Abstract
Background: Novel oral anticoagulants, including direct factor Xa inhibitors and direct factor IIa inhibitors, have been used to prevent stroke in patients with atrial fibrillation (AF) for a decade. In this study, the efficacy and safety of the novel oral anticoagulants were assessed in AF patients.
Methods: No language restrictions were applied. Study selection and data extraction were carried out by searching PubMed, EMBASE, OVID, the BIOSIS, the Web of Science, Clinical Trials Registers, Cochrane Central Register of Controlled Trials and the China Academic Library and Information System. Each database was searched from its inception date to June 2013. Using odds ratio (OR) as an indicator, we systematically evaluated the primary efficacy endpoints and safety endpoints, as well as 10 secondary endpoints.
Result: Compared to the control drugs, the novel oral anticoagulants showed an OR decreased by 26% (OR: 0.74, 95% confidence interval (CI): 0.62–0.88) for stroke or systemic embolism, decreased by 24% (OR: 0.76, 95% CI: 0.64–0.90) for major bleeding, decreased by 10% (OR: 0.90, 95% CI: 0.84–0.95) for death from any cause, decreased by 27% for disabling or fatal stroke (OR: 0.73, 95% CI: 0.54–0.97), decreased by 31% (OR: 0.69, 95% CI: 0.60–0.8) for fatal bleeding, and decreased by 8% (OR: 0.92, 95% CI: 0.88–0.95) for serious adverse events. However, there was no significant difference in acute myocardial infarction, systemic embolism, major bleeding or clinically relevant non-major, all bleeding events, all adverse events and liver function disorder, between the novel oral anticoagulants and control drugs (p > 0.05).
Conclusions: Compared to the control drugs, the novel oral anticoagulants showed higher efficiency and safety in patients with AF, as evidenced by their superior performance not only in reducing the risk of stroke or systemic embolism with a lower risk of major bleeding but also in decreasing the incidence of death from any cause, disabling or fatal stroke, serious adverse events and fatal bleeding.
Introduction
Atrial fibrillation (AF) is a common type of cardiac arrhythmia. The overall prevalence of AF is 0.4–1% and increases with age in people older than 60 years (Darby-Stewart et al., Citation2012). AF is one of the common causes for stroke, responsible for approximately one-sixth of strokes (Go et al., Citation2001; Stewart et al., Citation2001). Since the early 1990s, vitamin K antagonists (VKA) have become the major drug for stroke prevention in patients with AF (Darby-Stewart et al., Citation2012). However, due to a narrow therapeutic window and various interactions with drugs and foods, VKA therapy requires a close coagulation monitoring and dose adjustment (Ma et al., Citation2011; Apostolakis & Lip, Citation2012). Thus, only 50–60% of AF patients who meet the usage criteria have received VKA treatment (Connolly et al., Citation2007; Lam et al., Citation2011). In addition, it has been proposed that the advantages of VKA are counteracted by its limitations, such as increased bleeding, the need of coagulation monitoring and interaction with other drugs, leading to an underuse of VKA in clinic (Bassand, Citation2012).
Novel oral anticoagulants, including direct thrombin inhibitors and direct factor Xa inhibitors, have been widely used in clinic trials for stroke prevention in patients with AF in a recent decade due to less drug–food interacts and no need of coagulation monitoring and dose adjustment (Bassand, Citation2012). However, the results are inconsistent, and thus the overall efficacy and safety of novel oral anticoagulants remain uncertain. The primary objectives of this study were as follows: (1) to evaluate the value of novel oral anticoagulants by comparing them with all control drugs in terms of the endpoints of stroke or systemic embolism and major bleeding; (2) to evaluate the value of novel oral anticoagulants by comparing them with VKA; and (3) to evaluate the value of novel oral anticoagulants under other special conditions. The secondary objective of this study was to evaluate the value of novel oral anticoagulants by comparing them with all control drugs in terms of 10 endpoints including death from any cause, disabling or fatal stroke, major bleeding or clinically relevant non-major bleeding.
Methods
We completed the systematic review and meta-analysis under the guidance of the “Preferred Reporting Items for Systematic reviews and Meta-Analyses” published by Liberati et al. (Citation2009).
Search strategy
No language restrictions were applied. Databases were searched using the search terms “(new or novel oral direct factor Xa inhibitor OR apixaban OR rivaroxaban OR edoxaban OR betrixaban OR darexaban OR eribaxaban OR letaxaban OR TAK442 OR otamixaban OR new or novel oral direct factor IIa inhibitor OR direct thrombin inhibitor OR dabigatran OR ximelagatran OR AZD0837 OR LY-517717 OR melagatran OR flovagatran) AND (atrial fibrillation) AND (randomized controlled trial)” appearing in “title/abstract”. Databases included PubMed, EMBASE, OVID, the BIOSIS, the Web of Science for Randomized Controlled Trials, Clinical Trials Registers, The Cochrane Central Register of Controlled Trials and the China Academic Library and Information System. Each database was searched from its inception date to June 2013.
Trial selection
The inclusion criteria were as follows: (1) study subjects: patients older than 18 years who were diagnosed with AF by 12-lead electrocardiograms (ECG) and had a CHADS2 score (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke or transient ischemic attack (TIA) (doubled)) (Gage et al., Citation2001)≥1, without considering the type of heart valves involved and AF type (persistent, paroxysmal or permanent); the patients who had a creatinine clearance rate <30 mL/min, an aminotransferase level exceeding the upper normal limit by two times, a blood platelet count lower than 100 000/µL, a severe bleeding event within six months or a stroke event within 10 d, were excluded; (2) intervention measures: patients in the experiment group were administered novel oral anticoagulants, and the patients in the experiment group were administered placebo, VKA or aspirin (and/or clopidogrel), without considering the frequency and dose of the drug administration; (3) endpoints: primary endpoints included stroke or systemic embolism (combined endpoint) and major bleeding; secondary endpoints included acute myocardial infarction, systemic embolism, death from any cause, disabling or fatal stroke (combined endpoint), all bleeding events, fatal bleeding, major bleeding or clinically relevant non-major bleeding (combined endpoint), all adverse events, serious adverse events and liver function disorder; (4) trial types: the randomized controlled trials (RCTs) were included regardless of their sample size or conclusions. The exclusion criteria were as follows: the trials that were an observational study or in which the novel anticoagulants were not administered through the digestive system were excluded in this study.
