for prolonged period of hypoalgesia (Fusco & Giacovazzo, Citation1997). It has been reported that unilateral subcutaneous injection of capsaicin into the plantar surface of the hind paw prolongs tonic behavioral responses (Lariviere & Melzack, Citation1996). A weight loss in arthritis is an indication of abnormal stomach conditions which could be responsible for the decrease of weight, which returns to the normal level by releasing the hyperalgesia condition, feasible for access of food ingestion.
Skin adverse effect of capsaicin includes burning sensation, barely perceptible and redness on the skin after application is common and determinant to the patient withdrawal. The ethosomal system composed with phospholipid and ethanol may significantly reduce this adverse event. The mechanism of the reduction of adverse event could explain by the effect of an individual ingredient used in the system. Phosphotidylcholine derived from soybean lecithin is mainly used as a smoothing agent in skin/cosmetic preparation as emollient which nourishes the skin. Ethanol produces the cooling and desensitizing effect like a local anesthesia (Tappeiner et al., Citation2012). Furthermore, phosphotidylcholine likely minimizes the danger of allergic contact dermatitis (Gebhardt et al., Citation1994). This entire factor may contribute in reduction of adverse event in ethosomal capsaicin in the treated group.
Conclusion
Ethosomal carrier encapsulated with capsaicin has been successfully prepared and tailored for transdermal/topical application. Ethosomal system prepared with capsaicin was a novel nanocarrier for this irritant bioactive compound with great stability (in 1 year study). Advantage of ethosomal carrier for this bioactive molecule includes better penetration as well as reduction of irritation effect, which was successfully achieved. From the results of this study, ethosomal capsaicin significantly reduces the inflammation and pain. Ethosomal capsaicin can provide a number of important benefits including improving the drug’s efficacy and enhancing patient compliance.
Acknowledgements
Authors greatly thank Late Prof. (Dr.) A. K. Dolui for his novel ideas, righteous contribution and invaluable guidance made toward the work presented. They extend sincere thanks to Chillies Export House, Virudhanagar, India, for providing the gift sample of capsaicin, and Nattermann Phospholipid GmbH for providing gift samples of phosphatidylcholine (Phospholipon 90G).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. Authors are highly grateful to Indian Council of Medical Research (ICMR), New Delhi, for providing senior research fellowship to Mr. Khomendra Kumar Sarwa.
References
- Aranda FJ, Jos V, Comez-Fernfindez JC. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim Biophy Acta 1995;1234:225–34
- Backonja MM, Malan TP, Vanhove GF, Tobias JK. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med 2010;1:600–8
- Donnerer J, Amann R, Schuligoi R, Lembeck F. Absorption and metabolism of capsaicinoids following intragastric administration in rats. Naunyn Schmiedebergs Arch Pharmacol 1990;342:357–61
- Fusco BM, Giacovazzo M. Peppers and pain: the promise of capsaicin. Drugs 1997;53:909–14
- Gebhardt M, Reuter A, Knopf B. Allergic contact dermatitis from topical diclofenac. Contact Dermatitis 1994;30:183–4
- Jain SN, Jain D, Bhadra A, et al. Transdermal delivery of an analgesic agent using elastic liposomes: preparation, characterization and performance evaluation. Curr Drug Deliv 2005;2:223–33
- Kaithwas G, Gautam R, Jachak SM, Saklai A. Antiarthritic effect of Ajga bracteosa Wall ex Benth. in acute and chronic models of arthritis in albino rats. Asian Pac J Trop Biomed 2012;2:185–8
- Kawada T, Watanabe T, Katsura K, et al. J Chromatogr 1985;329:99–105
- Kuijk-Meuwissen ME, Junginger HE, Bouwstra JA. Interactions between liposomes and human skin in vitro, a confocal laser scanning microscopy study. Biochim Biophys Acta 1998;1371:31–9
- Lariviere WR, Melzack R. The bee venom test: a new tonic-pain test. Pain 1996;66:271–7
- Lee J, Kim KA, Jeong S, et al. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund's adjuvant-induced arthritis in rats. J Ethnopharmacol 2009;126:258–64
- Lodzki M, Godin B, Rakou L, et al. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. J Control Release 2003;93:377–87
- Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvants. Br J Pharmacol 1963;21:127–36
- Porreca E, Sergi R, Baccante G, et al. Peripheral blood mononuclear cell production of interleukin-8 and IL-8-dependent neutrophil function in hypercholesterolemic patients. Atherosclerosis 1999;146:345–50
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003;306:624–30
- Sarwa KK, Mazumder B, Rudrapal M, Verma VK. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv 2013a;Early Online: 1–9. doi: 10.3109/10717544.2013.871601
- Sarwa KK, Suresh PK, Debnath M, Ahmad MZ. Tamoxifen citrate loaded ethosomes for transdermal drug delivery system: preparation and characterization. Curr Drug Deliv 2013b;10:466–76
- Severcan F, Sahin I, Kazanci N. Melatonin strongly interacts with zwitterionic model membranes-evidence from Fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim Biophys Acta. 2005;1668:215–22
- Sharma VK, Sarwa KK, Mazumder B. Fluidity enhancement: a critical factor for performance of liposomal transdermal drug delivery system. J Liposome Res 2013;published online 25 October 2013. doi:10.3109/08982104.2013.847956
- Simionescus M. Ciba Found. Symp 71 (1980)
- Tappeiner C, Flueckiger F, Boehnke M, et al. Effect of topical anesthetic agents and ethanol on corneoepithelial wound healing in an ex vivo whole-globe porcine model. J Cataract Refract Surg 2012;38:519–24
- Touitou E, Dayan N, Bergelson L, et al. Ethosomes- novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 2000;65:403–18
- Tsuchiya H. Biphasic membrane effects of capsaicin, an active component in Capsicum species. J Ethnopharmacol 2001;75:295–9
- Verma DD, Fahr A. Synergistic penetration enhancement effect off ethanol and phospholipids on the topical delivery of cyclosporine A. J Controlled Release 2004;97:55–66
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci 2001;24:450–5
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in the hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med III 1962;544–7
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence based, expert consensus guidelines, Osteoarthr. Cartil 2008;16:137–62