Endpoints definition
In our study, stroke was defined as a local paroxysmal nervous functional defect including ischemic stroke, hemorrhage stroke and unspecified stroke (such as TIA). Systemic embolism was defined as an acute arterial vascular occlusion in limbs or organs evidenced by imaging examination, surgery or autopsy. Acute myocardial infarction was defined as an acute, typical chest pain lasting at least 20 min, accompanied with ECG changes of acute acute myocardial infarction and/or an increase of at least two times of the upper normal limit in myocardial enzyme level. Major bleeding referred to a fall of ≥2 g/dL hemoglobin within 24 h, requirement for transfusion of two or more units of blood/red cells or intracranial bleeding, hematorrhachis, intra-ocular hemorrhage (excluding conjunctival hemorrhage), pericardial hemorrhage, intra-joint bleeding, retroperitoneal hemorrhage or osteofascial compartment syndrome at injection sites or fatal bleeding. Clinically relevant non-major bleeding was defined as a significant bleeding leading to a decrease of <2 g/dL hemoglobin or requirement for transfusion of one unit blood/red cell, a temporary discontinuity of treatment or the bleeding with clinical implications resulting in medical consultation/intervention. Fatal bleeding is a subtype of intracranial major bleeding leading to a fall of ≥5 g/dL hemoglobin within 24 h, requirement of transfusion of four units blood/red cell or requirement for the vasopressor or surgical treatment. Disabling or fatal stroke referred to a stroke followed by death within 30 d or resulting in functional disability (modified Rankin score ≥3 or a decrease in the Barthel index <60 at 3 months after stroke). An adverse event was defined as the occurrence of an undesirable or worsened medical condition, while serious adverse events referred to the adverse events causing death or life threatening that require immediate medical treatments in a hospital or leading to permanent disabilities. Liver function disorder was defined as an event where the serum level of alanine aminotransferase or aspartate transarninase exceeds the upper normal limit by three times and/or the total bilirubin exceeds the upper normal limit by two times.
Data extraction
In accordance with the Jadad scoring system (Ashworth et al., Citation2011), two investigators evaluated the quality of all trials from the aspects of the randomization, blind method, drop-out and follow-up. We also evaluated the allocation concealment. The scoring for the above four indicators is as following: (1) two points were given when all criteria were met; (2) for a study in which a blinded method was used to evaluate the endpoint events, one point was given when the novel oral anticoagulants were double-blinded evaluated, while the control drugs were open-label evaluated; (3) one point was given for the trials where one above indicator was mentioned, and 0 was given for the trials without any mentions. The total score of the four indicators was 8 points. There were a total of three groups with two investigators in each for data extraction and database establishment; subsequently, the established database was compared and verified with the original data three times; finally, all three groups worked together to confirm the accuracy of data, with discrepancies resolved by discussion and consensus.
Extracted data included registration number or first author, year of publication or data uploading date, number of participating countries and research centres, sample size, age of patients, time of endpoint measurement, type of heart valves, proportion of patients with CHADS2 score, proportion of the patients with previous use of VKA or aspirin, proportion of the patients with stroke or TIA history, intervention measures as well as the numbers of primary and secondary endpoint events for the novel oral anticoagulants and control drugs in different subgroups.
Statistical analysis
Using odds ratio (OR) as an evaluation indicator, we performed a meta-analysis of the primary efficacy endpoint, the primary safety endpoint and 10 secondary endpoints to evaluate the efficacy and safety of novel oral anticoagulants. Prior to the meta-analysis on each endpoint, statistical heterogeneity across the various trials was tested using a Cochran's Q statistic Probability (QP) value of more than the nominal level of 0.10 for the Q statistic indicated a lack of heterogeneity across trials, allowing for the use of a fixed effect model, otherwise, a random effect model. All p values in the meta-analyses resulted from two-tailed tests. With only a few exceptions, data derived from the intention-to-treatment analysis were used for the meta-analysis in our study. Furthermore, subgroup analysis was conducted for the two primary endpoints, based on the type of novel oral anticoagulants and control drugs, frequency of drug regimen, sample size, time of endpoint measurement, gender, with/without the concomitant medication with antiplatelet agents, previous CHADS2 score, previous estimated glomerular filtration rate, with/without the history of stroke or TIA and with/without the previous use of VKA. Finally, we performed sensitivity analyses on the endpoints, which were confirmed to have a statistical significance, evaluated the publication bias. The fixed effect and random effect models were adopted for sensitivity analyses performed before and after the removal of certain trials. Publication bias was assessed using the fail-safe number calculation, Egger’s and Begg’s test.
Stata Version SE 12.0 (StataCorp LP, TX, USA) software was utilized for analyses in our study.
Results
Search results
A total of 1025 records were identified through database searches. We included 23 RCTs (Olsson, Citation2003; Albers et al., Citation2005; Ezekowitz et al., Citation2007; Tangelder et al., Citation2008; Connolly et al., Citation2009, Citation2011, Citation2013; Lip et al., Citation2009, Citation2011; Olsson et al., Citation2010; Weitz et al., Citation2010; Chung et al., Citation2011; Granger et al., Citation2011; Lip et al., Citation2011; Ogawa et al., Citation2011; Patel et al., Citation2011; Hori et al., Citation2012; The U.S. National Institutes of Health, Citation2012a,Citationd,Citatione,Citationf,Citationg; Yamashita et al., Citation2012), which met the inclusion criteria and nine extended reports (Petersen et al., Citation2003; Gomberg-Maitland et al., Citation2006; Diener et al., Citation2010; Ezekowitz et al., Citation2010; Fox et al., Citation2011; Oldgren et al., Citation2011; Diener et al., Citation2012; Easton et al., Citation2012; Hankey et al., Citation2012) for subgroup analysis. There were 12 records for direct factor Xa inhibitors, 11 for direct factor IIa inhibitors, 4 for ximelagatran, 19 for non-ximelagatran, 20 with warfarin as control drugs, 2 for aspirin or clopidogrel and 1 for placebo. There were 17 trials that had a sample size of <2000 and six trials with a sample size of ≥2000. The time of endpoint measurements was shorter than six months in 12 trials, while six months or longer in 11 trials. Characteristics of the 23 selected trials are shown in the online-only material (see eTable 1).
Primary efficacy endpoint
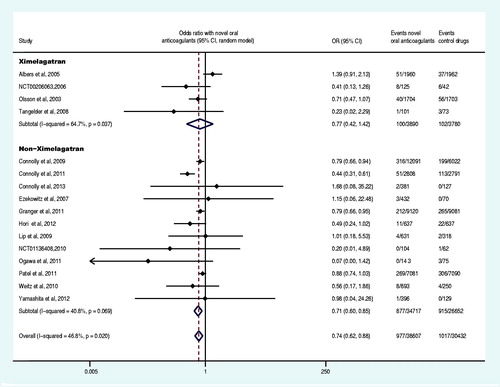
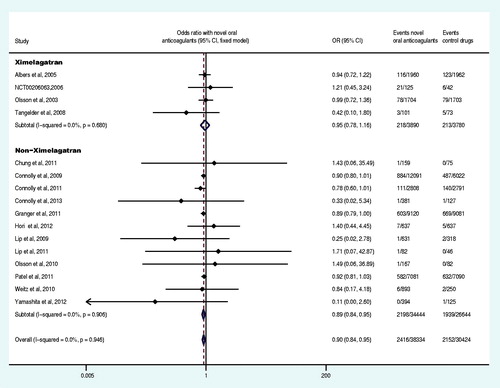
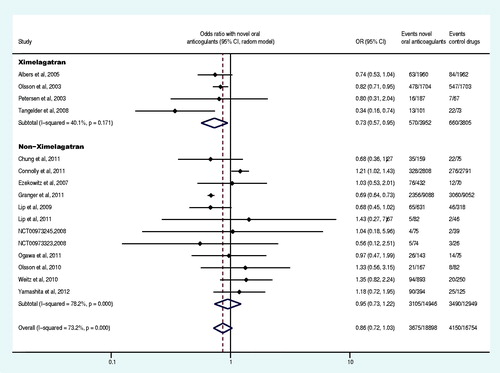
Sixteen trials reported at least one primary efficacy endpoint event (Olsson, Citation2003; Albers et al., Citation2005; Ezekowitz et al., Citation2007; Tangelder et al., Citation2008; Connolly et al., Citation2009, Citation2011; Lip et al., Citation2009; Weitz et al., Citation2010;; Granger et al., Citation2011; Ogawa et al., Citation2011; Patel et al., Citation2011; Hori et al., Citation2012; Yamashita et al., Citation2012; The U.S. National Institutes of Health Citation2012a,Citationg). The primary efficacy endpoint and the subgroup analysis data were shown in and . Compared with the control drug, the overall OR of stroke or systemic embolism decreased by 26% (OR: 0.74, 95% CI: 0.62–0.88) in the group of the novel oral anticoagulants. Specifically, the direct factor IIa inhibitors decreased the OR by 19% (OR: 0.81, 95% CI: 0.70–0.95), the direct factor Xa inhibitors group decreased the OR by 32% (OR: 0.68, 95% CI: 0.52–0.88), non-ximelagatran decreased the OR by 29% (OR: 0.71, 95% CI: 0.60–0.85) and ximelagatran group did not show significant difference (p = 0.406). Moreover, the OR decreased by 16% (OR: 0.84, 95% CI: 0.72–0.99) in the group of once daily regimen and 27% (OR: 0.73, 95% CI: 0.57–0.92) in the group of twice daily regimen. Novel oral anticoagulant in combination with aspirin or clopidogrel decreased the OR by 17% (OR: 0.83, 95% CI: 0.71–0.97), whereas it decreased by 35% (OR: 0.65, 95% CI: 0.51–0.84) in patents receiving only novel anticoagulants without aspirin or clopidogrel. Compared to the control drugs, the novel oral anticoagulants significantly decreased the OR of stroke or systemic embolism in patients with non-valvular disease, CHADS2 score ≥2, estimated glomerular filtration rate (GFR)<50 mL/min or ≥50 mL/min (p < 0.05). Regardless of the history of stroke or TIA as well as the previous use of VKA, novel oral anticoagulants significantly decreased the OR of stroke or systemic embolism compared to the control drugs (p < 0.05). Compared to VKA and aspirin (plus placebo), novel oral anticoagulants decreased the OR of stroke or systemic embolism by 18% and 57%, respectively (OR: 0.82, 95% CI: 0.75–0.9; OR: 0.43, 95% CI: 0.31–0.60).
Table 1. Meta-analysis results for the event rate of stroke or systemic embolism (novel oral anticoagulants versus control drugs).
Five trials reported at least one primary efficacy endpoint event in patients with large-dose or small-dose regimen of novel oral anticoagulants (Petersen et al., Citation2003; Connolly et al., Citation2009; Lip et al., Citation2009; Weitz et al., Citation2010; Granger et al., Citation2011). The meta-analysis results revealed that, compared to the small-dose, the large-dose regimen of novel oral anticoagulants reduced the OR of stroke or systemic embolism by 26% (large doses versus small doses: OR: 0.74, 95% CI: 0.60–0.91, p = 0.005). Two trials reported at least one primary efficacy endpoint event in patients administered with novel oral anticoagulants at two different frequencies (Lip et al., Citation2009; Weitz et al., Citation2010). The meta-analysis results demonstrated that there was no statistically significant difference between the two frequencies (twice daily versus once daily: OR: 1.66, 95% CI: 0.42–6.47, p = 0.468). Five trials reported at least one primary efficacy endpoint event for each gender (Petersen et al., Citation2003; Gomberg-Maitland et al., Citation2006; Connolly et al., Citation2009, Citation2011; Granger et al., Citation2011). The meta-analysis results showed that the OR of stroke or systemic embolism was significantly lower in males than females (male versus female, OR: 0.82, 95% CI: 0.68–0.99, p = 0.044) ().
Through comparison with Connolly et al. (Citation2009), we found that the sample size for CHADS2 score 3–6 (150 mg twice daily) should be 1981 but not 198 in in the report of Oldgren et al. (Citation2011).
Table 2. Meta-analysis results for the event rate of major bleeding (novel oral anticoagulants versus control drugs).
Connolly et al. (Citation2011) and NCT00412984 (TRIAL-RESULTS CENTER. ARISTOTLE study, 2011), apixaban 10 mg/d (5 mg/d if serum creatinine level of 1.5 mg per deciliter or more); Fox et al. (Citation2011) and NCT00494871 (The Circulation Journal, 2012), rivaroxaban 20 mg/d (15 mg/d if creatinine clearance 30–49 ml/min).
Primary safety endpoint
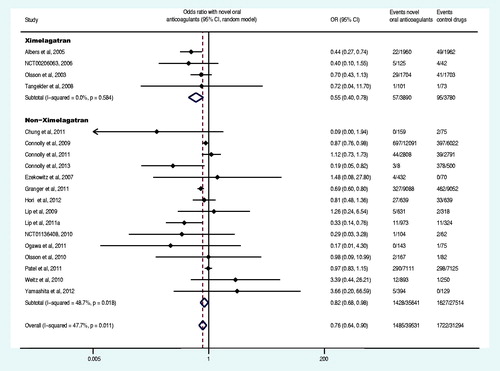
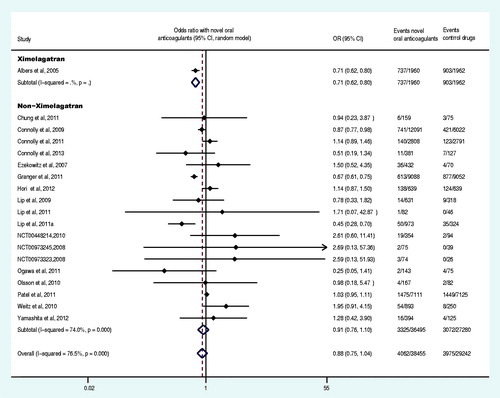
Nineteen trials reported at least one event of primary safety endpoint (Olsson et al., Citation2003, Citation2010; Albers et al., Citation2005; Ezekowitz et al., Citation2007; Tangelder et al., Citation2008; Connolly et al., Citation2009, Citation2011, Citation2013; Lip et al., Citation2009; Weitz et al., Citation2010; Chung et al., Citation2011; Granger et al., Citation2011; Lip et al., Citation2011; Ogawa et al., Citation2011; Patel et al., Citation2011; Hori et al., Citation2012; Yamashita et al., Citation2012; The U.S. National Institutes of Health, Citation2012a,Citationg). The primary safety endpoint and the subgroup analysis data were shown in and . Compared with control drugs, the novel oral anticoagulants decreased the OR of major bleeding by 24% (OR: 0.76, 95% CI: 0.64–0.90), among which, ximelagatran decreased the OR by 44% (OR: 0.56, 95% CI: 0.40–0.78), whereas non-ximelagatran decreased the OR by 18% (OR: 0.82, 95% CI: 0.68–0.98). Moreover, the group of once daily regimen no statistically significant difference was observed (p = 0.257), whereas the group of twice daily regimen decreased the OR by 26% (OR: 0.74, 95% CI: 0.60–0.91). Compared with control group, the novel oral anticoagulants significantly decreased the OR of major bleeding in patients with CHADS2 score at 1 and 2. The administration of novel oral anticoagulants did not result in significant differences in OR of major bleeding in AF patients, regardless of the history of stroke or TIA, the previous use of VKA, estimated GFR<50 ml/min or ≥50 ml/min or CHADS2 score ≥3 (p > 0.05). Compared with VKA, the novel oral anticoagulants decreased the OR of major bleeding by 27% (OR: 0.73, 95% CI: 0.61–0.87).
Nine trials reported at least one event of primary safety endpoint in patients with large-dose or small-dose of novel oral anticoagulants (Ezekowitz et al., Citation2007; Lip et al., Citation2009; Connolly et al., Citation2009, Citation2013; Olsson et al., Citation2010; Weitz et al., Citation2010; Granger et al., Citation2011; Lip et al., Citation2011; Yamashita et al., Citation2012). The meta-analysis results revealed that, the OR of major bleeding induced by the large-dose of novel oral anticoagulants was 2.03 times of the small-dose of novel oral anticoagulants (large doses versus small doses: OR: 2.03, 95% CI: 1.13–3.63, p = 0.018). The analysis on two trials, where the novel oral anticoagulants were administered with similar doses at both regimen frequencies (Lip et al., Citation2009; Lip et al., Citation2011), demonstrated that there was no statistically significant difference in the OR of major bleeding between the two frequencies (twice daily versus once daily: OR: 0.77, 95% CI: 0.29–2.09, p = 0.608). Two trials reported at least one event of primary safety endpoint of novel oral anticoagulants for each gender (Gomberg-Maitland et al., Citation2006; Connolly et al., Citation2011), and the meta-analysis results showed that there was no statistically significant difference in the OR of major bleeding between two genders (male versus female, OR: 0.95, 95% CI: 0.73–1.24, p = 0.706).
Secondary endpoints and adverse effects
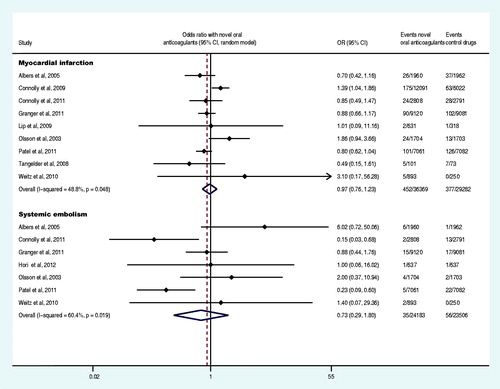
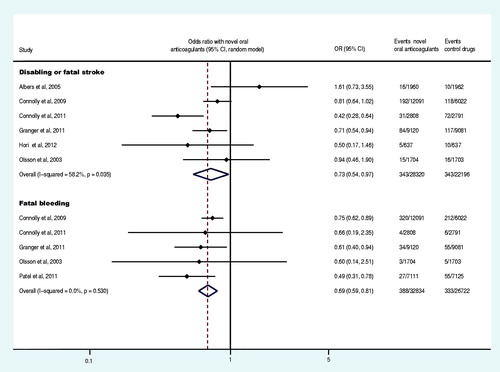
Nine trials reported at least one event of acute myocardial infarction (Olsson, Citation2003; Albers et al., Citation2005; Tangelder et al., Citation2008; Connolly et al., Citation2009, Citation2011; Lip et al., Citation2009; Weitz et al., Citation2010; Granger et al., Citation2011; Patel et al., Citation2011), and seven trials reported at least one event of systemic embolism (Olsson et al., Citation2003; Albers et al., Citation2005; Weitz et al., Citation2010; Connolly et al., Citation2011; Granger et al., Citation2011; Patel et al., Citation2011; Hori et al., Citation2012). The meta-analysis results demonstrated that there was no statistically significant difference in the OR of acute myocardial infarction and systemic embolism between the experiment group and control group (p = 0.775, p = 0.487) (). Six trials reported at least one case of the disabling or fatal stroke (Olsson, Citation2003; Albers et al., Citation2005; Connolly et al., Citation2009, Citation2011; Granger et al., Citation2011; Hori et al., Citation2012), and five trials reported at least one event of fatal bleeding (Olsson, Citation2003; Connolly et al., Citation2009, Citation2011; Granger et al., Citation2011; Patel et al., Citation2011). The meta-analysis demonstrated that, compared to the control drug group, the OR of the disabling or fatal stroke and fatal bleeding in the experiment group was decreased by 27% and 31% (OR: 0.73, 95% CI: 0.54–0.97; OR: 0.69, 95% CI: 0.60–0.81), respectively ().
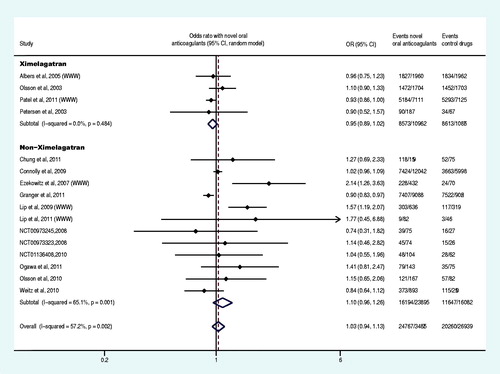
Sixteen trials reported at least one case of death from any cause (Olsson, Citation2003; Albers et al., Citation2005; Tangelder et al., Citation2008; Connolly et al., Citation2009, Citation2011, Citation2013; Lip et al., Citation2009, Citation2011; Olsson et al., Citation2010; Weitz et al., Citation2010; Chung, et al., Citation2011; Granger et al., Citation2011; Patel et al., Citation2011; Hori et al., Citation2012; Yamashita et al., Citation2012; The U.S. National Institutes of Health Citation2012g), and meta-analysis demonstrated that, compared to the control group, the OR of the death from any cause in the experiment group was decreased by 10% (OR: 0.90, 95% CI: 0.84–0.95) (). Nineteen trials reported at least one event of major bleeding or clinically relevant non-major bleeding (Albers et al., Citation2005; Ezekowitz et al., Citation2007; Connolly et al., Citation2009, Citation2011, Citation2013; Lip et al., Citation2009, Citation2011; Olsson et al., Citation2010; Weitz et al., Citation2010; Chung et al., Citation2011;; Granger et al., Citation2011; Lip et al., Citation2011; Ogawa et al., Citation2011; Patel et al., Citation2011; Hori et al., Citation2012; The U.S. National Institutes of Health, Citation2012d,Citatione,Citationf; Yamashita et al., Citation2012), 16 trials reported at least one case of all bleeding events (Olsson, Citation2003; Petersen et al., Citation2003; Albers et al., Citation2005; Ezekowitz et al., Citation2007; Tangelder et al., Citation2008; Lip et al., Citation2009, Citation2011; Olsson et al., Citation2010; Weitz et al., Citation2010; Chung et al., Citation2011; Connolly et al., Citation2011; Granger et al., Citation2011; Ogawa et al., Citation2011; Yamashita et al., Citation2012). The meta-analysis results revealed that there was no statistically significant difference in the OR of major bleeding or clinically relevant non-major bleeding and all bleeding events between the experiment group and control group (p = 0.139, p = 0.106) ( and ).
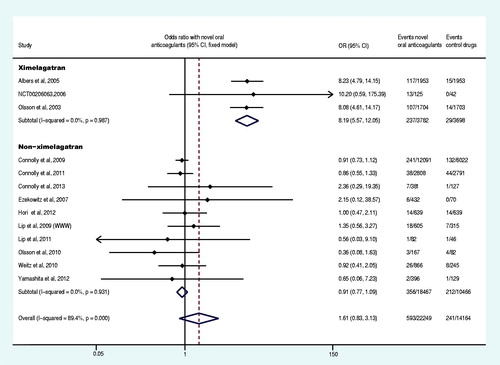
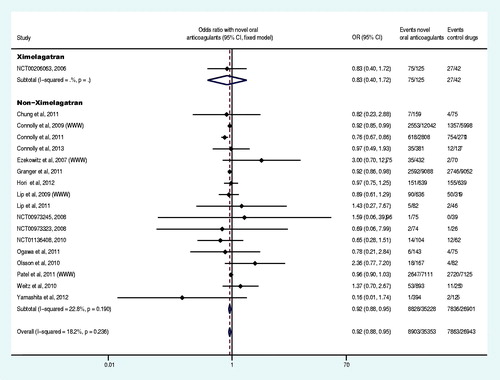
Sixteen trials reported at least one case of all adverse events (Olsson, Citation2003; Petersen et al., Citation2003; Ezekowitz et al., Citation2007; Connolly et al., Citation2009; Olsson et al., Citation2010; Weitz et al., Citation2010; Chung et al., Citation2011; Granger et al., Citation2011; Ogawa et al., Citation2011; The U.S. National Institutes of Health Citation2012a,Citationd,Citatione; TRIAL-RESULTS CENTER, 2005, SPORTIF V study), and 13 reported at least one case of liver function disorder (Olsson, Citation2003; Albers et al., Citation2005; Ezekowitz et al., Citation2007; Connolly et al., Citation2009, Citation2011, Citation2013; Lip et al., Citation2009; Olsson et al., Citation2010; Weitz et al., Citation2010; Lip et al., Citation2011; Hori et al., Citation2012; Yamashita et al., Citation2012; The U.S. National Institutes of Health, Citation2012g). Our meta-analysis results demonstrated that, compared with the control group, the overall OR of all adverse events and liver function disorder were not statistically significantly different in the experiment group (p = 0.480 and p = 0.148, respectively) ( and ), but the OR of liver function disorder was increased 719% (OR: 8.19, 95% CI: 5.57–12.05) by ximelagatran (Olsson, Citation2003; Albers et al., Citation2005; The U.S. National Institutes of Health Citation2012g). Eighteen trials reported at least one case of serious adverse events (Olsson et al., Citation2003; Ezekowitz et al., Citation2007; Connolly et al., Citation2009, Citation2011, Citation2013; Weitz et al., Citation2010; Chung et al., Citation2011; Granger et al., Citation2011; Lip et al., Citation2011; Ogawa et al., Citation2011; Hori et al., Citation2012; The U.S. National Institutes of Health, Citation2012a,Citationb,Citationc,Citationd,Citatione,Citationg,Citationi; Yamashita et al., Citation2012) and the meta-analysis demonstrated a decrease of 8% (OR: 0.92, CI: 0.88–0.95) in the OR of the experiment group compared to the control group ().
Heterogeneity test
The reanalysis revealed that there was no heterogeneity regarding the stroke or systemic embolism with the removal of two trials (Albers et al., Citation2005; Ogawa et al., Citation2011) (OR: 0.76, 95% CI: 0.70–0.84, QP = 0.139) as well as no heterogeneity regarding the major bleeding after removing another two trials (Albers et al., Citation2005; Granger et al., Citation2011) (OR: 0.88, 95% CI: 0.80–0.97, QP = 0.166).
Sensitivity analysis
Among seven endpoints confirmed to have statistical significance, the analysis using a fixed effect model showed that the point estimates of the OR for four endpoints increased with a narrow 95% CI, but p values (two-tailed tests) were still less than 0.05; as for other three endpoints, the results from the fixed effect model and random effect model were completely consistent. In addition, the reanalysis was performed after the removal of one trial to determine whether the meta-analysis result of an endpoint relied on this particular trial (). With the removal of any one trial, the conclusion derived from the reanalyses for each meta-analysis remained unchanged, that is, the upper limit of OR was smaller than 1 for the six endpoints that had the original point estimate of OR less than 1, the upper limit of OR was greater than 1 for one endpoint that had the original point estimate of OR more than 1, and all p values (one-tailed test) for the seven endpoints were smaller than 0.05 (). The sensitivity analysis using both methods mentioned above showed that the meta-analysis results for all the seven endpoints were solid and reliable with a low sensitivity.
Table 3. Results of sensitivity analysis (novel oral anticoagulants versus control drugs).
In addition, because ximelagatran has been withdrawn from the market due to liver toxicity, four trials involving ximelagatran were removed (Olsson, Citation2003; Albers et al., Citation2005; Tangelder et al., Citation2008; The U.S. National Institutes of Health, Citation2012g), and the removal did not reverse the evaluation results of the efficacy and safety of novel oral anticoagulants (stroke or systemic embolism: OR: 0.71, 95% CI: 0.60–0.85; major bleeding: OR: 0.82, 95% CI: 0.68–0.98). Moreover, the removal of the four ximelagatran trails did not reverse the other 10 endpoints (death from any cause: OR: 0.89, 95% CI: 0.84–0.95; disabling or fatal stroke: OR: 0.64, 95% CI: 0.48–0.86; fatal bleeding: OR: 0.69, 95% CI: 0.60–0.81; serious adverse events: OR: 0.92, 95% CI: 0.88–0.95; acute myocardial infarction: OR: 0.97, 95% CI: 0.83–1.13; systemic embolism: OR: 0.44, 95% CI: 0.18–1.07; major bleeding or clinically relevant non-major: OR: 0.92, 95% CI: 0.76–1.10; all bleeding events: OR: 0.95, 95% CI: 0.74–1.23; all adverse events: OR: 1.04, 95% CI: 0.94–1.16; liver function disorder: OR: 0.91, 95% CI: 0.77–1.09). Furthermore, we removed a trial that included the analysis of two endpoints and published as an abstract (Lip et al., Citation2011), and the removal of this trial along with the four above-mentioned trials involving ximelagatran did not reverse the results of the two endpoints (major bleeding: OR: 0.84, 95% CI: 0.77–0.91; major bleeding or clinically relevant non-major: OR: 0.96, 95% CI: 0.81–1.16). In addition, the removal of five phase I trials that stated “the study was not powered for stroke or systemic embolism” (Ezekowitz et al., Citation2007; Tangelder et al., Citation2008; Lip et al., Citation2009, Citation2011; Weitz et al., Citation2010) did not reverse the results of the efficacy of the novel oral anticoagulants (stroke or systemic embolism: OR: 0.74, 95% CI: 0.61–0.90). The analysis after the combined removal of the above five trials with four ximelagatran-involved trials (Olsson et al., Citation2003; Albers et al., Citation2005; Tangelder et al., Citation2008; The U.S. National Institutes of Health, Citation2012g) still confirmed the efficacy of the novel oral anticoagulants (stroke or systemic embolism: OR: 0.71, 95% CI: 0.58–0.86). Early termination of the trial could lead to the overestimation of the endpoints (Montori et al., Citation2005), therefore, one trial (Connolly et al., Citation2011) with the early termination was removed from our analysis, and the removal did not not reverse the conclusion on the efficacy of novel oral anticoagulants (stroke or systemic embolism: OR: 0.82, 95% CI: 0.74–0.90).
Publication bias proof
The analysis demonstrated that the fail-safe numbers for the eight endpoints were greatly larger than the actual number of included trials in the meta-analysis, and p values of the Egger’s and Begg’s test for each meta-analysis were greater than 0.05, indicating that there was no publication bias in these meta-analyses ().
Table 4. The results of the fail-safe number, Egger’s and Begg’s test (novel oral anticoagulants versus control drugs).
Discussion
Our study indicated that the primary efficacy and safety of the novel oral anticoagulants were superior to the control drugs as well as VKA. The novel oral anticoagulants demonstrated better performances than the control drugs in preventing death from any cause, disabling or fatal stroke, serious adverse events and fatal bleeding. However, there was no significant difference in reducing the risk of acute myocardial infarction, systemic embolism, major bleeding or clinically relevant non-major, all bleeding events and all adverse events. In addition, these effects were independent to the application of ximelagatran.
Our study included phase I–III clinical trials that meet three preconditions including “AF patients” and “novel oral anticoagulants”, as well as “randomized controls”. There were significant differences in the CHADS2 score between trials. For example, the CHADS2 scores in the studies by Ogawa et al. (Citation2011) and Granger et al. (Citation2011) were less than 2.1, whereas in the study from Albers et al. (Citation2005), more than 74% of the subjects had at least two stroke risk factors. The heterogeneity analysis indicated that these trials did result in the heterogeneity of the primary efficacy and safety endpoints in our study. The subgroup analysis showed that the safety of novel oral anticoagulants was superior to the control drugs in patients with CHADS2 score of 1 or 2 points, while there was no difference between the novel oral anticoagulants and control drugs in patients with the CHADS2 score ≥3. The subgroup analysis on the AVERROES trial (Connolly et al., Citation2011) and RE-LY trial (Connolly et al., Citation2009) also suggested that patients with a high CHADS2 score had an increased risk of stroke and bleeding, especially in anticoagulant therapy (Oldgren et al., Citation2011; Flaker et al., Citation2012). Due to the lack of matching antidotes against the novel anticoagulants, a great attention must be paid on the major bleeding during the anticoagulant treatment in patients with a CHADS2 score ≥3. In addition, our analysis indicated that previous administration of VKA did not reduce the safety of the novel oral anticoagulants compared with the control drugs, which is in agreement with a RE-LY trial report (Ezekowitz et al., Citation2010).
It is worth mentioning that the large-dose regimen of novel oral anticoagulants achieved a higher level of efficacy but a greatly lower level of safety than the small- dose regimen, which is in agreement with a report of the RE-LY trial (Eikelboom et al., Citation2011). The novel oral anticoagulants administered in male patients showed a higher efficacy than those in female patients, but no difference in safety between male and female patients, which is in agreement with a report of SPOR TIF trial (Gomberg-Maitland et al., Citation2006). In addition, our study revealed that novel oral anticoagulants exhibited a higher level of efficacy and safety to the control drugs for patients with non-valvular AF.
The previous study has revealed that the peak concentration is an important influencing factor of the bleeding risk (Eriksson et al., Citation2005); therefore, a twice-daily regimen can be adopted to reduce the difference between peak and trough drug levels (Weitz et al., Citation2010) (there was an opposite report that a trial for a weekly regimen was terminated because of the increased bleed events (Amadeus et al., Citation2008). In our study, the novel oral anticoagulants showed an improved safety compared to the control drugs when administered twice daily, while there was no difference between the two when administered once daily. However, in the self-comparison assessment of the novel oral anticoagulants, the levels of efficacy and safety are comparable between the twice-daily regimen and once-daily regimen. Our study did not support the superiority of twice-daily regimen, which might be related to the small sample size. Because our analysis confirmed that the efficacy levels of once-daily and twice-daily regimens are similar, from the perspective of safety, twice-daily regimens are recommended.
Different from the previous study conducted by Kwong et al. (Citation2013), our study confirmed not only the primary efficacy but also the primary safety of the novel oral anticoagulants. Although another two meta-analyses (Ntaios et al., Citation2012; Testa et al., Citation2012) have shown the efficacy and safety of novel oral anticoagulants, these studies repeatedly applied the control group data provided by the RE-LY trial (Connolly et al., Citation2009) (including the events and sample size) in the analysis of one endpoint, thus overestimating the effect of endpoint events of the control group and leading to the unreliable results. Compared with these studies (Kwong et al., Citation2013; Lega et al., Citation2013), the data applied in our study are more accurate. In addition, the data of each endpoint used in our study cover the events only within the period from the beginning of drug administration to the five half-lives of the drugs after the termination of drug administration, but exclude the events occurred in the follow-up period. Different from the study of Miller et al. (Citation2012), our analysis showed that the novel oral anticoagulants had a significantly higher level of safety than warfarin (major bleeding: relative risk (RR) = 0.79, 95% CI: 0.67–0.93, p = 0.003; Miller et al. (Citation2012): RR: 0.88, 95% CI: 0.71–1.09, p = 0.249). Compared to the study conducted by Dentali et al. (Citation2012), this study enrolled more clinical trials, including the controls of both VKA and non-VKA drugs; more endpoints were investigated; and, more subgroup analyses were accomplished to further explore primary efficacy endpoints and safety endpoints. In addition, we also compared the differences caused by gender, drug administration frequency and dose.
Unanswered questions
Due to the limited available data and access to the corresponding database, we could not to collect the raw data of each patient. As a result, some analyses could not be carried out, such as the analysis of time-to-event and INR (international normalized ratio)-to-event, the subgroup analysis on a basis of consistent characteristics of the trial and the subjects (including baseline CHADS2 score, valvular type, AF types (persistent, paroxysmal or permanent), the mean time in therapeutic range of INR, time of endpoint measurement, previous stroke or TIA, previous VKA use and estimated GFR). In addition, due to the high sensitivity of meta-analysis, we had to omit the analysis of death from vascular cause and hemorrhage.
Conclusions
The preventive use of the novel oral anticoagulants in AF patients showed higher primary efficacy and safety than the control drugs (also superior to VKA). In addition, the novel oral anticoagulants showed a superior performance to the control drugs in reducing the risk of death from any cause, disabling or fatal stroke, serious adverse events and fatal bleeding. The previous use of VKA did not reduce the safety of novel anticoagulants. However, for patients with CHADS2 score ≥3, a great attention should be paid in major bleeding during the anticoagulant treatment.
Declaration of interest
There were no conflicts of interest among authors.
This study received financial support from the Sowers Foundation for evidence-based medicine of Taihe Hospital (2013). All received funds were used in the search for related literature, the printing of materials and language translation; therefore, there was no impact from the funding on the results and conclusions in this study.
Supplementary Material
Download PDF (120.5 KB)Acknowledgements
We sincerely thank Mr. Ya Jun Li (the Library of Hubei University of Medicine) and Ms. Ya Ning Wang (Pfizer Medical Information) for their help with the literature searches. We also greatly appreciate the help of Dr. Hui Nie ([email protected]) with the translation of this paper.
References
- Albers GW, Diener HC, Frison L, et al. (2005). Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA 293:690–8
- Amadeus I, Bousser MG, Bouthier J, et al. (2008). Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet 371:315–21
- Apostolakis S, Lip GY. (2012). Novel oral anticoagulants: focus on the direct factor Xa inhibitor darexaban. Expert Opin Investig Drugs 21:1057–64
- Ashworth J, Konstantinou K, Dunn KM. (2011). Prognostic factors in non-surgically treated sciatica: a systematic review. BMC Musculoskelet Disord 12:208
- Bassand JP. (2012). Review of atrial fibrillation outcome trials of oral anticoagulant and antiplatelet agents. Europace 14:312–24
- Chung N, Jeon HK, Lien LM, et al. (2011). Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost 105:535–44
- Connolly SJ, Eikelboom J, Dorian P, et al. (2013). Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 34:1498–505
- Connolly SJ, Eikelboom J, Joyner C, et al. (2011). Apixaban in patients with atrial fibrillation. N Engl J Med 364:806–17
- Connolly SJ, Eikelboom J, O’Donnell M, et al. (2007). Challenges of establishing new antithrombotic therapies in atrial fibrillation. Circulation 116:449–55
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. (2009). Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–51
- Darby-Stewart A, Dachs R, Graber MA. (2012). Rivaroxaban vs. warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Am Fam Physician 85:577–86
- Dentali F, Riva N, Crowther M, et al. (2012). Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation 126:2381–91
- Diener HC, Connolly SJ, Ezekowitz MD, et al. (2010). Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 9:1157–63
- Diener HC, Eikelboom J, Connolly SJ, et al. (2012). Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 11:225–31
- Easton JD, Lopes RD, Bahit MC, et al. (2012). Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 11:503–11
- Eikelboom JW, Wallentin L, Connolly SJ, et al. (2011). Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 123:2363–72
- Eriksson BI, Dahl OE, Buller HR, et al. (2005). A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 3:103–11
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. (2007). Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 100:1419–26
- Ezekowitz MD, Wallentin L, Connolly SJ, et al. (2010). Dabigatran and warfarin in vitamin K antagonist-naive and -experienced cohorts with atrial fibrillation. Circulation 122:2246–53
- Flaker GC, Eikelboom JW, Shestakovska O, et al. (2012). Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke 43:3291–7
- Fox KA, Piccini JP, Wojdyla D, et al. (2011). Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 32:2387–94
- Gage BF, Waterman AD, Shannon W, et al. (2001). Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285:2864–70
- Go AS, Hylek EM, Phillips KA, et al. (2001). Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285:2370–5
- Gomberg-Maitland M, Wenger NK, Feyzi J, et al. (2006). Anticoagulation in women with non-valvular atrial fibrillation in the stroke prevention using an oral thrombin inhibitor (SPORTIF) trials. Eur Heart J 27:1947–53
- Granger CB, Alexander JH, McMurray JJ, et al. (2011). Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365:981–92
- Hankey GJ, Patel MR, Stevens SR, et al. (2012). Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 11:315–22
- Hori M, Matsumoto M, Tanahashi N, et al. (2012). Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J 76:2104–11
- Kwong JS, Lam YY, Yan BP, et al. (2013). Bleeding of new oral anticoagulants for stroke prevention in atrial fibrillation: a meta-analysis of randomized controlled trials. Cardiovasc Drugs Ther 27:23–35
- Lam YY, Ma TK, Yan BP. (2011). Alternatives to chronic warfarin therapy for the prevention of stroke in patients with atrial fibrillation. Int J Cardiol 150:4–11
- Lega JC, Mismetti P, Cucherat M, et al. (2013). Impact of double-blind vs. open study design on the observed treatment effects of new oral anticoagulants in atrial fibrillation: a meta-analysis. J Thromb Haemost 11:1240–50
- Liberati A, Altman DG, Tetzlaff J, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
- Lip G, Halperin J, Petersen P. (2011). Safety and tolerability of the oral factor Xa inhibitor, YM150vs.warfarin in 1297 patients with nonvalvular atrial fibrillation: a dose confirmation study (OPAL-2). J Thromb Haemost 9:748
- Lip GY, Rasmussen LH, Olsson SB, et al. (2011). Oral direct thrombin inhibitor AZD0837 for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation: a Phase II study of AZD0837 in patients who are appropriate for but unable or unwilling to take vitamin K antagonist therapy. Thromb Res 127:91–9
- Lip GY, Rasmussen LH, Olsson SB, et al. (2009). Oral direct thrombin inhibitor AZD0837 for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation: a randomized dose-guiding, safety, and tolerability study of four doses of AZD0837 vs. vitamin K antagonists. Eur Heart J 30:2897–907
- Ma TK, Yan BP, Lam YY. (2011). Dabigatran etexilate versus warfarin as the oral anticoagulant of choice? A review of clinical data. Pharmacol Ther 129:185–94
- Miller CS, Grandi SM, Shimony A, et al. (2012). Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 110:453–60
- Montori VM, Devereaux PJ, Adhikari NK, et al. (2005). Randomized trials stopped early for benefit: a systematic review. JAMA 294:2203–9
- Ntaios G, Papavasileiou V, Diener HC, et al. (2012). Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke 43:3298–304
- Ogawa S, Shinohara Y, Kanmuri K. (2011). Safety and efficacy of the oral direct factor xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation. The ARISTOTLE-J study. Circ J 75:1852–9
- Oldgren J, Alings M, Darius H, et al. (2011). Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med 155:660–7, W204
- Olsson SB, Executive Steering Committee of the SIIII. (2003). Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet 362:1691–8
- Olsson SB, Rasmussen LH, Tveit A, et al. (2010). Safety and tolerability of an immediate-release formulation of theoral direct thrombin inhibitor AZD0837 in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Thromb Haemost 103:604–12
- Patel MR, Mahaffey KW, Garg J, et al. (2011). Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–91
- Petersen P, Grind M, Adler J, et al. (2003). Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. SPORTIF II: a dose-guiding, tolerability, and safety study. J Am Coll Cardiol 41:1445–51
- Stewart S, Hart CL, Hole DJ, et al. (2001). Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86:516–21
- Tangelder MJ, Frison L, Weaver D, et al. (2008). Effect of ximelagatran on ischemic events and death in patients with atrial fibrillation after acute myocardial infarction in the efficacy and safety of the oral direct thrombin inhibitor ximelagatran in patients with recent myocardial damage (ESTEEM) trial. Am Heart J 155:382–7
- Testa L, Agnifili M, Latini RA, et al. (2012). Adjusted indirect comparison of new oral anticoagulants for stroke prevention in atrial fibrillation. QJM 105:949–57
- The Circulation Journal (2012). The supplementary files of “Rivaroxabanvs. Warfarin in Japanese Patients with Atrial Fibrillation”. Available from: https://www.jstage.jst.go.jp/article/circj/76/9/76_CJ-12-0454/_article/supplement [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012a). A dose response study of dabigatran etexilate (BIBR 1048) in pharmacodynamics and safety in patients with non-valvular atrial fibrillation in comparison to warfarin. Available from: http://ClinicalTrials.gov/show/NCT01136408 [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012b). An efficacy and safety study of rivaroxaban with warfarin for the prevention of stroke and non-central nervous system systemic embolism in patients with non-valvular atrial fibrillation. Available from: http://clinicaltrials.gov/ct2/show/results/NCT00403767?sect=X3015#evnt [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012c). Atrial fibrillation (AF) patients not taking vitamin-K antagonist (VKA). Available from: http://clinicaltrials.gov/show/NCT00623779 [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012d). BAY 59-7939 (Factor Xa inhibitor) Phase II low dose study in patients with atrial fibrillation. Available from: http://www.clinicalstudyresults.org/documents/company-study_8338_2.pdf [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012e). BAY 59-7939 (Factor Xa inhibitor) phase II once daily dose study in patients with atrial fibrillation. Available from: http://www.clinicalstudyresults.org/documents/company-study_8338_1.pdf [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012f). Direct factor Xa inhibitor YM150 for prevention of stroke in subjects with non-valvular atrial fibrillation – a double blind, parallel, dose-finding study in comparison with open label warfarin. Available from: http://spo.escardio.org/AbstractDetails.aspx?id=80552&eevtid=40 [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012g). Long-term treatment with the oral direct thrombin inhibitor ximelagatran, compared to warfarin, as stroke prophylaxis in patients with atrial fibrillation. A long term follow-up study stroke prevention by oral thrombin inhibition IV (SPORTIF IV). Available from: http://www.clinicaltrial.gov/ct/show/NCT00206063. [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012h). PETRO stroke prevention in patients with AF by treatment with dabigatran, with and without aspirin, compared to warfarin. Available from: http://clinicaltrials.gov/ct2/show/results/NCT01227629?sect=X76015 [last accessed 21 Oct 2012]
- The U.S. National Institutes of Health. (2012i). Prevention of stroke and systemic embolic events in patients with atrial fibrillation. Available from: http://clinicaltrials.gov/ct2/show/results/NCT00684307?sect=X43015 [last accessed 21 Oct 2012]
- TRIAL-RESULTS CENTER (2011). ARISTOTLE study (apixaban versus warfarin in patients with atrial fibrillation). Available from: http://www.trialresultscenter.org/study10339-ARISTOTLE. htm [last accessed 21 Oct 2012]
- TRIAL-RESULTS CENTER (2005). SPORTIF V study (ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial). Available from: http://www.trialresultscenter.org/study2638-SPORTIF-V.htm [last accessed 21 Oct 2012]
- Weitz JI, Connolly SJ, Patel I, et al. (2010). Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 104:633–41
- Yamashita T, Koretsune Y, Yasaka M, et al. (2012). Randomized, multicenter, warfarin-controlled phase II study of edoxaban in Japanese patients with non-valvular atrial fibrillation. Circ J 76:1840–7